Abstract
Thalidomide, an agent which inhibits biosynthesis of tumor necrosis factor alpha (TNF-α) and which is used to treat a variety of chronic inflammatory conditions, was investigated as therapy for experimental sepsis. Sepsis was induced by intraperitoneal injection of 107 CFU of Escherichia coli per kg of body weight to 80 Wistar rats divided into four groups. Group A consisted of 24 control animals that did not receive any pretreatment, group B consisted of 18 vehicle-treated control animals pretreated with seed oil, group C consisted of 30 rats administered thalidomide diluted in seed oil at a dose of 50 mg/kg 30 min before bacterial challenge, and group D consisted of eight animals that were not challenged with E. coli but that were used for white blood cell count determination. Sepsis was determined by measurement of vital signs before and 6 h after bacterial challenge. After 6 h the animals were killed and blood was sampled for culture; white blood cell count determination; and determination of endotoxin (lipopolysaccharide), TNF-α, interleukin-1β (IL-1β), and IL-6 levels. The levels of these cytokines were also estimated in the supernatants of human monocytes pretreated with thalidomide after exposure to the isolate. Sepsis developed in all vehicle-treated control animals and controls by 6 h after bacterial challenge but in only 10 animals (33.3%) pretreated with thalidomide (P < 0.0001). Six hours after bacterial challenge all animals had similar levels of endotoxemia, IL-1β, and IL-6. The mean white blood cell count for groups A, B, and C were 5,631.1, 2,638.9, and 8,169.3 cells/μl, respectively (P value between groups, <0.0001); the TNF-α levels were 77.3, 107.2, and 26.1 pg/ml, respectively (P values between groups, <0.0001). Pretreatment of human monocytes with thalidomide prevented the secretion of TNF-α and IL-1β but not that of IL-6. It is concluded that thalidomide exerts a considerable anti-inflammatory effect by preventing evolution to sepsis and by decreasing the level of production of TNF-α and therefore deserves to be further evaluated in research for the therapy of sepsis.
Over the last decade the idea of inhibiting tumor necrosis factor alpha (TNF-α) biosynthesis or TNF-α activity as an immunomodulatory therapy for sepsis caused by gram-negative organisms has been thoroughly explored with various animals and in clinical trials (4, 18). Results from recent human studies with the p55 tumor necrosis factor receptor protein (lenercept), however, were disappointing (1). Thalidomide is an old agent that in 1998 was approved for use by the Food and Drug Administration for the treatment of erythema nodosum leprosum. Since then, further knowledge on its immunomodulatory properties has been acquired. A major effect of this drug is reduction of the half-life of TNF-α mRNA in human monocytes (7). Thalidomide is used to treat a variety of dermatological conditions, such as cutaneous lupus erythematosus and Behçet's syndrome; various conditions associated with human immunodeficiency virus infection like aphthous ulcers, wasting syndrome, and diarrhea; and hematologic malignancies, such as multiple myeloma (7, 8).
Thalidomide has been considered potent in reducing the level of TNF-α biosynthesis in rats challenged with bacterial endotoxin (19). In the majority of animal studies on the immunomodulatory treatment of sepsis, sepsis is induced by injection of endotoxins; the present study was designed to investigate the effect of pretreatment with thalidomide on the development of sepsis in rats after bacterial challenge with an Escherichia coli clinical isolate from a patient with severe sepsis. Thalidomide was administered orally as pretreatment and not as treatment, since intestinal absorbance could be defective in a rat model of abdominal infection and a parenteral formulation of thalidomide is not available at present.
The effect of thalidomide was evaluated not only by determination of serum cytokine levels but also by evaluation of parameters related to the clinical conditions of the animals. To identify the probable clinical relevance of the present animal study, human monocytes pretreated with thalidomide at concentrations close to those achieved in humans were exposed to the E. coli test isolate in vitro.
MATERIALS AND METHODS
Animals.
A total of 80 male Wistar rats were enrolled in the study. Their mean ± standard deviation (SD) weights were 345.1 ± 75.1 g. The study was approved by the Veterinary Directorate of the Perfecture of Athens according to Greek legislation, in conformance with a Council Directive of the European Community (Directive K/18531). Rats were housed in metal cages and had access to tap water and standard balanced chow ad libitum. The temperature ranged between 18 and 22°C, the relative humidity was between 55 and 65%, and the light-dark cycle consisted of light from 6 a.m. to 6 p.m.
Bacterial isolate.
One E. coli isolate from the blood of a female patient dying from severe sepsis with underlying acute pyelonephritis and subsequent multiple-organ failure was used in the study. The isolate was stored at −70°C as multiple aliquots in skim milk (Becton Dickinson, Cockeysville, Md.); one aliquot was thawed before each experiment. Single colonies were incubated for 2 h at 37°C in 5 ml of Muller-Hinton broth (Oxoid Ltd., London, United Kingdom) in a shaking water bath and were grown to the log phase. The bacterial inoculum was then diluted in Mueller-Hinton broth to 5 × 107 CFU/ml to be used for bacterial challenge.
Thalidomide preparation.
Thalidomide was supplied as a white amorphous powder (ICN Biomedicals GmbH, Thüringer, Germany) insoluble in water. It was dissolved in sterilized commercial sunflower seed oil. For determination of the MIC of thalidomide for the isolate, the drug in seed oil was further diluted 1:20 in 99% ethanol to become water soluble at a concentration of 5.7 mg/ml. The MIC was determined by a microdilution technique with a final volume of 0.1 ml in Mueller-Hinton broth and was considered the lowest concentration of thalidomide that limited visible bacterial growth after an 18-h incubation at 35°C.
Study design.
Animals were divided into four groups (groups A to D). Group A comprised 24 control animals that did not receive any pretreatment and that were challenged with E. coli, group B comprised 18 control animals pretreated with seed oil 30 min before bacterial challenge, group C comprised 30 animals pretreated with thalidomide 30 min before bacterial challenge, and group D comprised 8 animals that were not exposed to E. coli and that were killed for determination of their white blood cell counts. Thalidomide was administered to group C animals via a gastric tube at a dose of 50 mg/kg of body weight in a volume of 1 ml per animal. The time interval selected for pretreatment was based on previous studies of thalidomide administration before mycobacterial challenge in rabbits (22). The dose used was equal to that used in previous studies with rats (5, 9, 11, 14). A maximum of six animals were enrolled on each day of the experiment; the animals in all groups were represented to minimize the interday biological variation of the results.
The vital signs, i.e., rectal temperature, pulse rate, and breathing rate, of each animal were recorded before and 6 h after bacterial challenge. The breathing rate was estimated by inspection before anesthesia, and the rectal temperature and pulse rate were determined after slight anesthesia with ethyl ether (Merck, Darmstadt, Germany). The temperature was estimated with a thermometer (Omron, Berlin, Germany) placed in the rectum, and the pulse rate was determined by Doppler measurement (DVM 4200; Hadeco, Tokyo, Japan). An inoculum of 107 CFU of E. coli per kg was injected intraperitoneally into each animal, as described by other investigators (12, 20).
Six hours after bacterial challenge blood was sampled from each animal for culture; determination of the white blood cell count; and determination of the endotoxin (lipopolysaccharide [LPS]), TNF-α, interleukin-1β (IL-1β), and IL-6 levels. The abdominal aorta was accessed through a midline incision and was punctured with a 19-gauge needle, and 8 ml of blood was collected in a pyrogen-free syringe. Animals were then euthanized by intramuscular injection of pentothal.
Blood culture and LPS and cytokine level determinations.
One milliliter of blood was added to flasks with thioglycolate growth medium (Becton Dickinson), and the flasks were incubated at 35°C for a total of 2 days. Identification of gram-negative isolates from blood cultures was done with the API 20E kit (bioMérieux, Marcy l' Etoile, France).
For counting of white blood cells, 2 ml of whole blood was added to EDTA-containing tubes (Vacutainer; Becton Dickinson), and the results were read with a Coulter counter.
The sampled blood was added to pyrogen-free tubes (Vacutainer; Becton Dickinson) and centrifuged; the serum was kept refrigerated at −70°C until it was assayed. For estimation of LPS levels, serum was inactivated after dilution 1:10 with pyrogen-free water (BioWhitaker, Walkersville, Md.) and incubated for 5 min at 70°C. LPS levels were then measured by the QCL-1000 Limulus amoebocyte lysate assay (lower detection limit, 0.1 EU/ml [1 EU = 10 ng]; BioWhitaker). TNF-α, IL-1β, and IL-6 levels were estimated by an enzyme immunoassay (Biotrack, London, United Kingdom). The lower limits of detection for TNF-α, IL-1β, and IL-6 were 15.1, 10, and 10 pg/ml, respectively. All determinations were performed in duplicate.
Definition of sepsis.
The presence of sepsis 6 h after bacterial challenge was defined in analogy to the criteria applied for humans (2). Any animal was considered to be septic if it satisfied at least two of the following criteria: (i) rectal temperature above 38°C or below 36°C, (ii) increased pulse rate, (iii) increased breathing rate, and (iv) more than 12,000 or less than 4,000 white blood cells per μl. The changes in the pulse and breathing rate were determined by comparison with their values before bacterial challenge.
In vitro stimulation of human monocytes.
Monocytes pretreated with thalidomide were exposed to the E. coli isolate used in the study to assess the ability of the isolate to trigger human monocytes and to test the effect of thalidomide in vitro. Human monocytes were isolated from a healthy 30-year-old volunteer, as described previously (13). Briefly, heparinized venous blood was layered over Ficoll Hypaque (Biochrom, Berlin, Germany) and centrifuged at 2,300 × g for 25 min. Isolated mononuclear cells were washed three times with phosphate-buffered saline (pH 7.2; Merck, Darmstadt, Germany) and resuspended in RPMI 1640 (Biochrom) supplemented with 10% fetal bovine serum (Biochrom) and 2 mM glutamine (Biochrom) in the presence of 100 U of penicillin G per ml and 0.1 mg of streptomycin (Sigma, St. Louis, Mo.) per ml. After 1 h in culture at 37°C in 5% CO2, nonadherent cells were removed and adherent monocytes were thoroughly washed with Haks solution (Biochrom). The cells were then removed by dilution with 0.25% trypsin-0.02% EDTA (Biochrom) and resuspended in a 12-well plate at a density of 105 cells per well in RPMI 1640 supplemented with 10% fetal bovine serum and 2 mM glutamine. The purity of the isolated cells in monocytes was >95%. The final volume of each well was 2.4 ml. Four wells were used as controls; the cells in four wells were pretreated for 30 min with seed oil dissolved in 99% ethanol; and the cells in another four wells were pretreated for 30 min with thalidomide in seed oil-ethanol. Ten microliters of the dilution of thalidomide used for MIC determination was added to each well so that the final concentration of thalidomide in each well was 2.38 μg/ml, in analogy to the levels reported in the serum of humans (6, 17, 23); the content of 99% ethanol in each well was 0.4% (vol/vol), and that of seed oil was 0.02% (vol/vol).
After pretreatment for 30 min at 37°C in 5% CO2, 20 μl of a log-phase inoculum of the E. coli isolate was added to each well to a final density of 5 × 106 CFU/well. After 2 h of incubation at 37°C in 5% CO2, the cell supernatant was removed and used for the determination of TNF-α, IL-1β, and IL-6 levels, as described above.
Statistical analysis.
The results for the vital signs, white blood cell counts, and LPS levels are expressed as means ± SDs. The concentrations of cytokines are expressed as means ± standard errors (SEs). Comparisons of all quantitative measurements between groups of animals were performed by analysis of variance with the Bonferroni correction. The advent of sepsis in each group was determined according to the criteria presented above. Comparisons were performed by the χ2 test. Any value of P less than or equal to 0.05 was considered significant. The cytokine concentrations after pretreatment are expressed as picograms per 104 monocytes and are given as the means ± SDs for the four wells.
RESULTS
The MIC of thalidomide for the E. coli test isolate was 712.5 μg/ml. The changes in the vital signs of the animals in groups A, B, and C after bacterial challenge are shown in Table 1. Six hours after bacterial challenge the rectal temperature was significantly decreased in the animals in groups A and B (P = 0.023 and P = 0.05, respectively), but it remained unaltered in the animals in group C (P was not significant [NS]). Over the same time interval, the pulse rate was significantly increased in animals in group A (P = 0.030), but it remained constant in animals in groups B and C (P was NS). The breathing rate was significantly increased in animals in group B (P = 0.030), but it remained constant in animals in group C (P was NS).
TABLE 1.
Changes in rectal temperatures, pulse rates, and breathing rates of Wistar rats in groups A, B, and C after intraperitoneal challenge with E. coli
| Group | Rectal temp (°C) | Pulse rate (min−1) | Breath rate (min−1) |
|---|---|---|---|
| Group A (n = 24) | |||
| Before bacterial challenge | 35.79 ± 1.34a | 276.5 ± 57.9 | 70.3 ± 14.5 |
| After bacterial challenge | 34.78 ± 2.99 | 291.9 ± 101.1 | 74.7 ± 20.0 |
| Group B (n = 18) | |||
| Before bacterial challenge | 37.29 ± 0.67 | 261.8 ± 50.5 | 67.7 ± 13.2 |
| After bacterial challenge | 34.27 ± 1.29 | 254.3 ± 39.3 | 87.3 ± 29.6 |
| Group C (n = 30) | |||
| Before bacterial challenge | 36.49 ± 0.85 | 239.2 ± 34.2 | 104.9 ± 149.0 |
| After bacterial challenge | 36.39 ± 0.21 | 274.6 ± 64.6 | 77.3 ± 14.6 |
The values are means ± SDs.
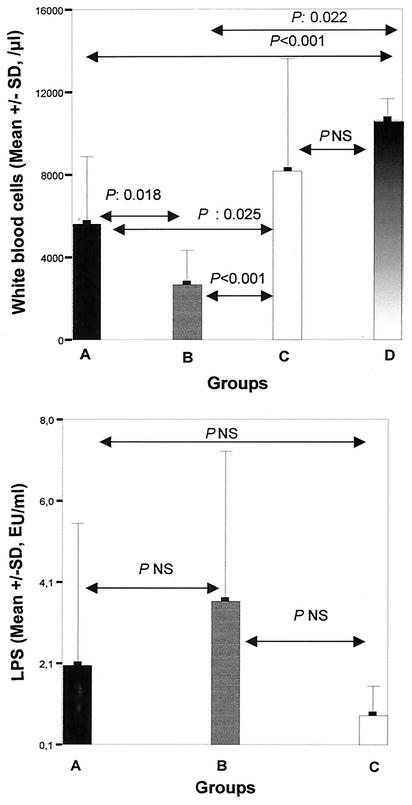
The LPS levels and the white blood cell counts for the various treatment groups 6 h after bacterial challenge and comparisons of those levels among the groups are shown in Fig. 1. The mean ± SD white blood cell count for group A was 5,631.1 ± 3,239.3/μl, that for group B was 2,638.9 ± 1,709.5/μl, that for group C was 8,169.3 ± 5,410.8/μl, and that for group D was 10,575 ± 1,096.6/μl. The differences in the white blood cell counts between the treatment groups involved polymorphonuclear cells. The mean ± SE LPS level for group A was 2.03 ± 0.72 EU/ml, that for group B was 3.59 ± 0.86 EU/ml, and that for group C was 0.87 ± 0.15 EU/ml.
FIG. 1.
Comparison of white blood cell counts and endotoxin (LPS) levels in the sera of controls animal (group A), animals pretreated with seed oil (group B), and animals pretreated with thalidomide (group C) and then killed 6 h after bacterial challenge. The white blood cell counts of animals not challenged with E. coli (group D) are also given.
Bacteremia was detected in all animals. The only species isolated from blood was E. coli. Sepsis was documented in 24 control animals (100%), 16 animals pretreated with seed oil (88.9%), and 10 animals (33.3%) pretreated with thalidomide (P values between groups, <0.0001).
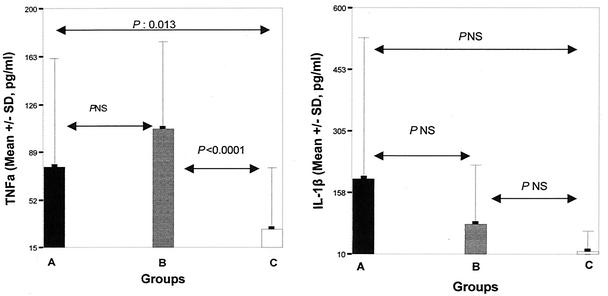
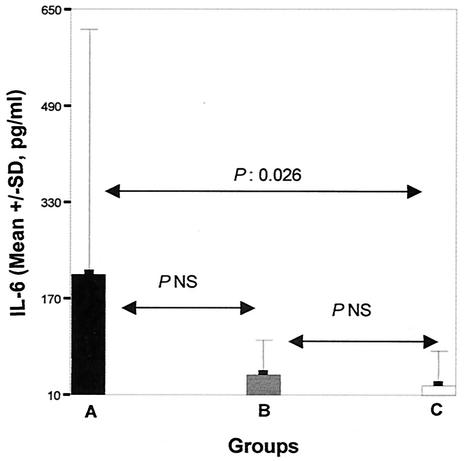
The influence of thalidomide pretreatment on the concentrations of TNF-α, IL-1β, and IL-6 in serum 6 h after bacterial challenge is shown in Fig. 2. The mean ± SE TNF-α concentrations were 77.3 ± 17.6 pg/ml for group A, 107.2 ± 15.9 pg/ml for group B, and 26.1 ± 8.7 pg/ml for group C. The concentrations of IL-1β were 191.7 ± 97.5, 85.0 ± 49.9, and 17.5 ± 11.9 pg/ml, respectively; and the concentrations of IL-6 were 210.2 ± 117.4, 44.5 ± 20.7, and 26.4 ± 13.4 pg/ml, respectively.
FIG. 2.
Comparisons of TNF-α, IL-1β, and IL-6 levels in the sera of controls animals (group A), animals pretreated with seed oil (group B), and animals pretreated with thalidomide (group C) and then killed 6 h after bacterial challenge.
The mean ± SD TNF-α concentrations secreted in the supernatants of the monocytes after 2 h of exposure to the E. coli isolate in vitro were 154.2 ± 13.9 pg/104 cells for the controls, 98.3 ± 13.7 pg/104 cells for cells pretreated with the ethanol-seed oil dilution, and 80.9 ± 18.2 pg/104 cells for cells pretreated with thalidomide. The IL-1β concentrations were 48.2 ± 15.7, 35.0 ± 8.1, and 27.9 ± 7.5 pg/104 cells, respectively. The IL-6 concentrations were 24.9 ± 2.4, 21.8 ± 1.1, and 26.9 ± 8.6 pg/104 cells, respectively.
DISCUSSION
Although thalidomide is considered a potent immunomodulatory agent in that it reduces the level of TNF-α biosynthesis (7), only two experimental studies have referred to its effect on sepsis induced after challenge with bacterial endotoxins (4, 19). Contrary to the usually applied model of sepsis induced after the intraperitoneal injection of endotoxins (10), the present study evaluated the effect of pretreatment with thalidomide on the advent of sepsis and on serum TNF-α levels after intraperitoneal injection of an E. coli isolate from a patient dying of severe sepsis. The application of a live strain attempted to create conditions similar to those encountered in clinical practice, in which immunomodulatory therapy might become necessary (18). Therefore, attention was focused on the vital signs of the animals.
Six hours after bacterial challenge all animals in the control groups (groups A and B) presented with clinical signs of sepsis. It should be emphasized that although all animals in groups A and B and all animals pretreated with thalidomide presented with bacteremia and with the same level of endotoxemia (Fig. 1), only 33% of the animals pretreated with thalidomide evolved to a septic state. Among the four criteria used to define sepsis, i.e., rectal temperature, heart rate, breath rate, and white blood cell count (2), thalidomide mainly prevented changes in rectal temperature and white blood cell counts (Table 1 and Fig. 1). The anti-inflammatory effects of thalidomide are most obvious on white blood cells; animals pretreated with thalidomide had the same counts 6 h after bacterial challenge as animals that were never exposed to the bacteria.
Determination of cytokine concentrations demonstrated that the reduction in the level of TNF-α production is a probable mechanism of action of thalidomide. Thalidomide acts through degradation of the mRNA of TNF-α, thus lowering the amount of circulating TNF-α (15). Although the action of thalidomide is mainly mediated through inhibition of TNF-α biosynthesis, it provokes a decrease in the levels of other proinflammatory cytokines, such as IL-1β and IL-6, to a lesser extent, as also proved in the present study (Fig. 2). Blocking of the early and rapid biosynthesis of proinflammatory cytokines (4) is an adequate explanation for the beneficiary effect of thalidomide on the progression to sepsis. It should be mentioned that the vehicle applied for the administration of thalidomide, i.e., seed oil, seemed to enhance systemic inflammation, as a decrease in white blood cell counts and a concomitant increase in TNF-α levels followed its administration. Because of this, the reduction of the inflammatory response after pretreatment with thalidomide compared to that after the administration of the vehicle is stronger evidence for the anti-inflammatory properties of thalidomide.
All three treatment groups presented with the same degree of endotoxemia (Fig. 1). The MIC of thalidomide for the test isolate was far higher than its maximum concentration in the serum of humans (6, 17, 23), thus excluding an antibacterial effect of thalidomide as an explanation for its anti-inflammatory effect.
The results presented here are in accordance with those of two other published experimental studies with mice and rats on the effect of thalidomide in sepsis induced after acute challenge with endotoxin (4, 19). In those studies, thalidomide decreased the level of TNF-α production, prolonged the length of survival, and prevented irreversible pathological tissue changes; however, the changes in the levels of other cytokines were not assessed. The present study differed from both of those previous studies in that sepsis was induced by a lethal bacterial isolate and clinical benefit was evaluated by parameters other than survival. Similar findings have also been reported after thalidomide pretreatment in an experimental model of meningitis caused by Mycobacterium bovis, in which thalidomide not only reduced TNF-α levels and white blood cell counts in plasma and cerebrospinal fluid, but it also ameliorated the clinical score for meningitis (21, 22). In contrast to the results of the present study, oral administration of thalidomide in a model of thigh infection caused by Klebsiella pneumoniae in mice did not have any influence on the serum cytokine levels measured (16). However, in that study, blood sampling was performed 24 h after bacterial inoculation; thalidomide might have an effect on the early secretion of TNF-α that leads to sepsis, as shown in the present study (Fig. 2).
It cannot be determined with certainty whether the dose of thalidomide used in this study results in drug levels comparable to those obtained in humans, since pharmacokinetic studies of thalidomide in rats have focused on the levels of its metabolites rather than on the levels of thalidomide in serum (3, 24). This is why in vitro studies on the effect of thalidomide in suppressing cytokine elaboration from human monocytes stimulated with the test strain of E. coli were performed. Pretreatment of monocytes with concentrations of thalidomide similar to those achieved in human serum (6, 17, 23) prevented the secretion of TNF-α and IL-1β.
The present study revealed that thalidomide might efficiently prevent evolution to sepsis after bacterial challenge with a lethal clinical isolate. Its activity is attributed to its immunomodulatory effect on TNF-α biosynthesis. The clinical benefit provided by thalidomide leads the authors to consider thalidomide, already prescribed for a variety of other inflammatory clinical states, as an agent deserving of further research for the therapy of sepsis.
REFERENCES
- 1.Abraham, E., P. F. Laterre, J. Garbino, S. Pingleton, T. Butler, T. Dugernier, B. Margolis, K. Kudsk, K. Zimmerli, P. Anderson, M. Reynaert, D. Law, W. Lesslauer, S. Passe, P. Cooper, A. Burdeska, M. Modi, A. Leighton, M. Salgo, and P. van der Auwera. 2001. Lenercept (p55 tumor necrosis factor receptor protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit. Care Med. 29:503-505. [DOI] [PubMed] [Google Scholar]
- 2.American College of Chest Physicians and Society of Critical Care Medicine. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-875. [PubMed] [Google Scholar]
- 3.Ando, Y., E. Fuse, and W. D. Figg. 2002. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 8:1964-1973. [PubMed] [Google Scholar]
- 4.Arndt, P., and E. Abraham. 2001. Immunological therapy for sepsis. Intensive Care Med. 27(Suppl. 1):S104-S115. [DOI] [PubMed] [Google Scholar]
- 5.Arrieta, O., A. Ortiz-Reyes, D. Rembao, M. Calvillo, E. Rivera, and J. Sotelo. 1999. Protective effect of pentoxifylline plus thalidomide against septic shock in mice. Int. J. Exp. Pathol. 80:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aweeka, F., C. Trapnell, M. Chernoff, A. Jayewardene, J. Spritzler, E. Bellibas, P. Lizak, and J. Jacobson. 2001. Pharmacokinetics and pharmacodynamics of thalidomide in HIV patients treated for oral aphthous ulcers: ACTG protocol 251. J. Clin. Pharmacol. 41:1091-1097. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese, L., and A. B. Fleitscher, Jr. 2000. Thalidomide: current and potential clinical applications. Am. J. Med. 108:487-495. [DOI] [PubMed] [Google Scholar]
- 8.Corral, L., and G. Kaplan. 1999. Immunomodulation by thalidomide and thalidomide analogues. Ann. Rheum. Dis. 58(Suppl. I):1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enomoto, N., Y. Takei, M. Horose, K. Ikejima, H. Miwa, T. Kitamura, and N. Sato. 2002. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization and TNF-alpha production. Gastroenterology 123:291-300. [DOI] [PubMed] [Google Scholar]
- 10.Fink, M. P., and S. O. Heard. 1990. Laboratory models of sepsis and septic shock. J. Surg. Res. 49:186-196. [DOI] [PubMed] [Google Scholar]
- 11.Kenet, G., J. Wardi, Y. Avni, H. Aeed, H. Shirin, L. Zaidel, F. Hershkovitz, and R. Bruck. 2001. Amelioration of experimental colitis by thalidomide. Isr. Med. Assoc. J. 3:644-648. [PubMed] [Google Scholar]
- 12.Larsson-Backström, C., L. Lindmark, and L. Svansson. 1995. Effects of dietary α- and γ-linolenic acids on liver fatty acids, lipid metabolism, and survival in sepsis. Shock 4:11-20. [DOI] [PubMed] [Google Scholar]
- 13.Liel, Y., A. Rudich, O. Nagauker-Shriker, T. Yermiyahu, and R. Levy. 1994. Monocyte dysfunction in patients with Gaucher disease: evidence for interference of glucocerebroside with superoxide generation. Blood 83:2646-2653. [PubMed] [Google Scholar]
- 14.Lopez-Talavera, J. C., G. Cadelina, J. Olchowski, W. Merrill, and R. J. Groszmann. 1996. Thalidomide inhibits tumor necrosis factor alpha, decreases nitric oxide synthesis, and ameliorates the hyperdynamic circulatory syndrome in portal-hypertensive rats. Hepatology 23:1616-1621. [DOI] [PubMed] [Google Scholar]
- 15.Moreira, A., E. Sampaio, A. Zmuidzinas, P. Frindt, K. Smith, and G. Kaplan. 1993. Thalidomide exerts its inhibitory action on TNF-α by enhancing mRNA degradation. J. Exp. Med. 177:1675-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netea, M. G., W. L. Blok, B. J. Kullberg, M. Bemelmans, M. T. E. Vogels, W. A. Buurman, and J. W. M. van der Meer. 1995. Pharmacological inhibitors of tumor necrosis factor production exert differential effects in lethal endotoxemia and in infection with live microorganisms in mice. J. Infect. Dis. 171:393-399. [DOI] [PubMed] [Google Scholar]
- 17.Piscitelli, S. C., W. D. Figg, B. Hahn, G. Kelly, S. Thomas, and R. E. Walker. 1997. Single-dose pharmacokinetics of thalidomide in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 41:2797-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinhart, K., and W. Karzai. 2001. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit. Care Med. 29(Suppl. 1):S121-S125. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt, H., B. Rush, G. Simonian, T. Murphy, J. Hsieh, and M. Condon. 1996. Thalidomide inhibits TNF response and increases survival following endotoxin injection in rats. J. Surg. Res. 63:143-146. [DOI] [PubMed] [Google Scholar]
- 20.Short, B. L., W. M. Gardiner, R. I. Walker, J. R. Fletcher, and J. E. Rogers. 1983. Rat intraperitoneal sepsis. A clinically relevant model. Circ. Shock 10:351-359. [PubMed] [Google Scholar]
- 21.Tsenova, L., B. Mangaliso, G. Muller, Y. Chen, V. H. Freedman, D. Stirling, and G. Kaplan. 2002. Use of ImiD3, a thalidomide analogue, as an adjunct to therapy for experimental tuberculous meningitis. Antimicrob. Agents Chemother. 46:1887-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsenova, L., K. Sokol, V. J. Freedman, and G. Kaplan. 1998. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J. Infect. Dis. 177:1563-1572. [DOI] [PubMed] [Google Scholar]
- 23.Wohl, D. A., F. T. Aweeka, J. Schmitz, R. Pomerantz, D. Weng Cherng, J. Spritzler, L. Fox, D. Simpson, D. Bell, M. K. Holohan, S. Thomas, W. Robinson, G. Kaplan, and H. Teppler. 2002. Safety, tolerability, and pharmacokinetic effects of thalidomide in patients infected with human immunodeficiency virus: AIDS clinical trials group 267. J. Infect. Dis. 185:1359-1363. [DOI] [PubMed] [Google Scholar]
- 24.Zhou, S., P. Kestell, M. D. Tingle, L. M. Ching, and J. W. Paxton. 2001. A difference between the rat and mouse in the pharmacokinetic interaction of 5,6-dimethylxanthenone-4-acetic acid with thalidomide. Cancer Chemother. Pharmacol. 47:541-544. [DOI] [PubMed] [Google Scholar]