Abstract
We recently demonstrated that lactoferrin, an antimicrobial glycoprotein, can inhibit adenovirus infection by competing for common glycosaminoglycan receptors. This study further characterizes the antiadenovirus activity of the protein, thus demonstrating that lactoferrin neutralizes infection by binding to adenovirus particles and that its targets are viral III and IIIa structural polypeptides.
Lactoferrin is a multifunctional glycoprotein that possesses a variety of physiological roles, such as promotion of iron absorption (26), immunomodulation (16), and inhibiting activity toward different pathogens (14, 16, 24, 26, 32-34), including viruses (1, 2, 7, 9-13, 17-19, 21, 25, 28-30, 38). For all viruses investigated to date, lactoferrin exerts its antiviral activity during the early phases of infection. Recently, we demonstrated a competition between bovine lactoferrin (bLf) and adenovirus for the attachment to cell membrane glycosaminoglycans, even though a binding between lactoferrin and viral particles could not be ruled out (6). The present study provides deeper insight into the mechanism of the antiadenoviral action of bLf, demonstrating that this glycoprotein also exerts its antiadenoviral activity by a direct interaction with adenovirus particles.
Human epidermoid carcinoma larynx (HEp-2) cells, obtained from the American Type Culture Collection (ATCC), and human adenovirus type 2 were grown as previously described (2). Viral particles were purified according to the methods of Wadell and coworkers (35).
bLf (Morinaga Milk Industry) and heparin (Sigma, 170 USP U/mg) were dissolved in phosphate-buffered saline (PBS; pH 7.2). bLf was biotinylated according to the manufacturer's instructions (Amersham). Dot blot assays were carried out by applying 50 μl of purified adenovirus, heparin, or PBS to nitrocellulose paper and using a Bio-Dot apparatus according to the manufacturer's instructions (Bio-Rad). Air-dried membranes were blocked in block solution, washed with PBS containing 0.1% Tween 20, and then incubated with biotinylated bLf. After washings in PBS containing 0.1% Tween 20, streptavidin-horseradish peroxidase (streptavidin-HRP) conjugate was added to the blot. Blot was revealed by an enhanced chemiluminescence kit (Amersham Biosciences) and the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate kit for peroxidase (Vector Laboratories) according to the manufacturer's instructions.
Neutralization of adenovirus binding to cells was carried out by incubating lactoferrin with adenovirus for 1 h at 37 or 4°C. The suspensions were then added to cells for 1 h at 4°C. Viral antigen synthesis was monitored 24 h after infection (37°C) by immunofluorescence as previously described (2). Statistical analysis was performed using the Student's t test for unpaired data. P values of <0.05 were considered significant.
Adenovirus proteins were resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) (15) under denaturing conditions as described by Wadell and coworkers (36). Coxsackievirus proteins were resolved by SDS-15% PAGE (15). Electrophoretic transfer of proteins onto nitrocellulose membranes was carried out by using a Semi-Dry transfer cell (Bio-Rad) according to the manufacturer's protocol. After incubation for 1 h at 37°C with biotinylated bLf diluted in PBS, the reaction was revealed by the enhanced chemiluminescence and TMB substrate kits as reported above.
Biotinylated bLf binding to adenovirus was visualized by transmission electron microscopy by utilizing a streptavidin-10-nm gold conjugate (Sigma).
As a preliminary step—to verify whether lactoferrin was able to bind to adenovirus—purified viral particles and relative buffer were fixed to nitrocellulose membranes and probed with biotinylated bLf. Heparin was used as positive control. Results of dot blot assays, shown in Fig. 1, demonstrated that biotinylated bLf specifically reacted with both intact virions and heparin.
FIG. 1.
Dot blot assay. Purified adenovirus particles (a), heparin (b), and PBS (c and d) were directly applied to the nitrocellulose paper, incubated with biotinylated bLf, and then incubated with streptavidin-HRP. Reaction was revealed by the enhanced-chemiluminescence kit and TMB kit.
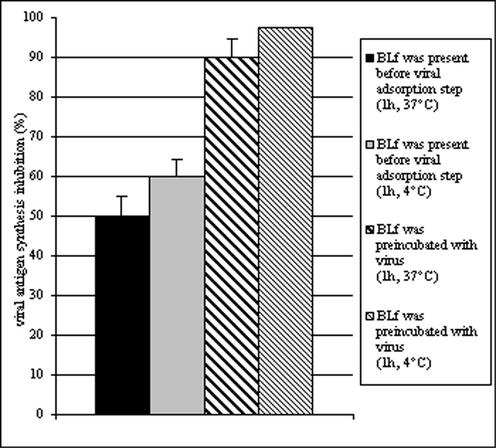
To then assess whether lactoferrin interaction with viral particles could produce a neutralization of infection, experiments were carried out in which lactoferrin and adenovirus were preincubated for 1 h before addition to the cells. Controls, in which cells were pretreated with lactoferrin for 1 h before infection, were included. The results obtained showed that preincubation of HEp-2 cells with bLf produced an inhibition of viral infection of about 60%, whereas when bLf and adenovirus were mixed together for 1 h and then added to the cells, viral antigen synthesis was inhibited by about 95% (Fig. 2).
FIG. 2.
Effect of 1 mg of bLf per ml on adenovirus antigen synthesis. Lactoferrin was incubated with the virus (1 h, 37 or 4°C) or with the cells (1 h, 37 or 4°C) before the viral adsorption step (1 h, 4°C). Virus antigen synthesis was monitored 24 h after infection by immunofluorescence. The data represent the mean of at least quadruplicate samples. For each sample, 600 cells were examined.
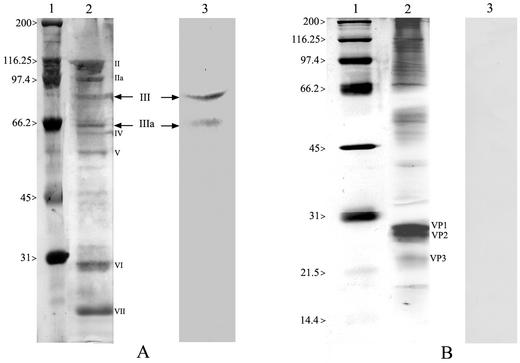
In order to better characterize the binding of bLf to viral particles, adenovirus-extracted proteins were separated by electrophoresis and Western blot assays were performed. In these experiments, coxsackievirus B3-extracted proteins were included as control. As shown in Fig. 3, biotinylated lactoferrin reacted with two adenovirus proteins of 85 and 66 kDa (panel A), corresponding to III and IIIa polypeptides (panel A, lane 3), respectively, whereas no interaction with coxsackievirus B3 outer proteins was detected (panel B, lane 3).
FIG. 3.
SDS-PAGE of adenovirus- and coxsackievirus-extracted proteins stained with silver stain (adenovirus [A], lane 2; coxsackievirus [B], lane 2); molecular weight standards (panels A and B, lanes 1). A western blot of separated proteins transferred to nitrocellulose and probed with biotinylated bLf followed by a reaction with streptavidin HRP-conjugate (panel A, lane 3 and panel B, lane 3) is shown. The arrows indicate 85- and 66-kDa adenovirus proteins corresponding to viral III and IIIa structural proteins, respectively.
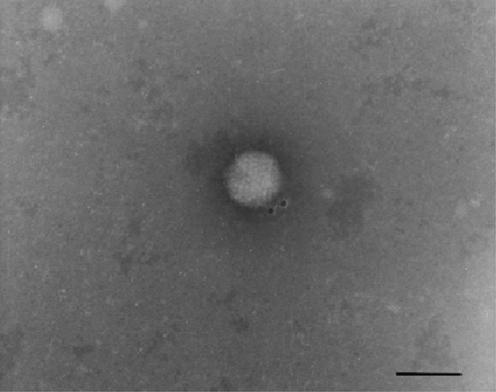
Finally, the binding of lactoferrin to adenovirus particles was visualized by transmission electron microscopy. Figure 4 shows a negative-stained biotinylated lactoferrin-treated adenovirus particle specifically labeled with streptavidin-gold. The 10-nm gold particles appear to be located in proximity to the vertex.
FIG. 4.
Electron photomicrograph of a negatively stained adenovirus particle. bLf binding to viral particles was visualized by streptavidin-10-nm gold conjugate. Bar, 100 nm.
It is well known that breast feeding protects against both respiratory and gastrointestinal infections in infants (20, 22). This protection is conferred through different mechanisms, as milk—besides immunoglobulins A and M—also contains nonantibody components with antimicrobial activity (8, 16, 20, 23, 24). On the basis of this observation, we analyzed the antiviral activity of one of these components, lactoferrin, on different respiratory or enteric viruses (2, 6, 18, 28, 29). Recently, we showed that bLf prevents adenovirus infection by competing with viral particles for cell membrane glycosaminoglycans inserted in target cell membranes (6). In the present study, we explored the possibility of a direct lactoferrin virus interaction in an attempt to better characterize the mechanism of the antiadenoviral activity of lactoferrin. For this purpose, a set of experiments was first carried out to verify a possible interaction of bLf with viral particles. Results from dot blot experiments demonstrated that bLf binds either heparin or adenovirus. Experiments on neutralization of virus attachment to target cells indicated that lactoferrin exerts its antiadenoviral action not only by competing with the virus for binding to a common cell receptor but also by specifically binding to viral particles. In particular, our results demonstrated that the incubation of HEp-2 cells with bLf before virus infection is not sufficient to totally prevent adenovirus infection—probably because the virus can also infect these cells by utilizing receptors other than glycosaminoglycans (27). As preincubation of viral particles with lactoferrin before infection resulted in a 95% inhibition of viral antigen synthesis, other experiments have been carried out to analyze the interaction of bLf with viral proteins.
The adenovirus shell is composed of 252 capsomeres: 240 hexons and 12 pentons (each containing a base and a projecting fiber). Electrophoretic analysis of viral proteins revealed different polypeptides—in particular, polypeptides II, III, and IV form the hexon, the penton base, and the fiber protein, respectively, and polypeptide IIIa is associated with hexon units surrounding the penton (27). Adenovirus 2 binds to cells through the interaction of the head domain of its fiber protein with a 42-kDa glycoprotein receptor, termed coxsackievirus and adenovirus receptor (3, 31), and is internalized through the interaction of the penton base protein with integrins (37). Recently, it has been demonstrated that heparan sulfate glycosaminoglycans (HS GAGs) expressed on cell surfaces are involved in the binding and infection of adenovirus 2 and 5 (4), although the viral structure responsible for adenovirus interaction with HS GAGs has not been identified (5). Our results from Western blot analysis demonstrated that bLf specifically binds to viral proteins and, in particular, to the adenovirus penton base. bLf-virus interaction was visualized by negative-stain electron microscopy, which showed that bLf interacts with adenovirus particles by binding to external viral structures. In conclusion, the data reported in this paper, together with our previous findings, demonstrate that, in spite of the adenovirus ability to use different receptors—such as coxsackievirus and adenovirus receptor, integrins, and HS GAGs—to infect host cells, lactoferrin is able to prevent at least two of these virus-cell interactions. Taken together, our findings provide further evidence that lactoferrin is an excellent candidate in the search for natural antiviral agents, as it mainly acts by hindering viral adsorption and internalization into the cells through a specific binding to both virus cell receptors and viral particles.
Acknowledgments
This work was supported by grants from the Italian Ministry of Health, Project 1%, the National Institutes of Health, MIUR-PRIN, and CNR.
REFERENCES
- 1.Andersen, J. H., S. A. Osbakk, L. H. Vorland, T. Traavik, and T. J. Gutteberg. 2001. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 51:141-149. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, D., A. M. Di Biase, M. Marchetti, A. Pietrantoni, P. Valenti, L. Seganti, and F. Superti. 2002. Antiadenovirus activity of milk proteins: lactoferrin prevents viral infection. Antiviral Res. 53:153-158. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 5.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus type 2 and 5. J. Virol. 75:8772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Biase, A. M., A. Pietrantoni, A. Tinari, R. Siciliano, P. Valenti, G. Antonini, L. Seganti, and F. Superti. 2003. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J. Med. Virol. 69:495-502. [DOI] [PubMed] [Google Scholar]
- 7.Fujihara, T., and K. Hayashi. 1995. Lactoferrin inhibits herpes simplex virus type-1 (HSV-1) infection in mouse cornea. Arch. Virol. 140:1469-1472. [DOI] [PubMed] [Google Scholar]
- 8.Hamosh, M. 1998. Protective function of proteins and lipids in human milk. Biol. Neonate 74:163-176. [DOI] [PubMed] [Google Scholar]
- 9.Hara, K., M. Ikeda, S. Saito, S. Matsumoto, K. Numata, N. Kato, K. Tanaka, and H. Sekihara. 2002. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol. Res. 24:228-235. [DOI] [PubMed] [Google Scholar]
- 10.Harmsen, M. C., P. J. Swart, M. P. de Béthune, R. Pawels, E. De Clercq, T. H. The, and D. K. F. Meijer. 1995. Antiviral effects of plasma and milk proteins: lactoferrin shows a potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J. Infect. Dis. 172:380-388. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa, K., W. Motsuchi, S. Tanaka, and S. Dosako. 1994. Inhibition with lactoferrin of in vitro infection with human herpesvirus. Jpn. J. Med. Sci. Biol. 47:73-85. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda, M., K. Sugiyama, T. Tanaka, K. Tanaka, H. Sekihara, K. Shimotohno, and N. Kato. 1998. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem. Biophys. Res. Commun. 215:744-749. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda, M., A. Nozaki, K. Sugiyama, T. Tanaka, A. Naganuma, K. Tanaka, H. Sekihara, K. Shimotohno, M. Saito, and N. Kato. 2000. Characterization of antiviral activity of lactoferrin against hepatitis C virus infection in human cultured cells. Virus Res. 66:51-63. [DOI] [PubMed] [Google Scholar]
- 14.Iwasa, M., M. Kaito, J. Ikoma, M. Takeo, I. Imoto, Y. Adachi, K. Yamauchi, R. Koizumi, and S. Teraguchi. 2002. Lactoferrin inhibits hepatitis C virus viremia in chronic hepatitis C patients with high viral loads and HCV genotype 1b. Am. J. Gastroenterol. 97:766-767. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Levay, P. F., and M. Viljoen. 1995. Lactoferrin: a general review. Haematologica 80:252-267. [PubMed] [Google Scholar]
- 17.Marchetti, M., C. Longhi, M. P. Conte, S. Pisani, P. Valenti, and L. Seganti. 1996. Lactoferrin inhibits herpes simplex virus type 1 adsorption to Vero cells. Antiviral Res. 29:221-231. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti, M., S. Pisani, G. Antonini, P. Valenti, L. Seganti, and N. Orsi. 1998. Metal complexes of bovine lactoferrin inhibit in vitro replication of herpes simplex virus type 1 and 2. BioMetals 11:89-94. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti, M., F. Superti, M. G. Ammendolia, P. Rossi, P. Valenti, and L. Seganti. 1999. Inhibition of poliovirus type 1 infection by iron-, manganese- and zinc-saturated lactoferrin. Med. Microbiol. Immunol. 187:199-204. [DOI] [PubMed] [Google Scholar]
- 20.May, J. T. 1988. Microbial contaminants and antimicrobial properties of human milk. Microbiol. Sci. 5:42-46. [PubMed] [Google Scholar]
- 21.Murphy, M. E., H. Kariwa, T. Mizutani, K. Yoshimatsu, J. Arikawa, and I. Takashima. 2000. In vitro antiviral activity of lactoferrin and ribavirin upon hantavirus. Arch. Virol. 145:1571-1582. [DOI] [PubMed] [Google Scholar]
- 22.Newburg, D. S. 1999. Human milk glycoconjugates that inhibit pathogens. Curr. Med. Chem. 6:117-127. [PubMed] [Google Scholar]
- 23.Peterson, J. A., S. Patton, and M. Hamosh. 1998. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol. Neonate 74:143-162. [DOI] [PubMed] [Google Scholar]
- 24.Portelli, J., A. Gordon, and J. T. May. 1998. Effect of compounds with antibacterial activities in human milk on respiratory syncytial virus and cytomegalovirus in vitro. J. Med. Microbiol. 47:1015-1018. [DOI] [PubMed] [Google Scholar]
- 25.Puddu, P., P. Borghi, S. Gessani, P. Valenti, F. Belardelli, and L. Seganti. 1998. Antiviral effect of bovine lactoferrin saturated with metal ions on early steps of human immunodeficiency virus type 1 infection. Int. J. Biochem. Cell Biol. 30:1055-1062. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez, L., M. Ismail, F. Y. Liew, and J. H. Brock. 1996. Iron transport across Caco-2 cell monolayers. Effect of transferrin, lactoferrin and nitric oxide. Biochim. Biophys. Acta 1289:291-297. [PubMed] [Google Scholar]
- 27.Shenk, T. E. 2000. Adenoviridae: the viruses and their replication, p. 2265-2300. In B. N. Fields, D. K. Knipe, P. M. Howley, (ed.), Virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 28.Superti, F., R. Siciliano, B. Rega, F. Giansanti, P. Valenti, and G. Antonini. 2001. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim. Biophys. Acta 1528:107-115. [DOI] [PubMed] [Google Scholar]
- 29.Superti, F., M. G. Ammendolia, P. Valenti, and L. Seganti. 1997. Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med. Microbiol. Immunol. 186:83-91. [DOI] [PubMed] [Google Scholar]
- 30.Swart, P. J., M. E. Kuipers, C. Smith, R. Pawels, M. P. De Béthune, E. De Clerck, D. K. F. Meijer, and J. G. Huisman. 1996. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro. AIDS Res. Hum. Retrovir. 12:769-775. [DOI] [PubMed] [Google Scholar]
- 31.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenti, P., M. Marchetti, F. Superti, M. G. Ammendolia, P. Puddu, S. Gessani, P. Borghi, F. Belardelli, G. Antonini, and L. Seganti. 1998. Antiviral activity of lactoferrin, p. 199-203. In G. Spik, D. Legrand, J. Mazurier, A. Pierce, and J. P. Perraudin (ed.), Advances in lactoferrin research. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 33.van der Strate, B. W., L. Beljaars, G. Molema, M. C. Harmsen, and D. K. Meijer. 2001. Antiviral activities of lactoferrin. Antiviral Res. 52:225-239. [DOI] [PubMed] [Google Scholar]
- 34.Vorland, L. H. 1999. Lactoferrin: a multifunctional glycoprotein. APMIS 107:971-981. [DOI] [PubMed] [Google Scholar]
- 35.Wadell, G., M. L. Hammarskjold, and T. Varsanyi. 1973. Incomplete virus particles of adenovirus type 16. J. Gen. Virol. 20:287-302. [DOI] [PubMed] [Google Scholar]
- 36.Wadell, G., G. Sundell, and J. C. de Jong. 1981. Characterization of candidate adenovirus 37 by SDS-polyacrylamide gel electrophoresis of virion polypeptides and DNA restriction site mapping. J. Med. Virol. 7:119-125. [DOI] [PubMed] [Google Scholar]
- 37.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αv β3 and αv β5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 38.Yi, M., S. Kaneko, D. Y. Yu, and S. Murakami. 1997. Hepatitis C virus envelope proteins bind lactoferrin. J. Virol. 71:5997-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]