Abstract
Children with acute otitis media underwent tympanocentesis and were given a single dose of 30 mg of azithromycin/kg of body weight. At day 28, the overall clinical cure rate was 206 of 242 (85%). Clinical cure rates for patients infected with Streptococcus pneumoniae (67 of 76; 88%) and Haemophilus influenzae (28 of 44; 64%) were consistent with historical rates for the 5-day dosing regimen.
On pharmacodynamic grounds, it is not the duration but rather the total dose of azithromycin that is predicted to correlate most closely with clinical efficacy (3). For azalides like azithromycin, as for the aminoglycoside and fluoroquinolone classes, the ratio of the area under the concentration-time curve (AUC) to the MIC for the pathogen appears to be the best predictor of efficacy. Based on the principle that greater 24-h AUC values and more rapid bacterial killing would have a favorable impact on the emergence of resistance, as well as on the likelihood of improved patient adherence, a study was undertaken to examine the efficacy of a single oral dose of 30 mg of azithromycin/kg of body weight in the treatment of acute otitis media in children.
Children were eligible for enrollment if they demonstrated one or more signs or symptoms of acute otitis media, including ear pain or fullness, discharge from the external auditory canal, decreased hearing, or fever. They must also have had one or more of the following: bulging or marked erythema of the tympanic membrane, loss of the normal light reflex or tympanic membrane landmarks, or impaired tympanic mobility on biphasic pneumatic otoscopy. The effusion was to be documented by acoustic reflectometry (8) with an abnormal reading of 3 or higher. Patients were excluded if they had a history of hypersensitivity to macrolides or azithromycin, had been treated with antibiotics in the previous 30 days, had symptoms of otitis media for longer than 4 weeks, had tympanostomy tubes present, or had been receiving antimicrobial prophylaxis. The institutional review board of each participating center reviewed and approved participation in the trial. Written informed consent was obtained for each patient.
Samples of middle ear fluid were obtained either by tympanocentesis or, in the case of a perforated tympanic membrane, with a swab. Patients then received 30 mg of azithromycin/kg in a single oral dose. Children who vomited within 30 min of dosing were redosed.
The samples of middle ear fluid were split for storage at a central laboratory and cultured locally. All pathogens isolated locally were subcultured and sent to a central laboratory (MDS Clinical Trial Laboratories) for confirmation and susceptibility testing. Phone contact with each patient occurred on study day 5 for assessment of adverse events; this was followed by visits on days 10 and 24 to 28, at which time detailed clinical assessments regarding clinical cure (complete resolution of all signs and symptoms of acute otitis media), improvement, or failure were made. Any patient who was considered to have failed therapy was to have a repeat tympanocentesis.
The final sample size was achieved by allowing for sufficient enrollment to accrue at least 25 patients with Streptococcus pneumoniae, 25 with Haemophilus influenzae, and 15 with Moraxella catarrhalis in the fluid samples from the baseline tympanocenteses. The primary endpoint of the trial was the investigator-designated cure rate at days 24 to 28, for which a 95% confidence interval (CI) was computed using the normal approximation to the binomial distribution. Other endpoints analyzed included the clinical response rate by baseline pathogen at days 24 to 28 and the overall clinical response at day 10. Only S. pneumoniae, M. catarrhalis, and H. influenzae were considered baseline pathogens for the purposes of these analyses.
A total of 248 patients, of whom 247 received azithromycin, were entered in the trial between December 1999 and May 2000. Children from 22 sites distributed among the United States (63%), Costa Rica (16%), Guatemala (13%), and Chile (8%) were enrolled. The mean age of the children was 3.4 years (range, 6 months to 12 years), and 86 of 247 (35%) were ≤2 years of age. At the baseline tympanocentesis, 95% had evidence of bulging, decreased mobility, or erythema of the tympanic membrane and 89% had all three signs. Children under 2 years of age were more likely than those older than 2 years to have these signs, including fever (53 versus 36%, respectively) and impaired mobility of the tympanic membrane (98 versus 91%, respectively). Seventy-two percent (178 of 248) of the children were noted to have had previous episodes of otitis media. The mean duration of symptoms related to the present episode of acute otitis media was 2.5 days.
The clinical cure rate, as determined by investigator, at days 24 to 28 was 206 of 242 (85%) patients (Table 1). Children 2 years of age or younger had a lower cure rate (64 of 83; 77%) than children older than 2 years (142 of 159; 89%). Similar results were seen at day 10, with an overall success rate (cure plus improvement) of 89% (213 of 240 patients). A total of 124 patients had a pathogen identified at the baseline tympanocentesis. The clinical cure rate at days 24 to 28 for these subjects was 100 of 124 (81%). Cure rates were highest for patients with M. catarrhalis isolated at baseline (10 of 10; 100%), followed by S. pneumoniae (67 of 76; 88%) and H. influenzae (28 of 44; 64%). At day 10, the clinical success rate for patients with a pathogen identified at baseline was 86% (105 of 122). By baseline pathogen, the success rates at day 10 were as follows: for M. catarrhalis, 10 of 10 patients (100%); for S. pneumoniae, 70 of 76 (92%); and for H. influenzae, 30 of 42 (71%). Ten children received another antibiotic because of an insufficient clinical response prior to day 5, resulting in a success rate of 96% up to that time point. Of these 10 failures, 5 had positive cultures identified at the baseline tympanocenteses, including 3 with H. influenzae infections, 1 with an S. pneumoniae infection, and 1 with a dual infection with S. pneumoniae and H. influenzae.
TABLE 1.
Clinical outcomes with azithromycin treatment by age range and baseline pathogen
| Subgroup of patients | No. of patients with outcome indicated/total no. (% [95% CI])a
|
|
|---|---|---|
| Clinical success at day 10b | Clinical cure at days 24 to 28 | |
| By age | ||
| ≤2 yr | 69/82 (84 [76, 93]) | 64/83 (77 [68, 87]) |
| >2 yr | 144/158 (91 [81, 96]) | 142/159 (89 [84, 94]) |
| Total | 213/240 (89 [85, 93]) | 206/242 (85 [80, 90]) |
| By pathogen identified at baseline | ||
| S. pneumoniae | 70/76 (92) | 67/76 (88) |
| M. catarrhalis | 10/10 (100) | 10/10 (100) |
| H. influenzae | 30/42 (71) | 28/44 (64) |
| Total | 105/122 (86) | 100/124 (81) |
Total number of patients, 247.
Clinical success is defined as cure plus improvement.
Ten patients considered clinical failures had positive cultures at both the baseline and follow-up tympanocenteses. Seven of these 10 patients had positive cultures for the same organisms for specimens taken from the same ear as in the initial tympanocentesis. The azithromycin MICs were similar (within one tube dilution) for both the baseline and follow-up specimens. Two of these cultures were positive for S. pneumoniae, and five were positive for H. influenzae. The specimens from the repeat tympanocenteses for two patients were sterile, and one patient's specimen grew S. pneumoniae at both the baseline (azithromycin MIC, 8 μg/ml) and repeat (azithromycin MIC, >256 μg/ml) tympanocenteses.
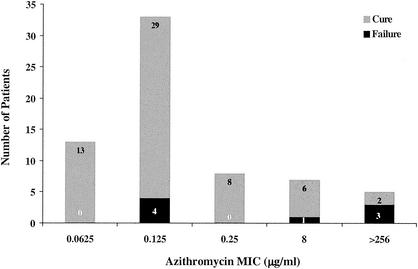
Twelve children were identified as having infections with macrolide-resistant S. pneumoniae isolates (Fig. 1). For 5 of the 12 isolates, the azithromycin MIC was >256 μg/ml, and all 12 were resistant to clindamycin; on PCR they were found to contain the erm(B) gene (11, 12). Only two of the five patients infected with these isolates were cured at day 28. For the seven remaining isolates, the azithromycin MIC was 8 μg/ml; these isolates were sensitive to clindamycin and were found to contain the mef(A) gene. Six of the seven children infected with these isolates were considered cured at day 28. Six of nine children with isolates of S. pneumoniae that were resistant to penicillin (MIC, ≥2 μg/ml) were cured at day 28. The three children who failed therapy were infected with isolates for which the macrolide MIC was >256 μg/ml; these isolates contained the erm(B) gene. All H. influenzae and M. catarrhalis strains that were isolated were susceptible to azithromycin.
FIG. 1.
Day 28 outcomes and MIC distributions for S. pneumoniae.
Ninety-two percent of the patients had complete resolution of their baseline symptoms by day 28. Tympanic membrane mobility was impaired in 93% of patients at baseline, but this frequency decreased to 27% by day 10 and to 12% at days 24 to 28.
As reported by the investigators, 30 of 248 (12.1%) subjects had adverse events that were possibly or probably related to azithromycin, with vomiting (6%) and diarrhea (3%) being the most common. One subject (0.4%) who vomited within 30 min of dosing was removed from therapy.
Azithromycin given in a single oral dose of 30 mg/kg of body weight resulted in clinical cure rates in this study that are consistent with those observed after a similar total dose was given over 5 days (1, 7, 9, 10) or over 3 days (M. W. Dunne, T. Latiolais, B. Lewis, B. Pistorius, G. Bottenfeld, W. Moore, and A. Shemer, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-1532, 2001). Response rates at days 24 to 28 for patients with S. pneumoniae and M. catarrhalis infections were high, and that for patients infected with H. influenzae was consistent with other similarly designed clinical trials (A. Arguedas, C. Loaiza, A. Perez, and A. Gutierrez, Abstr. 4th Int. Conf. Macrolides, Azalides, Streptogramins, Ketolides, abstr. 6.10, 1998; 2, 4, 6) (Table 2). The amount of drug delivered in the first 24 h is greater with a single-dose regimen than with either the 3-day or 5-day treatment options. While the contribution of the maximum concentration of drug in serum cannot be excluded (5), it is felt that the outcome is likely to be best predicted by the ratio of the AUC from 0 to 24 h to the MIC for the pathogen (3), supporting the overall comparability of these results to those of the 3-and 5-day regimens. Of particular interest was the efficacy in six of seven patients whose S. pneumoniae isolates were found to contain the mef(A) gene, which is responsible for efflux-mediated resistance. The possibility that efflux pump resistance could be overcome by exposing resistant organisms to higher concentrations of a drug earlier in the course of therapy warrants further evaluation.
TABLE 2.
Clinical outcomes by baseline pathogens at days 21 to 35 in studies of acute otitis media
| Outcome and treatment | Efficacy rate (%) (95% CI) by baseline pathogen
|
Reference or source | |
|---|---|---|---|
| H. influenzae | S. pneumoniae | ||
| Clinical successa | |||
| Azithromycin (5 days) | 64 (50, 78) | 71 (60, 83) | 9 |
| Amoxicillin-clavulanate | 67 (59, 76) | 74 (65, 82) | 4 |
| Clinical cure | |||
| Cefdinir | 62 (47, 78) | 56 (44, 68) | 2 |
| Azithromycin (single dose) | 67 (54, 80) | 88 (81, 94) | Present study |
| Ceftriaxone (3-day, i.m.b dose) | 69 (56, 81) | 81 (70, 92) | 6 |
| Azithromycin (3 days) | 69 (41, 97) | 94 (72, 100) | Arguedas et al.c |
Clinical success is defined as cure plus improvement.
i.m., intramuscular.
4th Int. Conf. Macrolides, Azalides, Streptogrimins, ketobides.
The results of this study support the conclusion that azithromycin is a safe and effective therapy for acute otitis media while offering the only orally available therapeutic alternative that maximizes compliance and minimizes the burden on the patient and the caregiver.
Acknowledgments
We acknowledge Cynthia McCoig for assistance with preparation of the manuscript.
This work was supported by Pfizer, Inc.
REFERENCES
- 1.Aronovitz, G. 1996. A multicenter, open label trial of azithromycin vs. amoxicillin/clavulanate for the management of acute otitis media in children. Pediatr. Infect. Dis. J. 15:S15-S19. [DOI] [PubMed] [Google Scholar]
- 2.Block, S. L., J. A. Hedrick, J. Kratzer, M. A. Nemeth, and K. J. Tack. 2000. Five-day twice daily cefdinir therapy for acute otitis media: microbiologic and clinical efficacy. Pediatr. Infect. Dis. J. 19:S153-S158. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A. 1997. Postantibiotic effects and the dosing of macrolides, azalides, and streptogramins, p. 27-38. In S. H. Zinner, J. F. Acar, and H. C. Neu (ed.), Expanding indications for the new macrolides, azalides, and streptogramins. Marcel Dekker, New York, N.Y.
- 4.Dagan, R., A. Hoberman, C. Johnson, E. L. Leibovitz, A. Arguedas, F. V. Rose, B. R. Wynne, and M. R. Jacobs. 2001. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr. Infect. Dis. J. 20:829-837. [DOI] [PubMed] [Google Scholar]
- 5.den Hollander, J. G., J. D. Knudsen, J. W. Mouton, K. Fuursted, N. Frimodt-Møller, H. A. Verbrugh, and F. Espersen. 1998. Comparison of pharmacodynamics of azithromycin and erythromycin in vitro and in vivo. Antimicrob. Agents Chemother. 42:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gehanno, P., L. Nguyen, B. Barry, M. Derriennic, F. Pichon, J. M. Goehrs, and P. Berche. 1999. Eradication by ceftriaxone of Streptococcus pneumoniae isolates with increased resistance to penicillin in cases of acute otitis media. Antimicrob. Agents Chemother. 43:16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khurana, C. M. 1996. A multicenter, randomized, open label comparison of azithromycin and amoxicillin/clavulanate in acute otitis media among children attending day care or school. Pediatr. Infect. Dis. J. 15:S24-S29. [DOI] [PubMed] [Google Scholar]
- 8.Kimball, S. 1998. Acoustic reflectometry: spectral gradient analysis for improved detection of middle ear effusion in children. Pediatr. Infect. Dis. J. 17:552-555, 580. [DOI] [PubMed] [Google Scholar]
- 9.McCarty, J. 1996. A multicenter, open label trial of azithromycin for the treatment of children with acute otitis media. Pediatr. Infect. Dis. J. 15:S10-S14. [DOI] [PubMed] [Google Scholar]
- 10.McLinn, S. 1996. A multicenter, double blind comparison of azithromycin and amoxicillin/clavulanate for the treatment of acute otitis media in children. Pediatr. Infect. Dis. J. 15:S20-S23. [DOI] [PubMed] [Google Scholar]
- 11.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tait-Kamradt, A., T. Davies, M. Cronan, M. R. Jacobs, P. C. Appelbaum, and J. Sutcliffe. 2000. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob. Agents Chemother. 44:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]