Abstract
The purpose of this study was to compare the concentrations of levofloxacin and azithromycin in steady-state plasma, epithelial lining fluid (ELF), and alveolar macrophage (AM) after intravenous administration. Thirty-six healthy, nonsmoking adult subjects were randomized to either intravenous levofloxacin (500 or 750 mg) or azithromycin (500 mg) once daily for five doses. Venipuncture and bronchoscopy with bronchoalveolar lavage were performed in each subject at either 4, 12, or 24 h after the start of the last antibiotic infusion. The mean concentrations of levofloxacin and azithromycin in plasma were similar to those previously published. The dosing regimens of levofloxacin achieved significantly (P < 0.05) higher concentrations in steady-state plasma than azithromycin during the 24 h after drug administration. The respective mean (± standard deviation) concentrations at 4, 12, and 24 h in ELF for 500 mg of levofloxacin were 11.01 ± 4.52, 2.50 ± 0.97, and 1.24 ± 0.55 μg/ml; those for 750 mg of levofloxacin were 12.94 ± 1.21, 6.04 ± 0.39, and 1.73 ± 0.78 μg/ml; and those for azithromycin were 1.70 ± 0.74, 1.27 ± 0.47, and 2.86 ± 1.75 μg/ml. The differences in concentrations in ELF among the two levofloxacin groups and azithromycin were significantly (P < 0.05) higher at the 4- and 12-h sampling times. The respective concentrations in AM for 500 mg of levofloxacin were 83.9 ± 53.2, 18.3 ± 6.7, and 5.6 ± 3.2 μg/ml; those for 750 mg of levofloxacin were 81.7 ± 37.0, 78.2 ± 55.4, and 13.3 ± 6.5 μg/ml; and those for azithromycin were 650 ± 259, 669 ± 311, and 734 ± 770 μg/ml. Azithromycin achieved significantly (P < 0.05) higher concentrations in AM than levofloxacin at all sampling times. The concentrations in ELF and AM following intravenous administration of levofloxacin and azithromycin were higher than concentrations in plasma. Further studies are needed to determine the clinical significance of such high intrapulmonary concentrations in patients with respiratory tract infections.
Intravenous formulations of fluoroquinolones with enhanced antipneumococcal activity and macrolides are recommended for the treatment of hospitalized patients with community-acquired pneumonia (5, 30). The in vitro antimicrobial activity of these agents includes classical (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis) and atypical (Chlamydia pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae) pathogens commonly associated with lower respiratory tract infections. Clinical trials in hospitalized patients receiving monotherapy for the treatment of community-acquired pneumonia have established that the clinical effectiveness and drug-related adverse effects of levofloxacin and azithromycin are similar to those of intravenous cephalosporins such as ceftriaxone or cefuroxime (15, 25, 34, 40).
Several studies have demonstrated that levofloxacin and azithromycin penetrate well into the lung tissues (7, 19, 24, 29, 41). Since these studies used whole tissue samples, intrapulmonary drug penetration into different compartments (e.g., extravascular and intracellular) of the lung was not determined. Recent investigations have established that antibiotic concentrations in epithelial lining fluid (ELF) and alveolar macrophages (AM) can be reliably assessed with techniques involving bronchoscopy and bronchoalveolar lavage (BAL) (3, 4, 31).
Intrapulmonary penetration studies for azithromycin and levofloxacin have been limited to oral drug administration and dose levels of 250 and 500 mg, respectively (1, 2, 11, 20, 32, 33, 36). The purpose of this study was to compare the concentrations of levofloxacin and azithromycin in steady-state plasma, ELF, and AM after intravenous administration of 500-mg doses to healthy adult subjects. In addition, the determination of the intrapulmonary penetration of levofloxacin at a dose of 750 mg was performed, since this once-daily dosage regimen is currently recommended for the treatment of nosocomial pneumonia (43).
(This work was presented in part at the 39th Annual Meeting of the Infectious Diseases Society of America, San Francisco, Calif., 25 to 28 October 2001.)
MATERIALS AND METHODS
Study design and subjects.
This was a randomized, open-label, single-center study of levofloxacin (Ortho-McNeil Pharmaceutical, Inc., Raritan, N.J.) and azithromycin (Pfizer Inc., New York, N.Y.). Nonsmoking, healthy adult subjects 18 years of age or older were considered eligible for this study. Nonsmoking was defined as an abstinence from cigarette smoking for the previous 12 months before enrollment in the study. All subjects must have met the inclusion and exclusion criteria and undergone screening procedures that included a medical history, physical examination, and assessment of clinical laboratory parameters (e.g., clinical chemistry, hematology, urinalysis, and pregnancy test [female subjects only]) prior to drug administration. Subjects were required to be within 10% of their acceptable range of weight according to height and frame tables of the Metropolitan Life Insurance Company (28). Exclusion criteria included evidence of significant organ dysfunction; known hypersensitivity to or intolerance of benzodiazepines, lidocaine, fluoroquinolones, or macrolides; concomitant treatment with drugs which might interact with fluoroquinolones or azithromycin; history of drug or alcohol dependence; and pregnancy or breastfeeding for women. Women of childbearing potential who were using effective means of contraception were allowed to participate. The study was approved by the Institutional Review Board, and written informed consent was obtained from each subject before study entry.
Subjects randomized to levofloxacin received one of two drug regimens: 500 mg (infused over 60 min) or 750 mg (infused over 90 min) once daily for a total of five doses. Subjects randomized to azithromycin received five intravenous doses of 500 mg administered as a 60-min infusion once daily. Subjects received verbal and written instructions regarding their scheduled outpatient visits for daily drug infusion and observation period. All doses were administered via a controlled infusion pump, and exact infusion times were recorded.
Bronchoscopy and BAL.
Each subject underwent one standardized bronchoscopy and BAL in the outpatient surgical facility at either 4, 12, or 24 h following the start of the last intravenous infusion of antibiotic. The sampling times were selected to provide concentration-time data over the 24-h dosing interval of each drug. The 4-h sampling time was selected to approximate the maximum (peak) intrapulmonary concentration, whereas the 12- and 24-h sampling times represent the midpoint and minimum (trough) concentrations of levofloxacin and azithromycin. In order to facilitate the scheduling of bronchoscopy, subjects randomized to the 4- or 24-h sampling time received their drug infusions between 6:30 and 11:30 a.m. The subjects randomized to the 12-h sampling time received their drug infusions between 6:00 and 8:30 a.m.
Two percent topical lidocaine was applied to the upper airway to prepare subjects for bronchoscopy. If needed, 1% lidocaine was used in the lower airway. A fiber optic bronchoscope (Olympus P-10; Olympus America Inc., Melville, N.Y.) was inserted into a subsegment of the right middle lobe. The bronchoscope was in place for an average of 5.7 min (range, 4 to 10 min).
Four 50-ml aliquots of sterile 0.9% normal saline were instilled into the middle lobe, and each specimen was immediately aspirated and placed in ice. The aspirate from the first 50-ml instillation (BAL 1) was collected separately and discarded, since a significant contamination with cells from the proximal airways has been reported (1, 2, 11, 20, 32, 33, 36). The aspirates recovered from the second, third, and fourth instillations were pooled (BAL 2). The volume of BAL 2 was measured and recorded. A 4-ml aliquot was removed from BAL 2 and immediately sent to the laboratory for cell count and differential. The remaining volume of BAL 2 was immediately centrifuged at 400 × g for 5 min. The supernatant and cells were separated and frozen at −70°C until the assays were performed. A single aliquot of supernatant was separated and frozen for the urea assay.
Blood pressure, heart rate, respiratory rate, and pulse were recorded before, at the end of, and 30 to 60 min following the end of bronchoscopy. A blood sample to determine drug and urea concentration was obtained just prior to scheduled bronchoscopy and kept on ice until centrifuged. In addition, blood samples to determine drug concentrations were obtained prior to and 5 min, 1 h, and 2.75 h after completion of the intravenous infusion of the fifth antibiotic dose. The 2.75-h blood sample for the 750-mg levofloxacin group was collected before the 4-h sampling time associated with bronchoscopy. The blood sampling times were determined by using an optimal sampling strategy for levofloxacin (35) (George Drusano, Albany Medical Center, personal communication). Blood samples were centrifuged at 1,000 × g for 10 min, and plasma was separated and stored at −70°C until the time of the assay. A physical examination and assessment of clinical laboratory parameters (e.g., clinical chemistry, hematology, and urinalysis) were repeated in all subjects following the end of bronchoscopy.
Sample preparation procedures.
Plasma samples of levofloxacin were ultrafiltered with an Amicon (W. R. Grace & Co., Beverly, Mass.) Centrifree apparatus based on a previously established procedure (20, 21). A displacing reagent containing the internal standard (A-57084 DNA gyrase inhibitor; Abbott Laboratories, Abbott Park, Ill.) was used to remove the fluoroquinolone from their protein binding site. The displacing reagent consisted of a mixture of acetonitrile-water (30:70 [vol/vol]) containing 0.5% sodium dodecyl sulfate and 0.075 M phosphate. The ultrafiltrates were injected onto a high-performance liquid chromatography (HPLC) column and eluted by using an ion pair mobile phase.
The sample preparation procedures for BAL fluid and macrophages were based on the detailed descriptions reported by Conte et al. and Patel et al. (11, 33). For the cell sample assay, cells were resuspended to a total of 10% of their recovered lavage fluid volume with a potassium buffer saline solution (pH = 8.0) and carried through three freeze-thaw cycles. After the third cycle, samples were sonicated (Vibracell sonicator; Sonics and Materials, Inc., Danbury, Conn.) at 50% power for 2 min. Macrophage samples were extracted by using the same procedure as for plasma samples. BAL fluid samples were filtrated through a 0.45-μm-pore-size filter (type HV syringe filters; Nihon Millipore Ltd., Yonezawa, Japan) before being injected into the HPLC system.
For azithromycin plasma samples, a liquid-liquid extraction based on previously established procedures was used (39). For the plasma assay curve of lower concentrations in plasma (range, 20.8 to 520 ng/ml), the internal standard was 9a-N-n-propyl azithromycin analogue (CP-65205; Central Research Division, Pfizer, Inc.). For the higher range of concentrations in plasma (range, 208 to 5,200 ng/ml), clarithromycin (USP reference standard; USPC Inc., Rockville, Md.) was used as the internal standard. After addition of the internal standard and 0.1 M sodium carbonate solution, plasma samples were extracted with t-butyl methyl ether. The ether layer was evaporated, and the residue was reconstituted in the mobile phase. The reconstituted samples were washed with hexane and were injected onto an HPLC column. The sample preparation procedures for BAL fluid and macrophages were the same as described for levofloxacin. Macrophage and BAL fluid samples of azithromycin were extracted by using the same procedure as the plasma assay curve for lower concentrations.
The prepared plasma, macrophage, and BAL fluid samples were stored at −70°C until thawed and analyzed. All samples were assayed within 8 months (range, 2 to 8 months) of the time of their collection. All drug and urea assays were performed at the Clinical Research Laboratory of the University of Illinois at Chicago College of Pharmacy.
Levofloxacin assay.
Concentrations of levofloxacin were measured by a reverse-phase HPLC method based on the previously established procedure reported by Granneman and Varga (21). Modifications of the original assay procedure involved a change in the analytical column and the mobile phase composition. These modifications were made to shorten the analysis time and to apply the assay procedure to BAL fluid and AM.
Briefly, the HPLC system consisted of an M510 solvent delivery system (Waters Associates, Milford, Mass.), a WISP model 712 automated sample processor system (Waters Associates), and a Spectroflow 980 programmable fluorescence detector (Applied Biosystems, Foster City, Calif.). The mobile phase was a mixture of acetonitrile-water (42:58 [vol/vol]) containing 0.04 M phosphoric acid, 0.01 M NaH2PO4, 0.4% sodium dodecyl sulfate, and 0.005 M N-acetohydroxamic acid. Prepared samples were pumped through a Symmetry C18 column (5-μm particle size, 3.9 by 150 mm; Waters Associates) at a flow rate of 1.5 ml/min at room temperature. Fluorescence detection was performed at wavelengths of 280 nm (excitation) and 389 nm (emission). The retention times for levofloxacin and A-57084 (internal standard) were 3.9 and 10.4 min, respectively, with a total run time of 13 min.
The standard curves for levofloxacin in plasma and BAL fluid were linear (r2 ≥ 0.99) in the range of concentrations from 8.76 to 5,408 ng/ml and 2.58 to 300 ng/ml, respectively. The intraday coefficients of variation for replicate plasma samples (n = 5) within these concentration ranges varied from 2.4 to 4.1% for plasma and 1.4 to 2.5% for BAL fluid. The interday coefficients of variation were from 1.8 to 2.5% for plasma and 1.3 to 2.5% for BAL fluid. The lower limits of detection were 8.90 and 2.55 ng/ml for plasma and BAL fluid, respectively.
The standard curves for cell suspension were linear (r2 ≥ 0.99) in the range of concentrations from 2.58 to 300 ng/ml for levofloxacin. The intraday coefficients of variation for replicate cell suspension quality control samples (n = 5) within the concentration range of the standard curves varied from 3.1 to 6.8% for levofloxacin. The interday coefficients of variation for cell suspensions varied from 7.1 to 7.7% for levofloxacin. The lower limit of detection for macrophage samples was 2.55 ng/ml for levofloxacin.
Azithromycin assay.
Concentrations of azithromycin were measured by an HPLC assay, with electrochemical detection based on the previously established procedure reported by Shepard et al. (39). Modifications of the original assay procedure involved a change in the analytical column and the mobile phase composition. These modifications were made to shorten the analysis time and to apply the assay procedure to BAL fluid and AM.
Briefly, the HPLC system consisted of an M580 solvent delivery system (ESA, Inc., Chelmsford, Mass.), a WISP model M-715+ automated sample processor system (Waters Associates), and a Coulochem II electrochemical detector with analytical cell M5010 and conditioning cell M5020 (ESA, Inc.). The mobile phase consisted of 60% (vol/vol) acetonitrile in a phosphate buffer containing 0.06 M NaH2PO4 and 0.02 M sodium perchlorate at pH 7.0. Prepared samples were pumped through a BetaBasic Cyano column (5-μm particle size, 4.6 by 150 mm; Keystone Scientific, Inc., Bellefonte, Pa.) at a flow rate of 1.0 ml/min at room temperature. The potentials for the analytical electrodes 1 and 2 were set at +600 and +850 mV, respectively, and the conditioning cell was set at +1,000 mV. The retention times for azithromycin, clarithromycin (internal standard I), and CP-65205 (internal standard II) were 8.0, 4.0, and 10.4 min, respectively. The total run times for the high and low curves were 10 and 12 min, respectively.
The low and high standard curves for azithromycin in plasma were linear (r2 ≥ 0.99) in the range of concentrations from 20.8 to 520 ng/ml and 208 to 5,200 ng/ml, respectively. The intraday coefficients of variation for replicate plasma samples (n = 5) within these concentration ranges varied from 1.7 to 6.9% for the low curve and 2.9 to 3.4% for the high curve. The interday coefficients of variation for the low and high curves ranged from 2.4 to 5.3% and 3.3 to 4.8%, respectively. The lower limits of detection for concentrations of azithromycin in plasma were 20.1 and 201 ng/ml for the low and high curves, respectively.
The standard curves for BAL fluid and cell suspension were linear (r2 ≥ 0.99) in the range of concentrations from 20.8 to 520 ng of azithromycin/ml. The intraday coefficients of variation for replicate quality control samples (n = 5) within the concentration ranges varied from 4.0 to 4.9% for BAL fluid and 4.4 to 7.1% for cell suspension. The interday coefficients of variation for varied from 3.6 to 6.7% for BAL fluid and 4.1 to 5.3% for cell suspension. The lower limit of detection was 20.1 ng/ml for BAL fluid and cell suspension.
Urea assay.
Concentrations of urea in plasma and BAL fluid were performed with a commercially available assay kit (urea nitrogen procedure no. 640; Sigma Diagnostics, St. Louis, Mo.) and measured on a Spectronic 70 spectrophotometer (Analytical Systems Division, Bausch and Lomb). The standard curve for plasma was prepared as recommended by the manufacturer and ranged from 0.75 to 7.50 mg/dl. For BAL samples, a modification of the manufacturer's procedure was made and standard curves were prepared in normal saline over a range of concentrations from 0.113 to 4.50 mg/dl. For both assays, standard curves were linear (r2 ≥ 0.99), interday coefficients of variations were <5%, and the relative accuracy ranged from 99.2 to 101.2%.
Calculations of ELF volume and antibiotic concentrations in ELF and AM.
The calculations of ELF volume and drug concentrations in ELF and AM were performed with fluid from BAL 2 (1, 2, 11, 20, 32, 33, 36).
The concentration of drug (ABXELF) in the ELF was determined as follows:
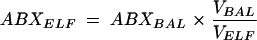 |
where ABXBAL is the measured concentration of antimicrobial agent in BAL fluid, VBAL is the volume of aspirated BAL fluid, and VELF is the volume of ELF sampled by the BAL. VELF is derived from the following equation:
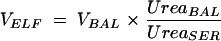 |
where UreaBAL is the concentration of urea in BAL fluid and UreaSER is the concentration of urea in plasma.
The concentration of drug (ABXAM) in the AM was determined as follows:
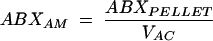 |
where ABXPELLET is the measured concentration of antimicrobial agent in the 1-ml cell suspension and VAC is the volume of alveolar cells in the 1-ml cell suspension. A differential cell count was performed to determine the number of macrophages and monocytes present. A mean macrophage cell volume of 2.42 μl/106 cells was used in the calculations for the volume of alveolar cells in the pellet suspension (2, 31).
Pharmacokinetic analysis.
The area under the concentration-time curve from 0 to 24 h (AUC0-24) was determined for the comparison of systemic exposure within the three matrices of each drug regimen. The AUC0-24 was determined with the linear trapezoidal method by using the microcomputer program WinNonlin (version 1.1; Scientific Consulting, Inc., Apex, N.C.). For concentrations in plasma, the means from each available sampling time (e.g., prior to [time zero] and 0.083, 1, and 2.75 h after completion of the intravenous infusion of the fifth antibiotic dose and during bronchopulmonary sampling times) were used to estimate an AUC0-24 for each drug regimen. The mean concentrations from bronchopulmonary sampling times (e.g., 4, 12, and 24 h) were used to estimate the AUC0-24 of ELF and AM. The 24-h sampling time was also used as a value at time zero for determining the area term of ELF and AM.
Statistical analysis.
Tests for normality and equality of variances were performed with Wilk-Shapiro and Levene's tests, respectively. Analysis of variance (ANOVA) methods were used to assess significant differences among the three study groups by using SAS-PROC GLM. The nonparametric analog to the standard parametric ANOVA was also used. This method involved ranking the data first (SAS-PROC RANK) and then using the ranked data as the response in the ANOVA model. For patient demographic and laboratory characteristics, parametric and nonparametric comparisons were performed by the Newman-Keuls (all pairwise) and Kruskal-Wallis (unblocked data) tests, respectively. Fisher's exact test was used to evaluate the patient variable of sex. For comparisons of drug concentrations, parametric and nonparametric testing were performed with the Newman-Keuls (all pairwise) test. Significance was determined at the P < 0.05 level. All data analyses were performed with PC SAS, version 8.0 (SAS Institute, Inc., Cary, N.C.).
RESULTS
Thirty-six healthy, nonsmoking adult subjects (14 males, 22 females) ranging in age from 18 to 55 years old completed the study (Table 1). A total of 44 subjects signed an informed consent document, and 38 subjects were enrolled in the study. Among the subjects not enrolled, an exclusion criterion was detected during the initial screening visit in three subjects and three subjects subsequently elected not to participate because of the required time commitment of the study. Two of the enrolled subjects were discontinued from the study because of an immediate skin rash of mild-to-moderate severity after the completion of the first intravenous infusion of 750 mg of levofloxacin.
TABLE 1.
Characteristics of 36 study subjectsa
| Drug regimen (n = 12 subjects per regimen) | Age (yr) | No. of subjects of each sex | Ht (in) | Wt (kg) | Total cell count in BAL fluid (cells/liter) | % Monocytes/ macrophages |
|---|---|---|---|---|---|---|
| Levofloxacin, 500 mg | 31.2 ± 10.5 | 7 F, 5 M | 66.3 ± 4.3 | 78.5 ± 12.0 | 1.01 × 108 ± 0.59 × 108 | 77.7 ± 11.4 |
| Levofloxacin, 750 mg | 30.8 ± 9.3 | 8 F, 4 M | 66.6 ± 4.4 | 72.1 ± 12.4 | 1.47 × 108 ± 0.76 × 108 | 74.5 ± 12.3 |
| Azithromycin, 500 mg | 38.2 ± 13.3 | 7 F, 5 M | 68.0 ± 4.5 | 79.6 ± 21.7 | 1.05 × 108 ± 0.51 × 108 | 65.4 ± 17.6 |
Data are expressed as means ± 1 SD (except for sex). Abbreviations: F, females; M, males. The differences in patient characteristics were not significant (P > 0.05) among the three drug regimens.
Levofloxacin and azithromycin were well tolerated in the 36 subjects completing all phases of the study, and no serious drug-related adverse effects were reported. Eight subjects reported one or more mild drug-related adverse effects. These effects included pruritus at the site of infusion (n = 2) and metallic taste (n = 1) in two subjects of the 500-mg levofloxacin group. Three subjects receiving 750 mg of levofloxacin described pruritus at the site of infusion (n = 2) and nausea (n = 1). For the azithromycin group, adverse effects in two subjects included pruritus (n = 1) and burning (n = 1) at the site of infusion, metallic taste (n = 1), and leukopenia (n = 1). Ten of the 36 subjects (28%) were observed to have transient crackles or rhonchi during chest examination after the bronchoscopy procedure. Renal and hepatic function tests were within the normal range for all subjects during the pre- and poststudy evaluations. One subject receiving azithromycin had a decrease in white blood cell count (5,900 cells/mm3 to 2,900 cells/mm3) at the poststudy evaluation, but this count returned to the normal range (4,700 cells/mm3) within 2 weeks.
The numbers (mean ± standard deviation [SD]) of cells recovered in the pooled BAL (BAL 2) were 1.01 × 108 ± 0.59 × 108, 1.47 × 108 ± 0.76 × 108, and 1.05 × 108 ± 0.51 × 108 cells/liter in the 500-mg levofloxacin, 750-mg levofloxacin, and azithromycin groups, respectively (Table 1). The mean (± SD) percentages of cells that were classified as monocytes and macrophages were 77.7 ± 11.4%, 74.5 ± 12.3%, and 65.4 ± 17.6% in the 500-mg levofloxacin, 750-mg levofloxacin, and azithromycin groups, respectively.
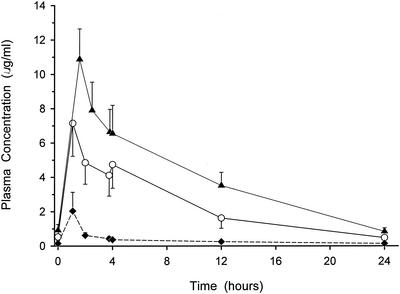
The plasma concentration-versus-time curve for each of the drug regimens is illustrated in Fig. 1. The mean (± SD) peak concentrations after the last infusion of 500-mg levofloxacin, 750-mg levofloxacin, and 500-mg azithromycin in plasma were 7.15 ± 1.92, 10.88 ± 1.77, and 2.04 ± 1.09 μg/ml, respectively. The respective trough concentrations in plasma before the start of the fifth infusion were 0.53 ± 0.22, 0.92 ± 0.34, and 0.15 ± 0.04 μg/ml. The AUC0-24s based on mean plasma concentrations from all available sampling times were 56.4, 95.4, and 8.2 μg · h/ml for 500-mg levofloxacin, 750-mg levofloxacin, and 500-mg azithromycin, respectively.
FIG. 1.
Mean (± SD) plasma concentration-versus-time profiles of 500-mg levofloxacin (open circles), 750-mg levofloxacin (closed triangles), and 500-mg azithromycin (closed diamonds) dosage regimens.
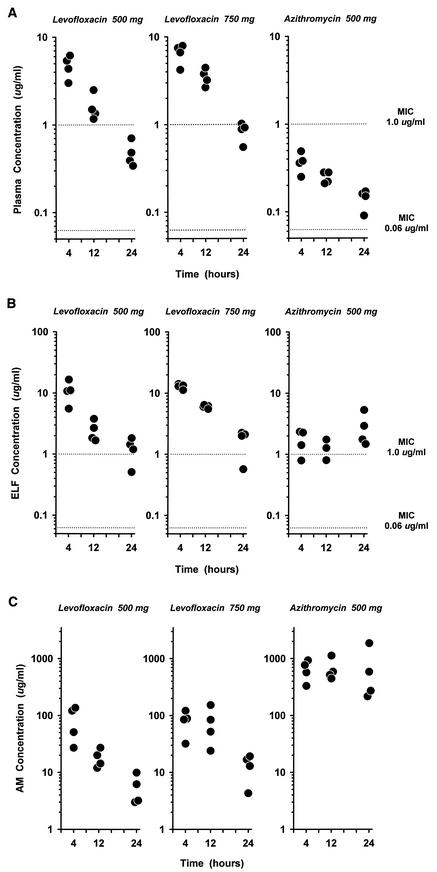
The concentrations of levofloxacin and azithromycin in plasma at the time of bronchoscopy are shown in Fig. 2A. The steady-state plasma concentrations of 500-mg levofloxacin at all sampling times were significantly (P < 0.05) higher than those of 500-mg azithromycin (Table 2) by 3.4 to 12.8 times. The mean concentrations of 750-mg levofloxacin in plasma were 1.4 to 2.2 times higher than those of 500-mg levofloxacin. The 12- and 24-h concentrations of 750-mg levofloxacin in plasma were significantly (P < 0.05) higher than those of 500-mg levofloxacin.
FIG.2.
(A to C) Individual steady-state concentrations of levofloxacin and azithromycin in plasma (A), ELF (B), and AM (C) at 4, 12, and 24 h after the start of the last antibiotic infusion. The y axis is in the log scale. In panels A and B, the dotted lines represent MICs (0.06 μg/ml applies to levofloxacin for H. influenzae and M. catarrhalis and to azithromycin for S. pneumoniae; 1.0 μg/ml applies to levofloxacin for S. pneumoniae and to azithromycin for H. influenzae).
TABLE 2.
Concentrations of levofloxacin and azithromycin in plasma, ELF, and AMa
| Sampling time (h) | Concn (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma
|
ELF
|
AM
|
|||||||
| Levofloxacin (500 mg) | Levofloxacin (750 mg) | Azithromycin (500 mg) | Levofloxacin (500 mg) | Levofloxacin (750 mg) | Azithromycin (500 mg) | Levofloxacin (500 mg) | Levofloxacin (750 mg) | Azithromycin (500 mg) | |
| 4 | 4.74 ± 1.37b | 6.55 ± 1.65c | 0.37 ± 0.10 | 11.01 ± 4.52b,e | 12.94 ± 1.21c,e | 1.70 ± 0.74e | 83.9 ± 53.2b,g,h | 81.7 ± 37.0c,g,h | 649.9 ± 259.1g,h |
| 12 | 1.63 ± 0.59b | 3.52 ± 0.77c,d | 0.25 ± 0.04 | 2.50 ± 0.97b,e | 6.04 ± 0.39c,d,e | 1.27 ± 0.47e,f | 18.3 ± 6.7b,g,h | 78.2 ± 55.4c,d,g,h | 669.4 ± 310.5g,h |
| 24 | 0.48 ± 0.16b | 0.84 ± 0.20c,d | 0.14 ± 0.04 | 1.24 ± 0.55e | 1.73 ± 0.78 | 2.86 ± 1.75e | 5.6 ± 3.2b,g,h | 13.3 ± 6.5c,d,g,h | 734.0 ± 769.8g,h |
Data are expressed as means ± 1 SD; n = 4 subjects at each sample collection time.
Differences between levofloxacin (500 mg) and azithromycin (500 mg) were significant (P < 0.05) during this sampling time of the same body fluid.
Differences between levofloxacin (750 mg) and azithromycin (500 mg) were significant (P < 0.05) during this sampling time of the same body fluid.
Differences between levofloxacin (750 mg) and levofloxacin (500 mg) were significant (P < 0.05) during this sampling time of the same body fluid.
Differences between ELF and plasma were significant (P < 0.05) during this sampling time for the same drug regimen.
Three of four subjects had concentrations equal to or above the quantitative limit of detection.
Differences between AM and plasma were significant (P < 0.05) during this sampling time for the same drug regimen.
Differences between AM and ELF were significant (P < 0.05) during this sampling time for the same drug regimen.
The concentrations of levofloxacin and azithromycin in ELF are displayed in Fig. 2B. The concentrations of 500- and 750-mg levofloxacin in ELF at the 4- and 12-h sampling times were significantly (P < 0.05) higher than those of 500-mg azithromycin (Table 2) by 2.0 to 7.6 times. Similar to those in plasma, mean concentrations of 750-mg levofloxacin in ELF were 1.2 to 2.4 times greater than 500-mg levofloxacin at all sampling times. The AUC0-24 values based on mean concentrations in ELF were 98.6, 151.5, and 45.8 μg · h/ml in the 500- and 750-mg levofloxacin and azithromycin groups, respectively. The concentrations in ELF for the three drug regimens were significantly (P < 0.05) greater than concurrent concentrations in plasma at all sampling times except for the 24-h period of 750-mg levofloxacin (Table 2). The range of mean and individual ratios of concentrations in ELF to concentrations in plasma of 500-mg levofloxacin were 1.5 to 2.6 and 1.0 to 3.7, respectively. In comparison, the mean and individual ratios of concentrations in ELF to concentrations in plasma for 750-mg levofloxacin ranged from 1.8 to 2.1 and 1.0 to 3.1, respectively. The range of mean and individual ratios of concentrations in ELF to concentrations in plasma for azithromycin were 4.7 to 20.0 and 2.2 to 33.6, respectively.
The concentrations of levofloxacin and azithromycin in AM are illustrated in Fig. 2C. The concentrations of azithromycin in AM were significantly (P < 0.05) greater than the concentrations of levofloxacin for either dosage regimen (Table 2). The concentrations of each drug in AM were significantly (P < 0.05) greater than concurrent concentrations in plasma and ELF at all sampling times. The 12- and 24-h concentrations of 750-mg levofloxacin in AM were significantly (P < 0.05) higher than those of 500-mg levofloxacin. The AUC0-24s of mean AM concentrations were 723, 1,378, and 14,944 μg · h/ml in the 500- and 750-mg levofloxacin and azithromycin groups, respectively. The range of mean and individual ratios of concentrations in AM to concentrations in plasma for 500-mg levofloxacin were 1.8 to 17.9 and 5.0 to 22.1, respectively. In comparison, the mean and individual ratios of concentrations in AM to concentrations in plasma for 750-mg levofloxacin ranged from 12.0 to 23.7 and 5.4 to 47.5, respectively. The mean ratio of concentrations in AM to concentrations in plasma for azithromycin was greater than 1,700, and the individual ratios ranged from 867 to 12,158.
DISCUSSION
The concentrations in plasma of levofloxacin for both dosage regimens were similar to those previously described for healthy subjects (8, 10). The mean (± SD) plasma concentrations before (Cmin) and after (Cmax) the last intravenous infusion of 500-mg levofloxacin were 0.53 ± 0.22 and 7.15 ± 1.92 μg/ml, respectively. The AUC0-24 based on mean plasma concentrations from all available sampling times was 56.4 μg · h/ml. The plasma concentration-time profile of once-daily dosing of intravenous levofloxacin (750 mg) resulted in a mean (± SD) Cmin of 0.92 ± 0.34 μg/ml, a Cmax of 10.88 ± 1.77 μg/ml, and an AUC0-24 of 95.4 μg · h/ml. The mean plasma concentrations during the bronchopulmonary sampling times were 1.4 to 2.2 times higher than those of 500-mg levofloxacin (Table 2).
The concentrations of 500-mg levofloxacin in ELF at the 4- and 24-h sampling periods were more than twofold higher than concurrent concentrations in plasma. The mean concentrations of 750-mg levofloxacin in ELF were 1.2 to 2.4 times higher than those of 500-mg levofloxacin at all sampling times (Table 2). The increase in levofloxacin dose by 1.5-fold resulted in a proportional increase in the AUC0-24 of ELF (98.6 to 151.5 μg · h/ml). These results expand our previous observations and confirm that a significant amount of levofloxacin is delivered to the extracellular site (e.g., ELF) of the lungs with either oral or intravenous dosing (20).
The ratio of unbound AUC0-24 to MIC is one of the most predictive pharmacodynamic parameters for clinical and microbiologic responses of fluoroquinolones (13, 37, 38). Minimum AUC0-24/MIC ratios of 125 and 30 have been suggested for gram-negative pathogens and S. pneumoniae, respectively. Combining these pharmacodynamic concepts with our observed ELF data for levofloxacin (750 mg) suggest that adequate coverage is provided at the site of extracellular infections caused by gram-negative pathogens and S. pneumoniae for which the MICs are ≤1 and ≤4 μg/ml, respectively. The higher concentrations in plasma and ELF associated with levofloxacin (750 mg) should be beneficial for the treatment of pathogens commonly causing nosocomial pneumonia.
Concentrations of azithromycin in plasma rapidly declined from a mean value of 2.04 to 0.61 μg/ml between 5 min and 1 h after the end of infusion (Fig. 1). The subsequent concentrations in plasma remained extremely low in value (range, 0.09 to 0.49 μg/ml) during the time of bronchoscopy (Fig. 2A). Our concentration-time data for plasma azithromycin are in good agreement with the limited published data involving intravenous azithromycin at doses of 500 mg to 4 g infused between 1 and 3 h (26, 27). Compared to the previous intrapulmonary penetration studies involving oral azithromycin, our concentrations in plasma are similar after differences in bioavailability (e.g., 37%) and dose (e.g., 250 versus 500 mg) are accounted for (32, 33, 36).
The concentration at the site of infection (e.g., ELF) has been suggested to be more predictive of the potential antimicrobial effects of azithromycin against extracellular pathogens associated with lower respiratory tract infections (16, 36). The concentrations of azithromycin in ELF in this study ranged from 0.79 to 5.32 μg/ml (Fig. 2B). These values are slightly higher than those observed with oral azithromycin (250 mg) (32, 36). The estimated AUC0-24 of azithromycin in ELF was 45.8 μg · h/ml, which is approximately 5.6-fold higher than the AUC0-24 in plasma.
The ratio of unbound AUC0-24 to MIC has also been suggested to be the most predictive pharmacodynamic parameter for the efficacy of azithromycin (13, 38; W. A. Craig, S. Kiem, and D. R. Andes, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1264, 2002). A plasma AUC0-24/MIC ratio of at least 10 (nonneutropenic host) and 25 to 30 (neutropenic host) have been suggested for the in vivo bacteriologic response of S. pneumoniae. Applying these concepts to our observed AUC0-24 in ELF lends support to the effectiveness of intravenous azithromycin (500 mg) for the treatment of susceptible pathogens associated with community-acquired lower respiratory tract infections. Our data would also suggest that the AUC0-24 of azithromycin in ELF is below the magnitude needed against resistant strains of S. pneumoniae (e.g., isolates with either low-level [efflux or Mef determinant] or high-level [ribosomal methylase or Erm determinant] macrolide resistance) for which MICs exceed 4 μg/ml. This would be particularly true in the patients who have serious pneumococcal pneumonia with bacteremia, since azithromycin also exhibits extremely low concentrations in plasma. The recent reports of treatment failures with azithromycin monotherapy in patients with pneumococcal pneumonia with bacteremia are consistent with these pharmacodynamic predictions (17, 23, 42).
Levofloxacin and azithromycin were both highly concentrated in AM (Fig. 2C). Our findings suggest that the 500-mg dose of intravenous azithromycin resulted in concentrations in AM that were approximately 1.4- to 1.9-fold higher than those observed after multiple oral doses of azithromycin (250 mg) (32, 33). The concentrations of intravenous regimens of levofloxacin in AM were similar in magnitude to those that we previously reported for oral regimens of 500 and 750 mg (20). The observed concentrations in AM for both agents were significantly higher than concurrent concentrations in plasma or ELF at all sampling times (Table 2).
There are no established pharmacodynamic relationships between drug concentrations and bacteriological or clinical outcomes for intracellular pathogens. It is likely that the high concentrations of levofloxacin and azithromycin in AM are in part responsible for the effectiveness of these agents in the treatment of intracellular pathogens such as C. pneumoniae and L. pneumophila. Additionally, the concentrations of azithromycin in AM were significantly greater than those observed with either dose regimen of levofloxacin. This difference in the magnitude of concentrations in AM between azithromycin and levofloxacin is probably not clinically significant because extremely high intracellular concentrations are achieved for the entire 24-h dosing interval with either agent and route of administration (oral versus intravenous).
The subjects in our study were healthy, nonsmoking adults without the presence of a significant infection and/or inflammatory process of the lungs. Because of that, we believe that our results are conservative and probably represent the minimal exposure of drug concentrations into the extracellular and intracellular compartments of the lung. Several observations would suggest that intrapulmonary concentrations of both drugs would be higher in patients with clinical conditions of inflammation and/or infection than in healthy volunteers. First, levofloxacin and azithromycin achieve high concentrations in the inflammatory infection model of blister fluid (9, 18). Azithromycin and fluoroquinolones have displayed extensive accumulation by phagocytes which may allow further drug delivery at sites of infections (6, 37). Subjects who smoke may also have higher concentrations of levofloxacin or azithromycin in AM, since several reports have suggested that smokers have increased intrapulmonary uptake of antibiotics that are highly concentrated in AM (12, 22). Finally, it has been demonstrated that infected patients receiving 500 mg of levofloxacin every 24 h have a higher systemic exposure of concentrations in plasma (e.g., AUC0-24 of 72.5 versus 54.6 μg · h/ml) than do healthy volunteers (35). Our study supports the premise that patients with higher concentrations of levofloxacin in plasma have higher concentrations in ELF and AM (e.g., observed when the dose was increased from 500 to 750 mg). This has also been shown by applying Monte Carlo simulations to patient-specific pharmacokinetic parameters and intrapulmonary penetration characteristics of oral levofloxacin (14). Obviously, further studies are warranted in patients with respiratory tract infections to confirm and explore the importance of high intrapulmonary concentrations, pharmacodynamic parameters, and clinical outcomes.
In summary, the concentrations of 500 and 750 mg of levofloxacin in steady-state plasma were significantly higher than those of 500 mg of azithromycin during the entire 24-h study time period. The mean concentrations of levofloxacin and azithromycin in ELF were higher than those in plasma except at the 24-h sampling time of 750-mg levofloxacin. Levofloxacin and azithromycin achieved significantly higher steady-state concentrations in AM than simultaneous concentrations in plasma and ELF throughout the 24-h period after drug administration. Further clinical studies are needed to assess the importance of maintaining and/or increasing the amount of drug exposure in ELF and AM for treatment of serious lower respiratory tract infections.
Acknowledgments
We thank Kelly Deyo and Jie Lu for analytical and technical assistance and Suzanne Hackett of StatWorks, Inc., for statistical assistance.
This work was funded in part by a research grant from Ortho-McNeil Pharmaceutical, Inc.
REFERENCES
- 1.Andrews, J. M., D. Honeybourne, G. Jevons, N. P. Brenwald, B. Cunningham, and R. Wise. 1997. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 40:573-577. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, D. R., R. Wise, J. M. Andrews, et al. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3:886-890. [PubMed] [Google Scholar]
- 3.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob. Agents Chemother. 36:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: observations and clinical relevance. Antimicrob. Agents Chemother. 36:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. M. File, D. M. Musher, and M. J. Fine. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet, M., and P. Van der Auwera. 1992. In vitro and in vivo intraleukocytic accumulation of azithromycin (CP-62,993) and its influence on ex vivo leukocyte chemiluminescence. Antimicrob. Agents Chemother. 36:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazzola, M., C. Siniscalchi, A. Vinciguerra, G. Santangelo, M. G. Matera, and F. Rossi. 1994. Evaluation of lung tissue and hilar lymph node concentrations of azithromycin. Int. J. Clin. Pharmacol. Ther. 32:88-91. [PubMed] [Google Scholar]
- 8.Chien, S.-C., M. C. Rogge, L. G. Gisclon, C. Curtin, F. Wong, J. Natarajan, R. R. Williams, C. L. Fowler, W. K. Cheung, and A. T. Chow. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 41:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Child, J., D. Mortiboy, J. M. Andrews, A. T. Chow, and R. Wise. 1995. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob. Agents Chemother. 39:2749-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow, A. T., C. L. Fowler, R. R. Williams, S. V. Callery-D'Amico, R. R. Williams, R. Nayak, and A. T. Chow. 2001. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 45:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte, J. E., J. Golden, S. Duncan, E. McKenna, E. Lin, and E. Zurlinden. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte, J. E., J. A. Golden, J. Kipps, E. T. Lin, and E. Zurlinden. 2001. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob. Agents Chemother. 45:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig, W. A. 2001. Re-evaluating current antibiotic therapy. Respir. Med. 95(Suppl. A):S12-S19. [DOI] [PubMed] [Google Scholar]
- 14.Drusano, G. L., S. L. Preston, M. H. Gotfried, L. H. Danziger, and K. A. Rodvold. 2002. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 46:586-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.File, T. M., J. Segreti, L. Dunbar, R. Player, R. Kohler, R. R. Williams, C. Kojak, and A. Rubin. 1997. A multicenter, randomized study comparing the efficacy and safety of intravenous and/or oral levofloxacin versus ceftriaxone and/or cefuroxime axetil in treatment of adults with community-acquired pneumonia. Antimicrob. Agents Chemother. 41:1965-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firsov, A. A., S. H. Zinner, S. N. Vostrov, O. V. Kononenko, Y. A. Portnoy, L. V. Shustova, and I. B. Kadenatsi. 2002. Comparative pharmacodynamics of azithromycin and roxithromycin with S. pyogenes and S. pneumoniae in a model that simulates in vitro pharmacokinetics in human tonsils. J. Antimicrob. Chemother. 49:113-119. [DOI] [PubMed] [Google Scholar]
- 17.Fogarty, C., R. Goldschmidt, and K. Bush. 2000. Bacteremic pneumonia due to multidrug-resistant pneumococci in 3 patients treated unsuccessfully with azithromycin and successfully with levofloxacin. Clin. Infect. Dis. 31:613-615. [DOI] [PubMed] [Google Scholar]
- 18.Freeman, C. D., C. H. Nightingale, D. P. Nicolau, P. P. Belliveau, M. A. Banevicius, and R. Quintiliani. 1994. Intracellular and extracellular penetration of azithromycin into inflammatory and noninflammatory blister fluid. Antimicrob. Agents Chemother. 38:2449-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita, A., T. Miya, R. Tanaka, S. Hirayama, H. Isaka, Y. Ono, Y. Koshiishi, and T. Goya. 1999. Levofloxacin concentrations in serum, sputum and lung tissue: evaluation of its efficacy according to breakpoint. Jpn. J. Antibiot. 52:661-666. [PubMed] [Google Scholar]
- 20.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114-1122. [DOI] [PubMed] [Google Scholar]
- 21.Granneman, G. R., and L. L. Varga. 1991. High-performance liquid chromatographic procedures for the determination of temafloxacin in biological matrices. J. Chromatogr. 568:197-206. [DOI] [PubMed] [Google Scholar]
- 22.Hand, W. L., R. M. Boozer, and N. L. King-Thompson. 1985. Antibiotic uptake by alveolar macrophages of smokers. Antimicrob. Agents Chemother. 27:42-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley, M. A., D. J. Weber, P. Gilligan, and M. S. Cohen. 2000. Breakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycin. Clin. Infect. Dis. 31:1008-1011. [DOI] [PubMed] [Google Scholar]
- 24.Lee, L. J., X. Sha, M. H. Gotfried, J. R. Howard, R. K. Dix, and D. N. Fish. 1998. Penetration of levofloxacin into lung tissues after oral administration to subjects undergoing lung biopsy or lobectomy. Pharmacotherapy 18:35-41. [PubMed] [Google Scholar]
- 25.Lipsky, B. A., and C. A. Baker. 1999. Fluoroquinolone toxicity profile: a review focusing on newer agents. Clin. Infect. Dis. 28:352-364. [DOI] [PubMed] [Google Scholar]
- 26.Luke, D. R., G. Foulds, S. F. Cohen, and B. Levy. 1996. Safety, toleration, and pharmacokinetics of intravenous azithromycin. Antimicrob. Agents Chemother. 40:2577-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke, D. R., and G. Foulds. 1997. Disposition of oral azithromycin in humans. Clin. Pharmacol. Ther. 61:641-648. [DOI] [PubMed] [Google Scholar]
- 28.Metropolitan Life Insurance Company. 1983. Metropolitan height and weight tables. Stat. Bull. 64:2-9. [Google Scholar]
- 29.Morris, D. L., A. De Souza, J. A. Jones, and W. E. Morgan. 1991. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur. J. Clin. Microbiol. Infect. Dis. 10:859-861. [DOI] [PubMed] [Google Scholar]
- 30.Niederman, M. S., L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, G. D. Campbell, N. Dean, T. File, M. J. Fine, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson, and V. L. Yu. 2001. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 31.Nix, D. E. 1998. Intrapulmonary concentrations of antimicrobial agents. Infect. Dis. Clin. North. Am. 12:631-646. [DOI] [PubMed] [Google Scholar]
- 32.Olsen, K. M., G. S. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel, K. B., D. Xuan, P. R. Tessier, J. H. Russomanno, R. Quintiliani, and C. H. Nightingale. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plouffe, J., D. B. Schwartz, A. Kolokathis, B. W. Sherman, P. M. Arnow, J. A. Gezon, B. Suh, A. Anzuetto, R. N. Greenberg, M. Niederman, J. A. Paladino, J. A. Ramirez, J. Inverso, C. A. Knirsch, and the Azithromycin Intravenous Clinical Trials Group. 2000. Clinical efficacy of intravenous followed by oral azithromycin monotherapy in hospitalized patients with community-acquired pneumonia. Antimicrob. Agents Chemother. 44:1796-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, F. A. Wong, and M. Corrado. 1998. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob. Agents Chemother. 42:1098-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodvold, K. A., and M. Neuhauser. 2001. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy 21(Suppl. 2):233S-252S. [DOI] [PubMed]
- 38.Rodvold, K. A. 2001. Pharmacodynamics of antiinfective therapy: taking what we know to the patient's bedside. Pharmacotherapy 21(Suppl. 2):319S-330S. [DOI] [PubMed]
- 39.Shepard, R. M., G. S. Duthu, R. A. Ferraina, and M. A. Mullins. 1991. High-performance liquid chromatographic assay with electrochemical detection for azithromycin in serum and tissues. J. Chromatogr. 565:321-337. [DOI] [PubMed] [Google Scholar]
- 40.Vergis, E. N., A. Indorf, T. M. File, J. Phillips, J. Bates, J. Tan, G. A. Sarosi, J. T. Grayston, J. Summersgill, and V. L. Yu. 2000. Azithromycin vs cefuroxime plus erythromycin for empirical treatment of community-acquired pneumonia in hospitalized patients: a prospective, randomized, multicenter trial. Arch. Intern. Med. 160:1294-1300. [DOI] [PubMed] [Google Scholar]
- 41.Von Baum, H., S. Bottcher, H. Hoffmann, and H. G. Sonntag. 2001. Tissue penetration of a single dose of levofloxacin intravenously for antibiotic prophylaxis in lung surgery. J. Antimicrob. Chemother. 47:729-730. [DOI] [PubMed] [Google Scholar]
- 42.Waterer, G. W., R. G. Wunderink, and C. B. Jones. 2000. Fatal pneumococcal pneumonia attributed to macrolide resistance and azithromycin monotherapy. Chest 118:1839-1840. [DOI] [PubMed] [Google Scholar]
- 43.West, M., B. R. Boulanger, C. Fogarty, A. Tennenberg, B. Wiesinger, M. Oross, S.-C. Wu, C. Fowler, N. Morgan, and J. B. Kahn. 2003. Levofloxacin compared with imipenem/cilastatin followed by ciprofloxacin in adult patients with nosocomial pneumonia: a multicenter, prospective, randomized, open-label study. Clin. Ther. 25:485-506. [DOI] [PubMed] [Google Scholar]