Abstract
Cefotaxime, given in two doses (each 100 mg/kg of body weight), produced a good bactericidal activity (−0.47 Δlog10 CFU/ml · h) which was comparable to that of levofloxacin (−0.49 Δlog10 CFU/ml · h) against a penicillin-resistant pneumococcal strain WB4 in experimental meningitis. Cefotaxime combined with levofloxacin acted synergistically (−1.04 Δlog10 CFU/ml · h). Synergy between cefotaxime and levofloxacin was also demonstrated in vitro in time killing assays and with the checkerboard method for two penicillin-resistant strains (WB4 and KR4). Using in vitro cycling experiments, the addition of cefotaxime in sub-MIC concentrations (one-eighth of the MIC) drastically reduced levofloxacin-induced resistance in the same two strains (64-fold increase of the MIC of levofloxacin after 12 cycles versus 2-fold increase of the MIC of levofloxacin combined with cefotaxime). Mutations detected in the genes encoding topoisomerase IV (parC and parE) and gyrase (gyrA and gyrB) confirmed the levofloxacin-induced resistance in both strains. Addition of cefotaxime in low doses was able to suppress levofloxacin-induced resistance.
One of the most challenging problems remains the permanent worldwide increase of penicillin-resistant pneumococcal strains. During the last years, resistance rates in the United States reached 51%, with 33% of the strains showing intermediate resistance (12). In some cases, additional resistance to cephalosporins has further reduced the therapeutic options for infections with penicillin-resistant strains. Furthermore, pneumococcal strains resistant to quinolones have already been isolated (2). Even more alarming is the report of treatment failure with quinolones due to the emergence of resistance during treatment (7). Until now, β-lactam antibiotics remain the drugs of choice for pneumococcal diseases, except when their penetration into infected tissues is limited, as is the case in meningitis. Based on actual guidelines, a combination of a cephalosporin with vancomycin is recommended for the empirical treatment of meningitis, especially when cephalosporin-resistant strains are involved (13). However, the occurrence of recently isolated vancomycin- and cephalosporin-tolerant strains might lead to treatment failures and jeopardize the utility of this antibiotic regimen (16). A highly bactericidal antibiotic combination which does not lead to the emergence of resistance would represent a major advantage. For several years, cefotaxime and levofloxacin have been well-established monotherapies for pneumococcal diseases (20). In this work we have studied the potential synergy between cefotaxime and levofloxacin in vitro and in experimental meningitis and the effect of cefotaxime on levofloxacin-induced resistance in vitro.
MATERIALS AND METHODS
Strains and MIC determination.
The two pneumococcal strains (Streptococcus pneumoniae WB4 and KR4) were originally isolated from two patients with pneumonia at the University Hospital of Bern, Bern, Switzerland. The MICs for WB4 were as follows: penicillin, 4 mg/liter; ceftriaxone, 0.5 mg/liter; cefotaxime, 1 mg/liter; vancomycin, 0.12 to 0.25 mg/liter; levofloxacin, 1 mg/liter; gatifloxacin, 0.12 to 0.25 mg/liter; moxifloxacin, 0.12 mg/liter; and garenoxacin, 0.03 mg/liter. The MICs for KR4 were as follows: penicillin, 4 mg/liter; ceftriaxone, 0.5 mg/liter; cefotaxime, 1 mg/liter; vancomycin, 0.12 to 0.25 mg/liter; levofloxacin, 1 mg/liter; gatifloxacin, 0.25 mg/liter; moxifloxacin, 0.12 mg/liter; and garenoxacin, 0.015 mg/liter.
MICs were determined by the macrodilution broth method. The MIC was defined as the lowest concentration that inhibited visible growth after 12 and 24 h of incubation at 37°C.
Rabbit meningitis model.
The meningitis model, originally described by Dacey and Sande (6) was used in this study. The experimental protocol was accepted by the local ethical committee (Veterinäramt des Kantons, Bern, Switzerland). Young New Zealand White rabbits weighing 2 to 2.5 kg were anesthetized by intramuscular injections of ketamine (30 mg/kg of body weight) and xylazine (15 mg/kg) and were immobilized in stereotaxic frames for the induction of meningitis and for CFU sampling. An inoculum containing approximately 105 CFU of penicillin-resistant strain WB4 was instilled in the cisterna magna. A long-acting anesthetic drug (ethyl carbamate [urethane], 3.5 g/rabbit) was injected subcutaneously, and animals were returned to their cages. Fourteen hours later, a catheter was introduced into the femoral artery for serum sampling and the cisterna magna was punctured again for periodic cerebrospinal fluid (CSF) sampling before and 1, 2, 4, 5, 6, and 8 h after initiation of therapy. Anesthesia was performed by repetitive injections of pentobarbital sodium (Nembutal). Blood samples for measuring cefotaxime and levofloxacin were drawn at 0.25, 0.5, 1, 1.5, 2, 3, 4, 4.25, 4.5, 5, 6, and 8 h after antibiotic administration in animals. Antibiotics were administered by a peripheral ear vein at the following concentrations: cefotaxime, 100 mg/kg; and levofloxacin, 10 mg/kg. Cefotaxime was injected at hours 0 and 4 and levofloxacin was injected only at hour 0. All antibiotics and anesthetic drugs were purchased commercially.
Bacterial titers were measured by 10-fold serial dilutions of CSF samples plated on blood agar plates containing 5% sheep blood and incubated overnight at 37°C. In parallel, 20 μl of undiluted samples was plated (limit of detectability, 50 CFU/ml). Comparisons between dilutions of CSF were used to exclude significant carryover effects during therapy. The antimicrobial activities of the different regimens during the 8-h treatment were calculated by linear regression analysis and expressed as the change in log10 CFU per milliliter per hour and as the change of viable count over 8 h. A value of 1.7 (log10 of the limit of detectability) was assigned to the first sterile CSF sample, and a value of 0 was assigned to any following sterile CSF sample. The results are expressed as means ± standard deviations. Statistical significance was determined by the Newman-Keuls test.
Determination of antibiotic levels in CSF.
Antibiotic concentrations in the CSF were measured by the agar diffusion method. Standard curves were performed in saline with 5% rabbit serum in order to mimic CSF protein concentration (18). Bacillus subtilis ATCC 6633 was used as the test strain for levofloxacin and cefotaxime (25). The inter- and intraday variability ranged between 5 and 10%. The limits of detection were 0.5 mg of cefotaxime/liter and 0.3 mg of levofloxacin/liter. The CSF penetration was calculated by comparison of serum and CSF areas under the curve (Systat software; SSPS, Inc., Evanston, Ill.)
In vitro killing assays.
The two pneumococcal strains (WB4 and KR4) were grown in C+Y medium (14) to an optical density of 0.3 at 590 nm and then diluted 40-fold to 106 CFU, corresponding approximately to the CSF bacterial titer in rabbits before the initiation of therapy. Cefotaxime was added in sub-MIC concentrations (one-half of the MIC for KR4 and one-fourth of the MIC for WB4), and levofloxacin was added in concentrations corresponding to the MIC. Bacterial titers were determined at 0, 2, 4, 6, and 8 h by serial dilution of samples plated on agar plates containing 5% sheep blood and incubated at 37°C for 24 h. Experiments were performed in triplicate, and results are expressed as means ± standard deviations.
Determination of FIC indices.
Fractional inhibitory concentration (FIC) indices were measured by using the checkerboard method as described previously (3). In brief, the two pneumococcal strains (WB4 and KR4) were grown in C+Y medium until the logarithmic growth phase (optical density of 0.3 at 590 nm) and were then diluted 1:40. Approximately 0.5 × 106 to 1 × 106 CFU was pipetted into microtiter trays containing concentrations of levofloxacin and cefotaxime which ranged from 1/32 to 2 times the MIC. Microtiter plates were incubated at 37°C for 24 h. After 6, 12, and 24 h, the plates were read for detection of inhibition of bacterial growth. The experiments were performed in duplicate and were repeated once. FIC indices were calculated by the method of Eliopoulos and Moellering (8). Synergy was defined as an FIC index of ≤0.5, indifference was defined as an FIC index of >0.5 to ≤4, and antagonism was defined as an FIC index of >4.
Selection of quinolone-resistant derivatives in vitro.
Experiments were designed to test the tendency of levofloxacin to select resistant strains in liquid cultures. Large inocula (ca. 108 CFU/ml) of either WB4 or KR4 were exposed to stepwise-increasing concentrations of antibiotics (9). Series of tubes containing twofold-increasing concentrations of levofloxacin were incubated with either WB4 or KR4 (ca. 108 CFU/ml), as for MIC determination. After 12 h of incubation, 0.1-ml samples from the tubes containing the highest antibiotic concentration and still showing turbidity were used to inoculate a new series of tubes containing antibiotic serial dilutions. The experiments were performed during 12 cycles. The MIC was determined after each cycle.
In parallel, the same experimental protocol was used but cefotaxime was added in low concentrations (0.125 mg/liter, corresponding to one-eighth of the MIC for the two strains) to the tubes containing serial dilutions of levofloxacin. After 12 h of incubation, the MIC was determined as described above in tubes containing only levofloxacin.
Preparation of chromosomal DNA, PCR amplification, and DNA sequence analysis.
Chromosomal pneumococcal DNA was prepared as described previously (23). PCR amplification of the parC, parE, gyrA, and gyrB genes was performed according to a published method (22). PCR amplification was performed with a GeneAmp PCR system 9700 apparatus (Perkin Elmer). After amplification, PCR products were purified by using a QIAquick PCR purification kit (Qiagen AG, Basel, Switzerland). Nucleotide sequencing for the PCR amplicons was carried out by using the ABI PRISM dye terminator cycle sequencing ready reaction kit according to the protocol of the manufacturer (Perkin Elmer). An ABI PRISM 377 DNA sequencer was used for sequencing. All testing was performed in duplicate.
RESULTS
After the first dose (100 mg/kg), levels of cefotaxime in serum peaked at 48 mg/liter after 15 min and decreased slowly to 1.5 mg/liter 4 h later. The second injection led to peak levels around 43 mg/liter, declining to 2.5 mg/liter after the end of the experimental period. After the first intravenous cefotaxime injection, the concentrations in CSF ranged between 4.7 and 1.3 mg/liter, and after the second injection at hour 4, the concentrations ranged between 4.5 and 2.4 mg/liter. During the entire treatment period, levels of cefotaxime in CSF remained above the MIC (1 mg/liter for WB4). The penetration of cefotaxime into the inflamed meninges was 17% (area under the concentration-time curve from 0 to 8 h [AUC0-8] in serum, 129 mg · h/liter; AUC0-8 in CSF, 22 mg · h/liter).
One injection of levofloxacin (10 mg/kg) produced peak levels in serum after 25 min of around 20 mg/liter, declining slowly to 10 mg/liter after 2 h, to 7 mg/liter after 4 h, and to 4 mg/liter after 6 h. At the end of the 8-h treatment period, the levels in serum ranged around 1.5 mg/liter (data not shown). In the CSF, the peak levels reached 3.7 mg/liter, decreasing continuously to 1.1 mg/liter. During the entire treatment period, the levofloxacin levels in CSF remained above the MIC (1 mg/liter). The CSF penetration of levofloxacin was 28% (AUC0-8 in serum, 57 mg · h/liter; AUC0-8 in CSF, 16 mg · h/liter).
The efficacies of the different regimens in the experimental meningitis model are summarized in Table 1. In untreated controls, bacterial titers increased only marginally during 8 h (+0.30 ± 0.11 log10 CFU/ml). Cefotaxime monotherapy produced a pronounced antibacterial activity (−0.49 ± 0.11 Δlog10 CFU/ml · h) but managed to sterilize the CSF of only 3 of 9 rabbits at the end of the treatment period. Levofloxacin monotherapy produced similar killing rates and sterilized the CSF of 1 of 9 rabbits. The combination treatment (levofloxacin combined with cefotaxime) was highly bactericidal (−1.04 ± 0.06 Δlog10 CFU/ml · h) and sterilized the CSF of all rabbits. The majority of the CSFs (7 of 9) were already sterile after 6 h.
TABLE 1.
Single-drug and combination therapy against penicillin-resistant S. pneumoniae in experimental meningitis
| Antibiotic | n | Initial titer (log10 CFU/ml) (mean ± SD) | Killing rate (Δlog10 CFU/ml · h) (mean ± SD) | Killing rate over 8 h (log10 CFU/ml) (mean ± SD) |
|---|---|---|---|---|
| None (control) | 7 | 6.15 ± 0.44 | +0.09 ± 0.60a | +0.30 ± 0.11a |
| Cefotaxime | 9 | 5.78 ± 0.84 | −0.49 ± 0.11b | −4.23 ± 0.11c |
| Levofloxacin | 9 | 6.09 ± 0.73 | −0.47 ± 0.15b | −3.57 ± 0.45c |
| Cefotaxime + levofloxacin | 9 | 6.07 ± 0.79 | −1.04 ± 0.06b | −6.07 ± 0.79c |
P < 0.05 versus all groups.
P < 0.001 for cefotaxime plus levofloxacin versus all monotherapies.
P < 0.05 for cefotaxime plus levofloxacin versus all monotherapies.
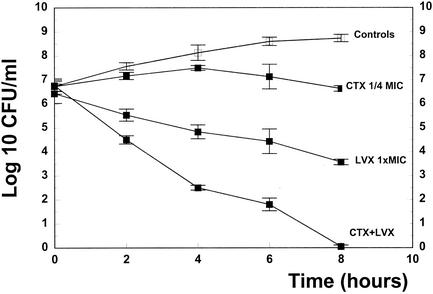
In time killing assays, the antibiotics were chosen at low concentrations, producing only a minimal bactericidal activity when used as monotherapies (cefotaxime, one-half to one-fourth of the MIC; levofloxacin, one times the MIC) in order to detect a potential synergy between the antibiotics. Against KR4, one-half of the MIC of cefotaxime produced a negligible decrease of the bacterial titer (−0.7 log10 CFU/ml over 8 h). Levofloxacin monotherapy (one times the MIC) produced a slightly more pronounced antibacterial effect (−1.9 log10 CFU/ml over 8 h). The combination regimen acted synergistically and was highly bactericidal (−5.8 log10 CFU/ml over 8 h), sterilizing almost all cultures (data not shown). Synergy was defined as the bactericidal effect of a drug combination significantly exceeding the sum of the bactericidal effects of each agent alone (8, 10). Against WB4, the combination regimen produced a similar synergy (Fig. 1) and managed to sterilize all cultures after 8 h. When used in higher doses (five times the MIC), monotherapies managed to sterilize the cultures after 6 and 8 h for levofloxacin and cefotaxime, respectively.
FIG. 1.
Killing rates of cefotaxime (CTX 1/4 MIC), levofloxacin (LVX 1× MIC), and cefotaxime combined with levofloxacin (CTX + LVX) for the penicillin-resistant strain WB4. Experiments were performed in triplicate, and killing rates were expressed as means ± standard deviation.
Synergy between cefotaxime and levofloxacin was also confirmed by FIC indices of 0.25 by using the checkerboard method for both strains.
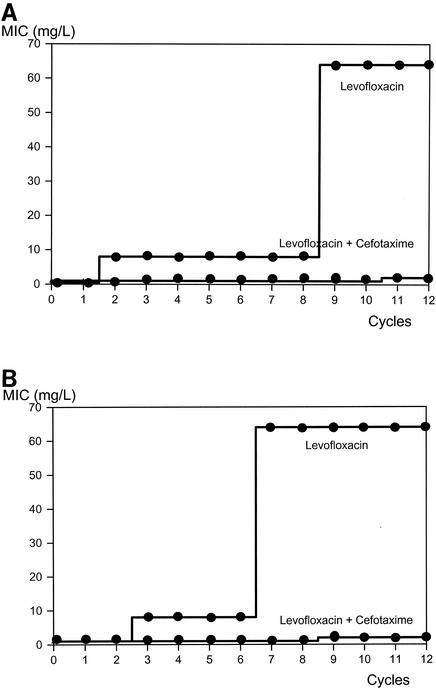
Levofloxacin-resistant mutants were selected by sequential incubation of both pneumococcal strains with different levofloxacin concentrations during 12 cycles, based on a previously described protocol (4). In WB4, the MIC of levofloxacin had already increased to 8 mg/liter after two cycles, and it reached 64 mg/liter after eight cycles and remained stable during the last four cycles. On the other hand, the addition of cefotaxime in low concentrations (one-eighth of the MIC) drastically reduced levofloxacin-induced resistance. After 10 cycles, only a twofold increase of the MIC was observed (Fig. 2A). The stepwise increase of the MIC by sequential incubation with levofloxacin correlated to mutations detected in the two target genes (Ser79→Tyr in parC and Glu85→Lys in gyrA) (Table 2). With the combination regimen, only one mutation in parC (Lys137→Asn) was detected. With the second strain (KR4), the development of levofloxacin-induced resistance was similar. The MIC of levofloxacin increased 8-fold after 2 cycles and 64-fold after 6 cycles and remained stable until the end of the experimental period (Fig. 2B). In analogy to the experiments with WB4, the addition of cefotaxime led to a minimal increase of the MIC of levofloxacin for KR4. Incubation with levofloxacin monotherapy induced two mutations in the parC gene (Ser79→Tyr and Asp 83→Tyr) and one mutation in the gyrA gene (Glu85→Lys). With the combination regimen, only one mutation was detected in the gyrA gene (Prol162→Thr). It is noteworthy that the addition of cefotaxime in low concentrations (one-eighth of the MIC) did not influence the MIC of levofloxacin. No cross-resistance between cefotaxime and levofloxacin was observed. Sequential incubation of the two pneumococcal strains with levofloxacin did not change the MIC of cefotaxime (Table 3).
FIG. 2.
Selection of levofloxacin-resistant mutants of S. pneumoniae WB4 (A) and KR4 (B) exposed to stepwise-increasing concentrations of levofloxacin alone or in combination with a sub-MIC concentration (one-eighth of the MIC) of cefotaxime.
TABLE 2.
Mutations in topoisomerase IV (parC and parE) and gyrase (gyrA and gyrB) genes before and after cyclic exposure to levofloxacin or either of these drugs plus cefotaxime in two penicillin-resistant pneumoccocal strains
| Straina | Mutation(s)
|
|||
|---|---|---|---|---|
| ParC | ParE | GyrA | GyrB | |
| WB4 | None | None | None | None |
| WB4 L | Ser79→Tyr | None | Glu85→Lys | None |
| WB4 CTX+L | Lys137→Asn | None | None | None |
| KR4 | None | None | None | None |
| KR4 L | Ser79→Tyr | None | Glu85→Lys | None |
| Asp83→Tyr | ||||
| KR4 CTX+L | None | None | Pro162→Thr | None |
WB4 and KR4, quinolone-susceptible but penicillin-resistant parent pneumococci; WB4 L and KR4 L, levofloxacin-resistant derivative selected by passages on this drug; WB4 CTX+L and KR4 CTX+L, same as WB4 L and KR4 L, respectively, cycled in the presence of subinhibitory concentrations of cefotaxime (one-eighth of the MIC).
TABLE 3.
MICs of levofloxacin alone and in combination with subinhibitory concentrations of cefotaxime for two penicillin-resistant strains (WB4 and KR4)
| Drug | MIC (mg/liter)a
|
|||||
|---|---|---|---|---|---|---|
| WB4 | WB4 LVX | WB4 LVX + CTX | KR4 | KR4 LVX | KR4 LVX + CTX | |
| Levofloxacin | 1 | 64 | 2 | 1 | 64 | 2 |
| Cefotaxime | 1 | 1 | 1 | 1 | 1 | 1 |
WB4, quinolone-susceptible but penicillin-resistant parent pneumococcus (MIC, 4 mg/liter); WB4 LVX, levofloxacin-resistant derivative selected by passages on this drug; KR4, quinolone-susceptible but penicillin-resistant parent pneumococcus (MIC, 4 mg/liter); KR4 LVX, levofloxacin-resistant derivative selected by passages on this drug; WB4 LVX+CTX and KR4 LVX + CTX, same as WB4 LVX and KR4 LVX, respectively, cycled in presence of subinhibitory concentrations of cefotaxime.
DISCUSSION
During the last decades, pneumococci have invented several strategies in order to resist the respective therapeutic modalities. They managed to resist the action of β-lactam antibiotics by modifying the structure of the targets, i.e., the penicillin-binding proteins, and the action of quinolones by point mutations in the target genes (gyrA, gyrB, parC, and parE) (21) jeopardizing the efficacy of this class of antibiotics. More alarming are recent reports about the emergence of vancomycin- and cephalosporin-tolerant strains leading to treatment failures in cases of pneumococcal meningitis (16). In addition, the emergence of quinolone resistance during treatment might limit the use of these antibiotics as monotherapy. The aim of this study was to test a highly bactericidal regimen for pneumococcal meningitis, reducing the risk of development of quinolone-induced resistance.
The antibiotic doses were chosen in order to mimic levels achieved in humans. Two injections of 100 mg of cefotaxime/kg led to levels in the CSF of rabbits ranging between 4.7 and 1.3 mg/liter, approximately corresponding to levels obtained in humans with bacterial meningitis (1, 15). One injection of levofloxacin in rabbits (10 mg/kg) led to slightly higher peak levels than those measured in humans after 500 mg twice a day (peak levels, 3.7 mg/liter in rabbits versus 2.56 to 1.29 mg/liter in humans) (24).
One of the most interesting aspects of this study was the highly bactericidal activity of the combination regimen compared to that of the monotherapies (−1.04 Δlog10 CFU/ml · h for the combination regimen versus −0.47 and −0.49 Δlog10 CFU/ml · h for the cefotaxime and levofloxacin monotherapies, respectively). The antibacterial efficacy of cefotaxime monotherapy was comparable to a previously published study with the same experimental setting (27), but levofloxacin was slightly more efficacious, although the peak levels in CSF were similar (19). Interestingly, the combination of cefotaxime with levofloxacin was almost twice as efficacious as the standard regimen based on ceftriaxone and vancomycin against the identical strain (WB4) in the same experimental model (circa −0.50 Δlog10 CFU/ml · h for the standard regimen versus −1.0 Δlog10 CFU/ml · h for cefotaxime combined with levofloxacin) (3, 5). The synergy observed in our experimental model was confirmed by FIC indices (0.25) for both strains with the checkerboard method and in time-killing assays over 8 h.
The most striking feature of this study was the effect of the addition of low concentrations of cefotaxime on the development of levofloxacin-induced resistance in vitro. The cefotaxime concentration (one-eighth of the MIC) was chosen based on results obtained with the checkerboard method. Cycling of the two pneumococcal strains in the presence of different concentrations of levofloxacin led to a stepwise increase of resistance until high-level resistance (MIC, 64 mg/liter) was reached after 12 cycles for both strains. This increase of resistance is based on point mutations in genes encoding the two target enzymes, i.e., topoisomerase IV (parC) and gyrase (gyrA). The mutations mentioned in Table 2 have been already described in pneumococci (11, 17, 22, 26). On the other hand, the addition of cefotaxime to the same pneumococcal cultures almost completely impeded levofloxacin-induced resistance, confirmed by an only twofold increase of the MIC after 12 cycles. In both strains, this MIC increase occurred after 10 cycles. This correlates with the point mutations observed in parC (Lys137→Asn) and in gyrA (Pro162→Thr) of these mutants (Table 2). The underlying mechanism of this effect is not completely understood, but two scenarios are conceivable. (i) Based on the synergy observed between these two antibiotics, the bacterial population might be lowered for a longer period below the critical level that allows selection of mutations (i.e., below 106 to 108 CFU/ml). (ii) The prevention of mutations might be due to the combined antibacterial effect of antibiotics with different targets (β-lactams and quinolones). This hypothesis seems more unlikely because the MIC was not influenced by the addition of cefotaxime at low concentrations, although a slight interaction on the cellular level might be missed by the MIC determination.
In analogy to these findings, we have previously shown in the same experimental setting that the addition of another antibiotic interfering with cell wall synthesis, i.e., vancomycin, reduced ciprofloxacin- and trovafloxacin-induced resistance in the same strain (WB4) but to lower orders of magnitude.
This combination produced a prompt and highly bactericidal effect, sterilizing the CSF of rabbits within 6 h, and drastically reduced the risk of quinolone-induced resistance. In summary, we have demonstrated that a combination of cefotaxime with levofloxacin fulfilled all prerequisites of a very efficacious therapeutic regimen needed in pneumococcal meningitis caused by resistant strains. In the future, a combination of β-lactam antibiotics and quinolones might play a central role in the empirical treatment of bacterial meningitis.
Acknowledgments
This study was supported by a grant from Aventis Pharma AG, Zurich, Switzerland.
REFERENCES
- 1.Belohradsky, B. H., K. Bruch, D. Geiss, D. Kafetzis, W. Marget, and G. Peters. 1980. Intravenous cefotaxime in children with bacterial meningitis. Lancet 12:61-63. [DOI] [PubMed] [Google Scholar]
- 2.Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canada surveillance network. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 3.Cottagnoud, P., F. Acosta, M. Cottagnoud, K. Neftel, and M. G. Täuber. 2000. Synergy between trovafloxacin and ceftriaxone against penicillin-resistant pneumococci in the rabbit meningitis model and in vitro. Antimicrob. Agents Chemother. 44:2179-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottagnoud, P., J. M. Entenza, M. Cottagnoud, Y.-A. Que, P. Moreillon, and M. G. Täuber. 2001. Sub-inhibitory concentrations of vancomycin prevent quinolone-resistance in a penicillin-resistance isolate of Streptococcus pneumoniae. BMC Microbiol. 1:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottagnoud, P., C. M. Gerber, M. Cottagnoud, and M. G. Täuber. 2002. Gentamicin increases the efficacy of vancomycin against penicillin-resistant pneumococci in the meningitis model. Antimicrob. Agents Chemother. 46:188-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey., R. G., and M. A. Sande. 1974. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob. Agents Chemother. 6:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumoniae. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos, G. M., and R. C. Moellering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 9.Entenza, J. M., U. Flückiger, P. M. Glauser, and P. Moreillon. 1997. Levofloxacin versus ciprofloxacin, flucloxacillin or vancomycin for treatment of experimental endocarditis due to penicillin-susceptible and -resistant streptococci. Antimicrob. Agents Chemother. 41:1662-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber, C. M., M. Cottagnoud, K. A. Neftel, M. G. Täuber, and P. Cottagnoud. 2000. Evaluation of cefepime alone and in combination with vancomycin against penicillin-resistant pneumococci in the rabbit meningitis model and in vitro. J. Antimicrob. Chemother. 45:63-68. [DOI] [PubMed] [Google Scholar]
- 11.Gootz, T. D., R. Zaniewski, S. Haskell, B. Schmieder, J. Tankovic, D. Girard, P. Courvalin, and R. J. Polzer. 1996. Activity of the new fluoroquinolone trovafloxacin (CP-99219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob. Agents Chemother. 40:2691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs, M. R. 1999. Drug-resistant Streptococcus pneumoniae: rational antibiotic choices. Am. J. Med. 106(Suppl.):19-25. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan, S. L., and E. O. Mason. 1998. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 11:628-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lack, S., and R. D. Hotchkiss. 1960. A study of genetic material determining an enzyme activity in pneumococcus. Biochem. Biophys. Acta 39:508-518. [DOI] [PubMed] [Google Scholar]
- 15.Landesman, S. H., P. M. Schah, M. Armengaud, M. Barza, and C. E. Cherubin. 1981. Past and current roles for cephalosporin antibiotics in treatment of meningitis. Am. J. Med. 71:693-703. [DOI] [PubMed] [Google Scholar]
- 16.McCullers, J. A., B. K. English, and R. Novak. 2000. Isolation and characterization of vancomycin-tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J. Infect. Dis. 181:369-373. [DOI] [PubMed] [Google Scholar]
- 17.Munoz, R., and A. G. de la Campa. 1996. parC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperated with DNA gyrase A subunit in forming resistance phenotype. Antimicrob. Agents Chemother. 40:2252-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nau, R., M. Kaye, E. Sachdeva, M. Sande, and M. G. Täuber. 1994. Rifampin for the therapy of experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 38:1186-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nau, R., T. Schmidt, K. Kaye, J. L. Froula, and M. G. Täuber. 1995. Quinolone antibiotics in therapy of experimental meningitis in rabbits. Antimicrob. Agents Chemother. 39:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niedermann, M. S., L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, D. G. Campbell, N. Dean, T. File, M. File, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson, and V. L. Yu. 2001. Guidelines for the management of adults with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 21.Pan, W. S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan, X. S., and L. M. Fisher. 1996. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J. Bacteriol. 178:4060-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Scotton, P. G., F. Pea, M. Giobbia, M. Baraldo, A. Vaglia, and M. Furlanut. 2001. Cerebrospinal fluid penetration of levofloxacin in patients with spontaneous acute bacterial meningitis. Clin. Infect. Dis. 33:109-111. [DOI] [PubMed] [Google Scholar]
- 25.Simon, H. J., and E. Y. Yin. 1970. Microassay of antimicrobial agents. Appl. Microbiol. 19:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tankovic, J., B. Perichon, J. Duval, and P. Courvalin. 1996. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob. Agents Chemother. 40:2505-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Täuber, M. G., C. J. Hackbarth, K. G. Scott, M. G. Rusnak, and M. A. Sande. 1985. New cephalosporins cefotaxime, cefpimizole, BMY 28142, and HR 810 in experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 27:340-342. [DOI] [PMC free article] [PubMed] [Google Scholar]