Abstract
The in vitro activities of 25 quinolones and fluoroquinolones against erythrocytic stages of Plasmodium falciparum and against liver stages of Plasmodium yoelii yoelii and P. falciparum were studied. All compounds were inhibitory for chloroquine-sensitive and chloroquine-resistant P. falciparum grown in red blood cells. This inhibitory effect increased with prolonged incubation and according to the logarithm of the drug concentration. Grepafloxacin, trovafloxacin, and ciprofloxacin were the most effective drugs, with 50% inhibitory concentrations of <10 μg/ml against both strains. Only grepafloxacin, piromidic acid, and trovafloxacin had an inhibitory effect against hepatic stages of P. falciparum and P. yoelii yoelii; this effect combined reductions of the numbers and the sizes of schizonts in treated cultures. Thus, quinolones have a potential for treatment or prevention of malaria through their unique antiparasitic effect against erythrocytic and hepatic stages of Plasmodium.
The spread of multidrug-resistant Plasmodium falciparum has highlighted the urgent need to develop new antimalarial drugs (14). Quinolones and fluoroquinolones have already been proposed for treatment of malaria, as these drugs were proven to have in vitro antimalarial activity against chloroquine-sensitive and chloroquine-resistant P. falciparum (5, 8, 13, 20, 23). However, all these studies were restricted to the erythrocytic stages of P. falciparum and so far there is no information concerning the potential effects of the drugs against the hepatic development of the parasite.
In this study we assessed the in vitro inhibitory effects of 25 quinolones and fluoroquinolones against blood stages of P. falciparum and hepatic stages of Plasmodium yoelii yoelii and P. falciparum.
The 25 quinolones studied were cinoxacin, enoxacin, flumequine, nalidixic acid, norfloxacin, oxolinic acid, pipemidic acid, piromidic acid, sparfloxacin, temafloxacin, trovafloxacin (Sigma Aldrich, Paris, France), ciprofloxacin, moxifloxacin (Bayer Pharma), marbofloxacin (Vetoquinol), irloxacin (Laboratoire Dr. Esteve), grepafloxacin (Glaxo-Wellcome), gatifloxacin (Grünental), levofloxacin, ofloxacin, pefloxacin (Aventis), rufloxacin (Mediolanum Farmaceutic), lomefloxacin (Monsanto Searle), clinafloxacin (Parke-Davis), fleroxacin (Roche), and rosafloxacin (Sanofi Synthelabo). From a stock solution at a concentration of 1 mg/ml, serial dilutions were prepared in culture medium for in vitro tests against blood and hepatic stages of Plasmodium.
In vitro drug susceptibility assays of blood stages of P. falciparum.
Cultures of the 3D7 chloroquine-sensitive clone and NF54-R chloroquine-resistant strain derived from the NF54 strain were maintained in continuous culture according to a modified version of the method of Trager and Jensen (19).
The in vitro activities of the drugs were evaluated by using the method of Desjardins et al. (4) with modifications. In brief, 200 μl of ring stage parasitized erythrocytes (parasitemia, 0.5%; hematocrit, 1.8%) was distributed in 96-well plates preloaded with nine concentrations (0.025 to 166 μg/ml) of each drug in triplicate and with serial dilutions of chloroquine in positive-control wells. After 72 h, [3H]hypoxanthine was added to each well and then plates were incubated for an additional 24 h. Parasites were harvested, and incorporation of radioactivity was determined by liquid scintillation counting. Experiments were repeated twice.
In vitro drug susceptibility assays of hepatic stages of P. yoelii yoelii and P. falciparum.
The in vitro activities of the 25 quinolones against hepatic stages of P. yoelii yoelii were studied, and only drugs with an activity against P. yoelii yoelii were examined for activity against P. falciparum. Mouse or human hepatocyte cultures were prepared in Lab-Tek slides (Nalge Nunc International, Naperville, Ill.) as previously described (1, 10, 15) and then incubated for 24 h before sporozoite inoculation. Sporozoites were obtained by dissection of the salivary glands of Anopheles stephensi mosquitoes infected with P. yoelii yoelii (265 BY) or P. falciparum (NF54). Dilutions of drugs were made in culture medium (1 to 100 μg/ml), and 8 × 104 sporozoites of P. yoelii yoelii or 2 × 105 sporozoites of P. falciparum were added to hepatocyte cultures. Each drug was tested in four replicates. The culture medium containing the drug was renewed every 24 h, thus maintaining a correct concentration. Cultures were incubated for 48 h for P. yoelii yoelii and for 72 to 120 h for P. falciparum and then fixed with cold methanol. Schizonts were evaluated using an immunofluorescence test with an anti-HSP-70 antibody, as previously described (1, 15). Both the numbers and sizes of schizonts in the cultures were taken into account to assess drug activity. Experiments were repeated twice.
Statistical analysis.
For the erythrocytic stage, a linear regression model was used to summarize the concentration-effect relationship and to determine the 50% inhibitory concentrations (IC50) (3). For the hepatic stage, the IC50 of each compound was determined from the mean values of schizont counts calculated from four replicate cultures.
In vitro activities of quinolones and fluoroquinolones with blood stages of P. falciparum.
All the quinolones and fluoroquinolones tested were inhibitory against the chloroquine-sensitive clone (3D7) and chloroquine-resistant strain (NF54-R) of P. falciparum. A progressive inhibitory effect was observed as judged according to the log10 of the concentration and the duration of incubation with the drug. A marked chloroquine inhibitory effect was noted as early as 48 h and did not significantly vary with time, whereas the quinolone effect progressively increased at 72 and 96 h (data not shown). Table 1 gives the IC50 values of the different drugs from two separate experiments. For 3D7, IC50 values ranged between 4.5 and 142.9 μg/ml. Three quinolones presented an IC50 of <10 μg/ml; the most active quinolone was grepafloxacin. ICs were spread over a narrower range of concentrations for NF54-R than for the 3D7 clone. The molecules that were found to be most active against the 3D7 clone were also found to be among the most active against this strain. However, we found that enoxacin, rosafloxacin, and pefloxacin were more active against this strain than against the 3D7 clone.
TABLE 1.
Antimalarial activities (IC50) of quinolones and fluoroquinolones against chloroquine-sensitive (3D7) and chloroquine-resistant (NF54-R) strains of P. falciparum in vitro
| Drug | IC50 (μg/ml)a
|
|
|---|---|---|
| 3D7 | NF54-R | |
| Chloroquine | 0.011 ± 0.002 | 0.24 ± 0.194 |
| Grepafloxacin | 4.5 ± 1.7 | 3.1 ± 1.8 |
| Ciprofloxacin | 9.2 ± 2.9 | 3.4 ± 1.5 |
| Trovafloxacin | 9.6 ± 2.2 | 9.2 ± 4.8 |
| Clinafloxacin | 13.7 ± 1.8 | 3.3 ± 1.1 |
| Norfloxacin | 17.2 ± 5.9 | 6.7 ± 6.1 |
| Marbofloxacin | 30.8 ± 11.3 | 21.6 ± 6.6 |
| Fleroxacin | 34.6 ± 2.5 | 12.9 ± 2.3 |
| Gatifloxacin | 34.8 ± 9.3 | 10.9 ± 0.3 |
| Temafloxacin | 34.9 ± 1.2 | 19.1 ± 7.2 |
| Flumequine | 38.3 ± 0.7 | 14.1 ± 1.4 |
| Enoxacin | 38.8 ± 0.3 | 1.4 ± 0.3 |
| Pipemidic acid | 40.9 ± 2.0 | 10.5 ± 3.6 |
| Piromidic acid | 41.4 ± 0.4 | 14.4 ± 1.4 |
| Moxifloxacin | 44.8 ± 1.7 | 18.0 ± 7.1 |
| Irloxacin | 49.4 ± 11.9 | 10.5 ± 0.7 |
| Rufloxacin | 81.9 ± 6.9 | 30.1 ± 18.3 |
| Pefloxacin | 83.7 ± 8.8 | 6.5 ± 2.1 |
| Rosoxacin | 87.9 ± 14.9 | 10.6 ± 2.3 |
| Lomefloxacin | 107.9 ± 10.1 | 29.7 ± 17.1 |
| Levofloxacin | 111.6 ± 21.5 | 40.3 ± 15.9 |
| Ofloxacin | 113.1 ± 26.5 | 58.6 ± 33.9 |
| Nalidixic acid | 116.6 ± 7.7 | 59.1 ± 24.8 |
| Cinoxacin | 118.2 ± 18.1 | 28.5 ± 6.4 |
| Oxolinic acid | 129.7 ± 7.9 | 42.2 ± 1.3 |
| Sparfloxacin | 142.9 ± 34.1 | 73.9 ± 1.6 |
Means ± standard deviations from two separate experiments (each one performed in triplicate for each concentration).
In vitro activities of quinolones and fluoroquinolones with hepatic stages of P. yoelii yoelii and P. falciparum.
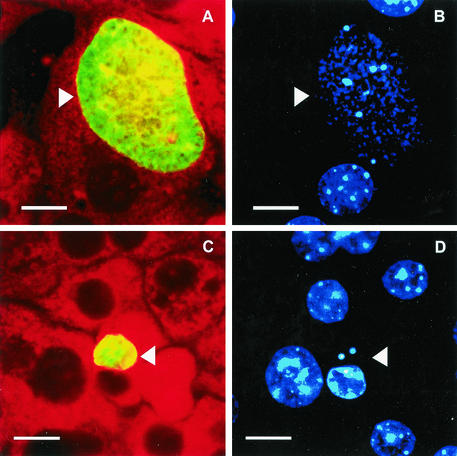
On the basis of schizont counts, 12 out of 25 quinolones were found to be inactive with P. yoelii yoelii. IC50 values were able to be estimated (Table 2) for 12 drugs, whereas they could not be determined for rosoxacin, which was toxic on hepatocyte cultures. Grepafloxacin, piromidic acid, norfloxacin, trovafloxacin, and cinoxacin were the five most effective compounds, with IC50 values ranging from 4.4 μg/ml for grepafloxacin to 36.3 μg/ml for cinoxacin. For grepafloxacin, trovafloxacin, and piromidic acid, this inhibitory effect was associated with marked alteration of schizont morphology (see, e.g., the trovafloxacin results shown in Fig. 1). Treated parasites were significantly smaller than untreated control parasites when examined by immunofluorescence assay (Table 3); staining with DAPI (4′,6′diamidino-2-phenylindole) showed that treated schizonts contained much less nuclear material than controls (Fig. 1). After these results of P. yoelii yoelii experiments were collected, grepafloxacin, trovafloxacin, and piromidic acid were tested with P. falciparum. The same profile of activity was found. The IC50 values were estimated at 5.0 ± 0.2 μg/ml for grepafloxacin, 21.6 ± 2.6 μg/ml for trovafloxacin, and 21.6 ± 7.2 μg/ml for piromidic acid. We observed a significant reduction of the schizont sizes in treated cultures compared to those of controls (Table 3), with a marked alteration of parasitic morphology (data not shown).
TABLE 2.
Antimalarial activities of quinolones and fluoroquinolones against hepatic stages of P. yoelii yoelii at 48 h
| Drug | IC50 (μg/ml)a | Effect on schizont size |
|---|---|---|
| Grepafloxacin | 4.4 ± 0.2 | Yes |
| Norfloxacin | 17.8 ± 2.6 | No |
| Piromidic acid | 21.6 ± 7.2 | Yes |
| Trovafloxacin | 30.8 ± 19.9 | Yes |
| Cinoxacin | 36.3 ± 19.8 | No |
| Ciprofloxacin | 44.0 ± 9.3 | No |
| Rufloxacin | 47.8 ± 8.0 | No |
| Sparfloxacin | 53.0 ± 4.2 | No |
| Ofloxacin | 64.0 ± 1.2 | No |
| Temafloxacin | 72.2 ± 13.6 | No |
| Pefloxacin | 86.3 ± 3.2 | No |
| Clinafloxacin | 90.2 ± 9.5 | No |
| Moxifloxacin | >100 | No |
| Gatifloxacin | >100 | No |
| Flumequin | >100 | No |
| Enoxacin | >100 | No |
| Levofloxacin | >100 | No |
| Lomefloxacin | >100 | No |
| Fleroxacin | >100 | No |
| Marbofloxacin | >100 | No |
| Nalidixic acid | >100 | No |
| Pipemidic acid | >100 | No |
| Oxolinic acid | >100 | No |
| Irloxacin | >100 | No |
| Rosoxacin | NDb | ND |
IC50 values were calculated from the average number of schizonts in four replicate wells for 9 concentrations of each drug in comparison to that of controls.
ND, not determined because of cytotoxicity of rosoxacin on hepatocyte monolayers.
FIG. 1.
Hepatic schizonts of P. yoelii yoelii (arrowheads) at 48 h after infection of mouse hepatocyte cultures treated with trovafloxacin (25 μ/ml) (C and D) or left untreated (A and B) and stained with anti-HSP70 antibodies (A and C) and DAPI (B and D). Bars, 10 μm.
TABLE 3.
Mean diameters of P. yoelii yoelii and P. falciparum schizonts in treated and untreated cultures
| Compound (μg/ml) | Mean diam (μm)± SD of schizonts in cultures for P. yoelii yoeliic | P value vs controld | Mean diam (μm) ± SD of schizonts in cultures for P. falciparumc | P value vs controld |
|---|---|---|---|---|
| Control | 25.7 ± 7.8 | 9.0 ± 3.1a/17.7 ± 9.1b | ||
| Grepafloxacin | ||||
| 3.125 | 12.4 ± 3.6 | <0.0001 | 4.1 ± 1.3a | <0.0001 |
| 6.25 | 8.3 ± 8.0 | <0.0001 | 3.6 ± 0.1a | <0.0001 |
| Trovafloxacin | ||||
| 12.5 | 12.9 ± 5.2 | <0.0001 | 3.7 ± 0.7a | <0.0001 |
| 25 | 9.7 ± 5.0 | <0.0001 | 3.9 ± 1.0a | <0.0001 |
| Piromidic acid | ||||
| 25 | 9.4 ± 3.8 | <0.0001 | 14.4 ± 3.5b | 0.09 |
| 50 | 4.3 ± 1.4 | <0.0001 | 6.3 ± 1.6b | <0.0001 |
Assessment of in vitro activity at 72 h.
Activity evaluated at 120 h.
Measurements of 25 schizonts were performed using an immunofluorescence test with an anti-HSPi72 antibody (magnification, ×250).
Student t test; significant for P < 0.05.
Our results show that all of the quinolones and fluoroquinolones tested have an antimalarial activity on the blood stages of a chloroquine-sensitive clone (3D7) and a chloroquine-resistant strain (NF54-R) of P. falciparum. The inhibitory effect increases according to the logarithm of the concentration in the culture. Our results are in good agreement with previously published data for trovafloxacin, ciprofloxacin, pefloxacin, and temafloxacin tested against sensitive and resistant strains of P. falciparum (5, 8, 20, 23). In our study, ciprofloxacin, trovafloxacin, and grepafloxacin were among the most active drugs against both the chloroquine-sensitive clone and the chloroquine-resistant strain of P. falciparum.
One remarkable characteristic of the effect of quinolones or fluoroquinolones on Plasmodium growth was a progressive inhibitory effect corresponding to the duration of the incubation with the drug. This has already been observed by other groups for ciprofloxacin, norfloxacin, and ofloxacin (13, 23) and for other protozoa such as Toxoplasma gondii (7, 9). The reasons for this delayed effect are still unknown. By analogy with what has been observed with T. gondii, one can be curious about the activity of the drug with the apicoplast of Plasmodium (6, 17), affecting the parasitic viability at the second or even the third generation. The fact that the reproductive cycle of P. falciparum is about 48 h and that we saw a meaningful effect at 96 h tends to support this hypothesis.
For the first time, we showed that seven quinolones and fluoroquinolones have a significant inhibitory effect on the hepatic stage of P. yoelii yoelii and P. falciparum. Grepafloxacin was clearly the most active drug. For grepafloxacin, piromidic acid, and trovafloxacin, this inhibitory effect combined a significant reduction of the number and size of schizonts in treated cultures compared to untreated controls and marked morphological alterations of the parasite. For the most active quinolones, concentrations that were found to be inhibitory for erythrocytic and hepatic stages of P. falciparum were in the range of those that can be obtained in human serum and liver (2, 12). This favors a possible clinical application of the quinolones for treatment or prophylaxis of human malaria. Previous studies already showed that treatment of acute P. falciparum malaria with norfloxacin or ciprofloxacin was efficient but was inferior to that with chloroquine (11, 16, 21, 22). This lower activity can be explained by the in vitro inhibitory effect of quinolone, which is largely dose dependent and delayed in time. This indicates that higher doses (or loading doses) are probably necessary to obtain a good clinical efficacy. Penetration of quinolones in red blood cells may represent a limiting factor for treatment efficacy. One study performed using ciprofloxacin for three patients treated for malaria showed that this drug readily diffuses across the erythrocytic membrane, with a mean erythrocyte-to-plasma drug concentration ratio of 1.5 (18). Obviously, further studies are required to confirm this finding on a large number of patients and for other quinolones and fluoroquinolones.
The demonstrated activity of quinolones on the hepatic stage of Plasmodium could be of particular interest for prophylaxis. Quinolones diffuse well into liver tissues (2), and liver concentration can reach levels that can markedly inhibit P. falciparum growth in vitro. Given this activity against hepatic schizonts, we suggest that these drugs could be used to block the development of the parasite before the blood stage and prevent erythrocytic invasion. Although this needs to be assessed in experimental models of in vivo infection, we consider that quinolones have a potential for treatment or prevention of malaria through their unique antiparasitic effect against chloroquine-sensitive and chloroquine-resistant strains.
Acknowledgments
This work was supported by a grant from PAL+ and a grant from the Ministère de l'Enseignement Supérieur et de la Recherche.
We thank D. Walliker for providing clone 3D7 and strain NF54 of P. falciparum, L. Hannoun for providing human surgical liver biopsies (Service de Chirurgie Digestive Hépato-Bilio-Pancréatique et de Transplantation Hépatique, Hôpital Pitié-Salpétrière, Paris, France), and I. Vouldoukis for providing the marbofloxacin. We thank J. Nitcheu, S. Blazquez, and D. Dorin for critical reading of the manuscript. We thank A. Chevance de Bois Fleury for technical assistance for the culture of hepatic stages of P. yoelii yoelii.
REFERENCES
- 1.Basco, L. K., P. Ringwald, J. F. Franetich, and D. Mazier. 1999. Assessment of pyronaridine activity in vivo and in vitro against the hepatic stages of malaria in laboratory mice. Trans. R. Soc. Trop. Med. Hyg. 93:651-652. [DOI] [PubMed] [Google Scholar]
- 2.Bryskier, A. 1999. Antibiotiques, agents antibactériens et anti-fongiques, vol. 16, p. 683-816. Ellipses, Paris, France.
- 3.Derouin, F., and C. Chastang. 1988. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrob. Agents Chemother. 32:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divo, A. A., A. C. Sartorelli, C. L. Patton, and F. J. Bia. 1988. Activity of fluoroquinolone antibiotics against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 32:1182-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature 390:407-409. [DOI] [PubMed] [Google Scholar]
- 7.Gozalbes, R., M. Brun-Pascaud, R. Garcia-Domenech, J. Galvez, P.-M. Girard, J.-P. Doucet, and F. Derouin. 2000. Anti-Toxoplasma activities of 24 quinolones and fluoroquinolones in vitro: prediction of activity by molecular topology and virtual computational techniques. Antimicrob. Agents Chemother. 44:2771-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamzah, J., T. Skinner-Adams, and T. M. Davis. 2000. In vitro antimalarial activity of trovafloxacin, a fourth-generation fluoroquinolone. Acta Trop. 74:39-42. [DOI] [PubMed] [Google Scholar]
- 9.Khan, A. A., F. G. Araujo, K. E. Brighty, T. D. Gootz, and J. S. Remington. 1999. Anti-Toxoplasma gondii activities and structure-activity relationships of novel fluoroquinolones related to trovafloxacin. Antimicrob. Agents Chemother. 43:1783-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazier, D., R. L. Beaudoin, S. Mellouk, P. Druilhe, B. Texier, J. Trosper, F. Miltgen, I. Landau, C. Paul, O. Brandicourt, et al. 1985. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227:440-442. [DOI] [PubMed] [Google Scholar]
- 11.McClean, K. L., D. Hitchman, and S. D. Shafran. 1992. Norfloxacin is inferior to chloroquine for falciparum malaria in northwestern Zambia: a comparative clinical trial. J. Infect. Dis. 165:904-907. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell, J. A., and S. P. Gelone. 2000. Fluoroquinolones. Infect. Dis. Clin. N. Am. 14:489-513. [DOI] [PubMed] [Google Scholar]
- 13.Pradines, B., C. Rogier, T. Fusai, J. Mosnier, W. Daries, E. Barret, and D. Parzy. 2001. In vitro activities of antibiotics against Plasmodium falciparum are inhibited by iron. Antimicrob. Agents Chemother. 45:1746-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridley, R. G. 2002. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415:686-693. [DOI] [PubMed] [Google Scholar]
- 15.Semblat, J. P., O. Silvie, J. F. Franetich, L. Hannoun, W. Eling, and D. Mazier. 2002. Laser capture microdissection of Plasmodium falciparum liver stages for mRNA analysis. Mol. Biochem. Parasitol. 121:179-183. [DOI] [PubMed] [Google Scholar]
- 16.Stromberg, A., and A. Bjorkman. 1992. Ciprofloxacin does not achieve radical cure of Plasmodium falciparum infection in Sierra Leone. Trans. R. Soc. Trop. Med. Hyg. 86:373.. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan, M., J. Li, S. Kumar, M. J. Rogers, and T. F. McCutchan. 2000. Effects of interruption of apicoplast function on malaria infection, development, and transmission. Mol. Biochem. Parasitol. 109:17-23. [DOI] [PubMed] [Google Scholar]
- 18.Teja-Isavadharm, P., D. Keeratithakul, G. Watt, H. K. Webster, and M. D. Edstein. 1991. Measurement of ciprofloxacin in human plasma, whole blood, and erythrocytes by high-performance liquid chromatography. Ther. Drug Monit. 13:263-267. [DOI] [PubMed] [Google Scholar]
- 19.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 20.Tripathi, K. D., A. K. Sharma, N. Valecha, and S. Biswas. 1993. In vitro activity of fluoroquinolones against chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Indian J. Malariol. 30:67-73. [PubMed] [Google Scholar]
- 21.Tripathi, K. D., A. K. Sharma, N. Valecha, and D. D. Kulpati. 1993. Curative efficacy of norfloxacin in falciparum malaria. Indian J. Med. Res. 97:176-178. [PubMed] [Google Scholar]
- 22.Watt, G., G. D. Shanks, M. D. Edstein, K. Pavanand, H. K. Webster, and S. Wechgritaya. 1991. Ciprofloxacin treatment of drug-resistant falciparum malaria. J. Infect. Dis. 164:602-604. [DOI] [PubMed] [Google Scholar]
- 23.Yeo, A. E., and K. H. Rieckmann. 1994. Prolonged exposure of Plasmodium falciparum to ciprofloxacin increases anti-malarial activity. J. Parasitol. 80:158-160. [PubMed] [Google Scholar]