Abstract
We investigated the in vitro activity of micafungin against clinical Aspergillus isolates (n = 37) (Aspergillusfumigatus [n = 21], Aspergillusflavus [n = 14], and Aspergillus niger [n = 2]) by using NCCLS M38A microdilution and an investigational disk diffusion assay. Microdilution assay results were evaluated by using the end points of a MIC-2 (measured in micrograms per milliliter) and minimum effective concentration (MEC, measured in micrograms per milliliter; the lowest concentration of micafungin that produces short and aberrant hyphal branchings microscopically). Disk diffusion results were interpreted by measuring the zone(s) of inhibition (ZOI, measured in millimeters). Micafungin proved to be similarly active against all Aspergillus species tested. At 24 h, MIC-2s and MECs were identical. At 48 h, however, MIC-2s increased unpredictably, leading to the loss of a consistent correlation between the two end points. MECs and ZOI remained consistent and correlated at both reading times, suggesting their use as relevant end points in susceptibility testing of micafungin against Aspergillus. All Aspergillus isolates yielded intrazonal growth on disk diffusion agar plates. The intrazonal colonies contained short, aberrant hyphal branchings microscopically. The in vivo significance of these findings remains to be further investigated.
Micafungin (formerly FK463) is a novel echinocandin undergoing phase III clinical trials. Similar to the other newly developed echinocandins, caspofungin and anidulafungin, micafungin exerts antifungal activity via inhibition of (1,3)-β-d-glucan synthesis (4, 6, 15, 16). This distinctive mode of action provides two significant advantages: first, selective antifungal activity and reduced rate of toxicity to the mammalian cells, and second, possible favorable activity against polyene- and azole-resistant isolates (10; P. A. Warn, A. Sharp, G. Morrissey, and D. Denning, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-187, 2002) as well as the susceptible ones.
The in vitro antifungal spectrum of micafungin covers various fungal genera, including most Candida spp. (11, 12, 14), Aspergillus (14), and the mycelial phase of Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis (T. Nakai, J. Uno, and M. Miyaji, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1509, 1999). Its activity is more limited against the dematiaceous fungi (Cladosporium trichoides, Exophiala dermatitidis, Exophiala spinifera, and Fonsecaea pedrosoi). Micafungin has limited or no activity against Cryptococcus neoformans, Trichosporon spp., Fusarium spp., Pseudallescheria boydii, zygomycetes, and the yeast phases of H. capsulatum, B. dermatitidis, and C. immitis (14; Nakai et al., 39th ICAAC). The in vivo efficacy of micafungin in infections due to Candida (7; D. P. Kontoyiannis, D. Buell, S. Frisbee-Hume, B. T. Reddy, and K. V. Rolston, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1629, 2001; J. Suleiman, M. Della Negra, A. Llanos-Cuentas, E. Ticona, J. H. Rex, and D. N. Buell, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-892, 2002) and Aspergillus (7; S. Kohno, T. Masaoka, and H. Yamaguchi, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-834, 2001) and its use in the prophylaxis of invasive fungal infections (J. Van Burik, V. Ratanatharathorn, J. Lipton, C. Miller, N. Bunin, T. J. Walsh, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1238, 2002) and in combination with other antifungal agents (2, 5; J. Capilla Luque, K. V. Clemons, and D. Stevens, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1834, 2001; E. K. Manavathu, L. T. Ganesan, J. L. Jutright, and P. H. Chandrasekar, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-125, 2001) are under investigation.
While micafungin has proven to be active in vitro against Aspergillus spp., the optimal testing method and the end point to be used in micafungin susceptibility tests for this particular group of fungi have not been fully standardized. The current NCCLS reference method does not include echinocandins. On the other hand, and more importantly, there is evidence from previous work suggesting that the use of a distinctive parameter, minimum effective concentration (MEC, measured in micrograms per milliliter), is a relevant and consistent end point for susceptibility testing of echinocandins (8), as reported for caspofungin (1) against Aspergillus. The initial report for caspofungin verified that the drug exerts antifungal activity at actively growing tips and branching points of Aspergillus hyphae, resulting in the flattening and swelling of tips (C. M. Douglas, J. C. Bowman, G. K. Abruzzo, A. M. Flattery, C. J. Gill, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, M. B. Kurtz, and H. Rosen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-1683, 2000). The lowest concentration of the drug yielding this appearance under the microscope is referred to as the MEC. Related data for micafungin, obtained by using a flourescent dye-staining method (E. Watabe, T. Nakai, S. Matsumoto, K. Hatano, and F. Ikeda, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-490, 2002) and scanning and transmission electron microscopy (SEM and TEM, respectively) (T. Nakai, K. Hatano, and F. Ikeda, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1511, 2002), were reported more recently. These data similarly suggest that micafungin also results in the emergence of markedly flattened, thick and short hyphal cells and a killing effect on Aspergillus.
In the interim and in addition to microdilution assays, a disk diffusion assay has been explored for caspofungin (3) but not for micafungin. In vitro data obtained so far for micafungin against Aspergillus used the NCCLS microdilution method and MIC-2 (prominent decrease in turbidity) end point (14). No data are available on the use of other end points and/or susceptibility methods other than microdilution. This study was undertaken to compare the reference microdilution and an investigational disk diffusion method in testing the in vitro activity of micafungin against Aspergillus and to explore the use and relevance of three different end points: MIC-2, MEC, and zone(s) of inhibition (ZOI).
(This work was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, 27 to 30 September 2002, San Diego, Calif. [S. Arikan, P. Yurdakul, and G. Hascelik, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1490, 2002]).
The in vitro activity of micafungin was tested against clinical isolates (n = 37) of various Aspergillus spp. (Aspergillus fumigatus [n = 21], Aspergillus flavus [n = 14], and Aspergillus niger [n = 2]). The test isolates were identified to the species level by standard methods (9) and maintained at −70°C on Sabouraud dextrose agar slants until tested. Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, and a reference Fusarium solani (item code 85) isolate were included in each run of susceptibility tests for quality control and assessment of reproducibility. Micafungin was provided by its manufacturer (Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan) as a standard powder and rendered soluble in water. The susceptibility tests were performed by using microdilution and disk diffusion assays. The results of the susceptibility tests were assessed and interpreted by three separate investigators.
The microdilution assay employed NCCLS M38A reference microdilution methodology (13). Micafungin was tested in a concentration range of 64 to 0.125 μg/ml. The results were read after 24 and 48 h of incubation and by using two end points: the visual MIC-2 (measured in micrograms per milliliter), the lowest concentration of micafungin that results in ≥50% reduction in turbidity compared to the growth control well, and the microscopic MEC (measured in micrograms per milliliter), the lowest concentration of micafungin which produces short and aberrant hyphal branchings. For evaluation of the results for the Candida American Type Culture Collection isolates, the MIC-0 (measured in micrograms per milliliter), the lowest concentration of micafungin that results in complete inhibition of growth, was used as the end point. The MIC determination for the Fusarium reference isolate, on the other hand, used the MIC-2 and MEC. Each clinical and reference isolate was tested in duplicate in each run of microdilution assays.
The disk diffusion assay employed an investigational method that used the reference RPMI 1640 agar supplemented to 2% glucose as the test medium. This method was similar to that previously used for caspofungin susceptibility testing (3), except that an inoculum size of 106 CFU/ml was used instead of 104 CFU/ml. The higher inoculum size of 106 CFU/ml was preferred in the present study since sufficient growth could not be attained at 24 h in our previous work with caspofungin by using the inoculum size of 104 CFU/ml. Micafungin disks were prepared by using blank paper disks (6.3 mm in diameter) to yield a disk content of 1 μg/disk. The disks were allowed to dry at room temperature prior to their use in disk diffusion assays. The inoculum density to be used in the test was adjusted spectrophotometrically to 106 CFU/ml for Aspergillus and Fusarium. For this purpose, the test strain was suspended in saline and adjusted spectrophotometrically at a 530-nm wavelength to ∼81 and ∼70% transmittance for Aspergillus and Fusarium, respectively. For Candida American Type Culture Collection strains, the inoculum size was adjusted to ∼77% transmittance, providing a final concentration of 106 CFU/ml. The performance of the assay followed the conventional rules of disk diffusion tests. The adjusted inoculum was swabbed onto the test medium and was left to dry at room temperature for about 20 min. The previously prepared micafungin disks were then placed on the centers of the inoculated plates, and the plates were incubated at 35°C. The results were read after 24 and 48 h of incubation and by measuring the ZOI (in millimeters) at the outermost point of marked decrease in fungal density. For analysis of the results, geometric means (GM) and ranges of MIC-2s and MECs and arithmetic means and ranges of ZOI were calculated. Both on-scale and off-scale results were included in the analysis. The high off-scale MIC-2s and MECs were converted to the next highest concentrations and the low off-scale MIC-2s and MECs were left unchanged. The results obtained by using the three end points, MIC-2, MEC, and ZOI, were comparatively evaluated for each species and at both 24 and 48 h.
Microdilution and disk diffusion results for the clinical isolates are shown in Table 1. Visual examination of the microdilution plates showed that the growth pattern was granular at concentrations at and above the MEC while it was filamentous at concentrations below the MEC. Noteworthy, a paradoxical effect was observed in 4 A. fumigatus isolates at the highest concentration of micafungin.
TABLE 1.
Microdilution and disk diffusion results of the clinical isolatesa
| Species (n) | Incubation period (h) | MIC-2 (μg/ml)
|
MEC (μg/ml)
|
ZOI (mm)
|
|||
|---|---|---|---|---|---|---|---|
| GM | Range | GM | Range | AM | Range | ||
| A. fumigatus (21) | 24 | 0.125 | ≤0.125 | 0.125 | ≤0.125 | 30 | 25-35 |
| 48 | 3.4 | ≤0.125->64 | 0.125 | ≤0.125 | 30 | 24-35 | |
| A. flavus (14) | 24 | 0.125 | ≤0.125 | 0.125 | ≤0.125 | 33 | 29-38 |
| 48 | 17.7 | ≤0.125->64 | 0.125 | ≤0.125 | 32 | 28-36 | |
| A. niger (2) | 24 | ND | ≤0.125 | ND | ≤0.125 | ND | 29-34 |
| 48 | ND | ≤0.125 | ND | ≤0.125 | ND | 25-32 | |
AM, arithmetic mean; ND, not determined due to low numbers of isolates.
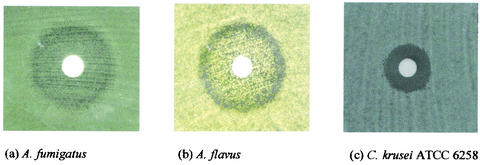
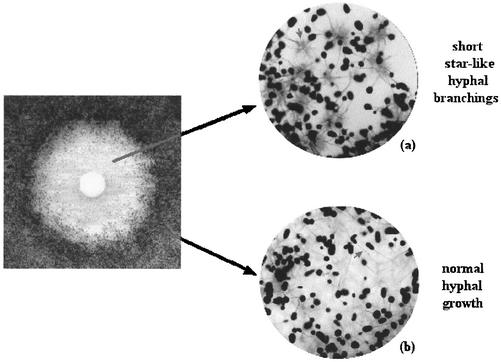
The growth patterns for some of the clinical and quality control isolates on disk diffusion plates are shown in Fig. 1. Colonies were observed inside the inhibition zones for all Aspergillus isolates (Fig. 1a and b). Microscopic examination of these colonies revealed short, aberrant hyphal branchings, an appearance identical to that observed for MEC (Fig. 2a). In contrast, the colonies outside the inhibition zone yielded normal, elongated, branching hyphal growth microscopically (Fig. 2b). Isolates of A. flavus yielded a double zone-like appearance around the disk (Fig. 1b). The number of the intrazonal colonies was lower for A. niger than for the other two species. Subcultures of these intrazonal colonies yielded a pattern identical to that of the parent strain when retested by disk diffusion assay.
FIG. 1.
Growth pattern on disk diffusion plates at 48 h.
FIG. 2.
Microscopic appearance of the colonies inside (a) and outside (b) the inhibition zone.
In accordance with the data reported previously by Tawara et al. (14), our results suggested that micafungin is similarly active in vitro against the tested Aspergillus species. There are differences between the two studies in terms of the reading time and the inoculum size. Tawara et al. reported 72-h MIC readings versus our data at 24 and 48 h, and we used an inoculum size of 104 CFU/ml as recommended in NCCLS M38A while 103 CFU/ml was used in the previous study. When our 48-h readings are compared to their 72-h readings, the MIC ranges generated in our hands appeared to be wider and attained higher levels (≤0.125 to >64 versus 0.0078 to 0.0313 for A. fumigatus, ≤0.125 to >64 versus 0.0078 to 0.0156 for A. flavus, ≤0.125 versus <0.0039 to 0.0156 for A. niger). These differences may originate from the higher inoculum size used in our study as well as the strain-dependent variations.
As shown in Table 1, we demonstrated that MIC-2s were identical to MECs at 24 h. While the MIC-2s at 24 h tended to be similar in all strains and species tested, this was not the case at 48 h. MIC-2s increased unpredictably as the incubation period was extended to 48 h. The most striking finding about the effect of the incubation period was observed for MECs. In contrast to MIC-2s, MECs tended to remain consistent as the incubation period was extended. Similar to MECs, the ZOI also appeared to be similar at 24 and 48 h, resulting in a good correlation between MECs and ZOI at both reading times. These findings suggest that MEC and ZOI are the consistent end points at both 24 and 48 h while MIC-2 appears as a relevant end point only at 24 h in determination of in vitro activity of micafungin against Aspergillus. In vivo experiments are required to validate the optimal end point to be used. These findings have been previously reported for caspofungin (1, 3), and to our knowledge, this report is the first to demonstrate their validity for micafungin as well. Importantly, we also observed that MECs can be predicted visually based on the clearly visible change in the growth pattern. This provides an easy and practical way of determining MECs compared to the microscopic method, which is time consuming and labor intensive. The significance and meaning of the paradoxical effect observed in some A. fumigatus isolates, on the other hand, need to be further investigated.
Our findings related to the growth pattern on disk diffusion agar plates were also noteworthy. In contrast to the clear inhibition zones observed for Candida isolates, intrazonal growth was consistently observed for all Aspergillus strains tested. This particular growth pattern was reproducible when the intrazonal colonies were subcultured and retested. Interestingly, the microscopic examination of the intrazonal colonies showed that the hyphal structure was distorted to yield short, aberrant hyphal branchings in contrast to the healthy, long, branching hyphae of extrazonal colonies. The distorted structure of hyphae in the intrazonal colonies was identical to that observed at the MEC. The intrazonal growth detected by disk diffusion assay and the microscopic findings detected at the MEC by microdilution assay both suggest that micafungin possibly exerts a partial inhibitory effect on Aspergillus. These findings have previously been reported for caspofungin as well (3). Nevertheless, the clinical significance of these observations remains yet unclear. The meaning of the double zone-like appearance observed for A. flavus isolates, on the other hand, is also unclear and awaits further investigation.
In conclusion, micafungin is similarly active in vitro against A. fumigatus, A. flavus, and A. niger. MECs and ZOI appear as the consistent end points to be used in assessment of its activity against Aspergillus at both 24 and 48 h. The findings at the MEC and the intrazonal growth pattern suggest a partial inhibitory effect, the clinical significance of which demands further investigation.
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2002. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob. Agents Chemother. 46:245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., V. Paetznick, and J. H. Rex. 2002. Comparative evaluation of disk diffusion with microdilution assay in susceptibility testing of caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 46:3084-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arikan, S., and J. H. Rex. 2002. New agents for the treatment of systemic fungal infections-current status. Expert Opin. Emerg. Drugs 7:3-32. [DOI] [PubMed] [Google Scholar]
- 5.Chiou, C. C., N. Mavrogiorgos, E. Tillem, R. Hector, and T. J. Walsh. 2001. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45:3310-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larone, D. H. 1995. Medically important fungi—a guide to identification, 3rd ed. ASM Press, Washington, D.C.
- 10.Maesaki, S., M. A. Hossain, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 2000. Efficacy of FK463, a (1,3)-beta-d-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob. Agents Chemother. 44:1728-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikamo, H., Y. Sato, and T. Tamaya. 2000. In vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptide. J. Antimicrob. Chemother. 46:485-487. [DOI] [PubMed] [Google Scholar]
- 12.Muller, F. M. C., O. Kurzai, J. Hacker, M. Frosch, and F. Muhlschlegel. 2001. Effect of the growth medium on the in vitro antifungal activity of micafungin (FK-463) against clinical isolates of Candida dubliniensis. J. Antimicrob. Chemother. 48:713-715. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumo, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanden Bossche, H. 2002. Echinocandins—an update. Expert Opin. Ther. Patents 12:151-167. [Google Scholar]
- 16.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38:335-347. [PubMed] [Google Scholar]