Abstract
Medium conditioned by tumor necrosis factor alpha (TNF-α)-stimulated polymorphonuclear leukocytes (PMN) (CM-TNF) suppresses PMN migration. Therefore, we wished to identify the agent(s) in CM-TNF that mediated antichemotactic activity. CM-TNF was fractionated by high-performance liquid chromatography, and one fraction with antichemotactic activity contained the bactericidal protein human neutrophil protein 1 (HNP-1). We showed that HNP-1 suppresses PMN migration to formyl-methionyl-leucyl-phenylalanine but not to interleukin 8.
Polymorphonuclear leukocyte (PMN) migration from the circulation, through the vessel wall, and through the extracellular milieu to an infectious site is a widely studied process that is crucial to PMN function (reviewed by Wagner and Roth [16]). Bacterial products and inflammatory mediators, primarily cytokines, regulate this process by acting as chemotactic agents or by upregulating proteins on the cell surface of endothelial cells that promote PMN adherence and migration.
Little is known about the ability of PMNs to regulate their own migration or that of surrounding PMNs (paracrine regulation). We have shown that medium (Hanks balanced salt solution [HBSS]) conditioned for 2 h by PMNs stimulated with tumor necrosis factor alpha (TNF-α) (CM-TNF) suppressed migration across an ECM-coated surface to formyl-methonyl-leucyl-phenylalanine (fMLP), leukotriene B4, and Gro-α but not to interleukin 8 (IL-8) (6). While several agents that inhibit PMN migration have been identified (1, 9, 13, 15), the agent(s) in CM-TNF is as yet unknown. The characterization of the antichemotactic agent in CM-TNF is limited to the knowledge that its secretion does not require de novo synthesis and occurs within an hour of TNF-α stimulation (6). Therefore, the purpose of this study was to identify the antichemotactic agent in CM-TNF.
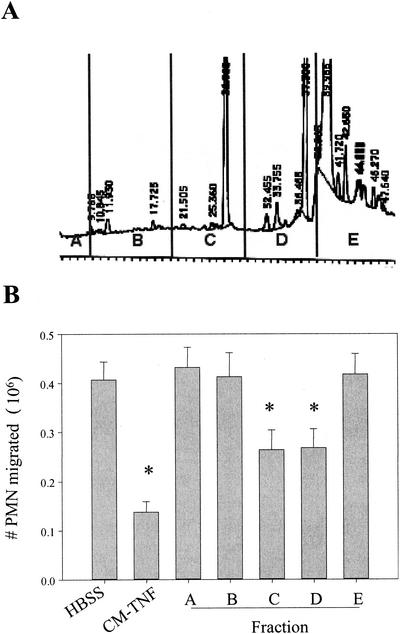
To identify the agents present in CM-TNF that suppress PMN migration to fMLP, >100 ml of CM-TNF was prepared as previously described (6) and concentrated by using Centriprep YM-3 and Centricon YM-3 centrifugal filter devices (Millipore Corp., Bedford, Mass.) sequentially to reduce the volume to <500 μl. The proteins were fractionated by reverse-phase high-performance liquid chromatography (HPLC) by utilizing a C4 column (Grace Vydac, Hesperia, Calif.) with a gradient of trifluoroacetic acid and acetonitrile (Sigma, St. Louis, Mo.). The resulting profile of the HPLC elution is shown in Fig. 1A. Fractions were collected at 1-min intervals, dried, and dissolved in HBSS. The fractions were divided at approximately 10-min intervals and pooled to form fractions A to E as illustrated in Fig. 1A. Fractions A to E were then assayed for their ability to suppress PMN migration across Transwell inserts (3.0-μm pore size; Corning Inc., Corning, N.Y.) coated with Biomatrix I (Biomedical Technologies Inc., Stoughton, Mass.). This method has been described previously in detail (6).
FIG. 1.
HPLC fractionation of CM-TNF. CM-TNF was fractionated by reverse-phase HPLC. The HPLC profile (A) was divided into five sections, A to E. The proteins in each section were pooled, dried, and solubilized in HBSS. These fractions were then tested for antichemotactic activity (B). Results shown are means ± standard errors of the means (n = 6). Asterisks indicate results that are significantly different from those of the HBSS controls (P < 0.05, one-way analysis of variance followed by Fisher's test of protected least significant difference).
PMNs from healthy human volunteers were isolated as previously described (6) and resuspended in HBSS, unfractionated CM-TNF, or fractions A to E, and migration to fMLP (Sigma) was determined. As seen previously, PMNs suspended in HBSS readily migrated to fMLP, and this migration was significantly suppressed by CM-TNF (Fig. 1B). PMNs resuspended in fractions A and B also migrated readily (Fig. 1B), which was not a surprise considering the lack of protein peaks present in the HPLC profile. Fraction E, which contained primarily albumin and residual TNF-α, also had no activity. In contrast, both fractions C and D exhibited significant suppression of PMN migration (Fig. 1B).
Since the profile for fraction C contained one major peak, we electrophoresed the sample on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel to determine if the peak represented one protein or several proteins that eluted at the same time. Coomassie blue staining of the gel revealed a single protein that was less than 10 kDa in size (data not shown). This band was excised and identified by matrix-assisted laser desorption ionization-time-of-flight analysis (performed by John Leszyk at the University of Massachusetts, Worcester) as human neutrophil protein 1 (HNP-1). HNP-1, or α-defensin 1, is a small protein (30 amino acids) found in abundance in PMNs, constituting 30 to 50% of the total protein content of the azurophilic granules (11). In addition to its microbicidal activity, HNP-1 is chemotactic for naive T cells (17), immature dendritic cells (17), and monocytes (11, 14). Here we provide data that suggest another role for HNP-1 in the regulation of immune cell migration, i.e., the suppression of PMN migration.
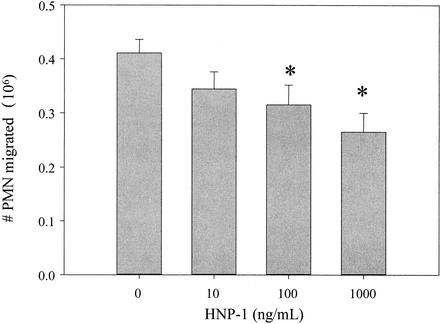
We verified the presence of HNP-1 in CM-TNF and fraction C by Western blot analysis with an antibody that recognizes HNP-1, -2, and -3 (clone DEF3; Bachem Biosciences, Inc., King of Prussia, Pa.) (data not shown). To further characterize the ability of HNP-1 to suppress PMN migration, recombinant HNP-1 (Bachem Biosciences) was used in the migration assay. In agreement with previously published studies (3, 18), CM-TNF and recombinant HNP-1 had no chemotactic activity for PMNs (data not shown). However, when PMNs were resuspended in HBSS plus 0 to 1,000 ng of HNP-1/ml, a range that encompassed the amount of HNP-1 measured in CM-TNF (833.8 ± 43.3 ng/ml, n = 6) (HNP1-3 enzyme-linked immunosorbent assay kit; Cell Sciences, Inc., Norwood, Mass.), and allowed to migrate to fMLP, recombinant HNP-1 suppressed migration in a dose-dependent manner (Fig. 2), with a 35% reduction exhibited at the highest dose.
FIG. 2.
Recombinant HNP-1 suppresses PMN migration. To test the ability of HNP-1 to suppress migration, PMNs were resuspended in HBSS with or without various doses of HNP-1 and tested for antichemotactic activity. Results shown are means ± standard errors of the means (n = 6). Asterisks indicate results that are significantly different from those of the HBSS controls (P < 0.05, one-way analysis of variance followed by Fisher's test of protected least significant difference).
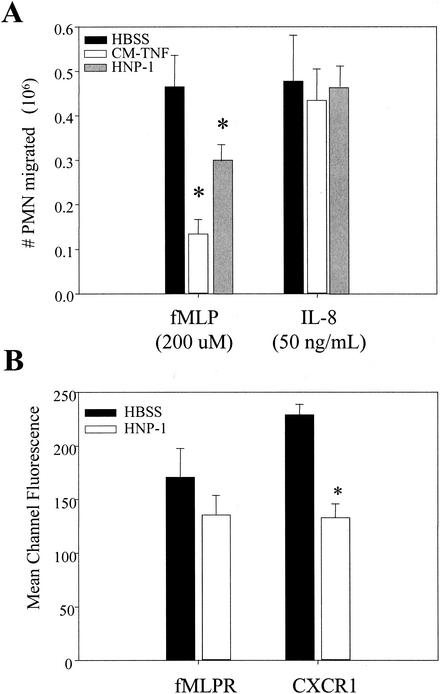
It has previously been shown that CM-TNF suppresses migration to fMLP, LTB4, and Gro-α but not to IL-8 (6). Therefore, we tested the ability of recombinant HNP-1 to suppress PMN migration to IL-8 (R&D Systems, Minneapolis, Minn.). PMNs were resuspended in HBSS, CM-TNF, or HBSS plus HNP-1 (1 μg/ml) and allowed to migrate to either fMLP or IL-8. As expected, both CM-TNF and HNP-1 suppressed migration to fMLP. In contrast, neither CM-TNF nor HNP-1 had any effect on the ability of PMNs to migrate to IL-8 (Fig. 3A). To test whether this lack of effect was due to selective downregulation of the fMLP receptor, fMLPR, and not the IL-8 receptor, CXCR1, we incubated PMNs in HBSS plus HNP-1 (1 μg/ml) for 30 min. The cell surface expression of fMLPR was measured by utilizing a fluorescein-conjugated formyl peptide as previously described (12). CXCR1 expression was measured by sequential incubation of the PMNs with anti-CXCR1 antibodies (Pharmingen, San Diego, Calif.) and fluorescein isothiocyanate-labeled secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.). As shown in Fig. 3B, HNP-1 suppressed migration towards fMLP independent of fMLPR expression. In contrast, HNP-1 did significantly downregulate CXCR1 expression; however, since migration was unaffected, this downregulation was physiologically insignificant as far as migration is concerned. However, we cannot rule out the possibility that HNP-1 may affect other IL-8-mediated responses in PMNs.
FIG. 3.
HNP-1 activity dependent on chemoattractant. (A) PMNs were resuspended in HBSS, CM-TNF, or HNP-1 (1 μg/ml in HBSS) and tested for antichemotactic activity. Results shown are means + standards errors of the means (n = 4). (B) The expression of the fMLP and IL-8 receptors, fMLPR and CXCR1, respectively, on PMNs incubated in HBSS with or without HNP-1 for 30 min was examined by fluorescence-activated cell sorting. Results shown are means ± standard errors of the means (n = 6). Asterisks indicate results that are significantly different from those of the HBSS controls (P < 0.05, one-way analysis of variance followed by Fisher's test of protected least significant difference).
While the receptor for HNP-1, -2, and -3 is unknown, Yang et al. has suggested that they may use G-protein-linked receptors since their activity is pertussis toxin sensitive (17). One hypothesis for the antichemotactic activity on PMNs is that HNP-1 acts by desensitizing the PMNs to subsequent chemotactic stimulation by fMLP or Gro-α in a manner similar to that in which fMLP, IL-8, and Gro-α have the ability to desensitize PMNs to each other (8, 16). While these agents all use G-protein-linked receptors and exhibit similarities in their pathways (8), they have been shown to diverge (4, 7), giving each receptor specific sensitivity to the actions it elicits and possibly divergent mechanisms of regulation. One possible mediator is protein kinase C, which is inhibited by HNP-1 (2) and is required for fMLP-directed migration (5). In contrast, only specific isoforms of protein kinase C are utilized by IL-8-mediated migration (10). Therefore, the ability of HNP-1 to suppress fMLP-mediated chemotaxis, but not migration to IL-8, may be due to selective regulation of one or more members of crucial signal transduction pathways. However, until further testing can be done, this is only speculation.
In summary, HNP-1 is a small neutrophil granule protein that, in addition to its microbicidal activity, is chemotactic for naive T cells, immature dendritic cells, and monocytes. The data presented here demonstrate that PMNs downregulate the migration of other PMNs by secreting HNP-1, as well as other as-yet-unidentified proteins (as indicated by fraction D), in response to inflammatory factors such as TNF-α. These data clearly show that PMNs not only kill invading pathogens through the secretion of HNP-1 but also have the ability to regulate several aspects of the inflammatory process.
Acknowledgments
We thank John Leszyk at the University of Massachusetts, Worcester, for his professional guidance in regards to the preparation of the samples that we sent to him for matrix-assisted laser desorption ionization-time-of-flight analysis.
This work was supported by NIH grant GM53114 (H.H.S.) and the Rhode Island Hospital Department of Surgery.
REFERENCES
- 1.Benjamim, C. F., S. H. Ferreira, and F. d. Q. Cunha. 2000. Role of nitric oxide in the failure of neutrophil migration in sepsis. J. Infect. Dis. 182:214-223. [DOI] [PubMed] [Google Scholar]
- 2.Charp, P. A., W. G. Rice, R. L. Raynor, E. Reimund, J. M. Kinkade, Jr., T. Ganz, M. E. Selsted, R. I. Lehrer, and J. F. Kuo. 1988. Inhibition of protein kinase C by defensins, antibiotic peptides from human neutrophils. Biochem. Pharmacol. 37:951-956. [DOI] [PubMed] [Google Scholar]
- 3.Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. [DOI] [PubMed] [Google Scholar]
- 4.Damaj, B. B., S. R. McColl, K. Neote, C. A. Hébert, and P. H. Naccache. 1996. Diverging signal transduction pathways activated by interleukin 8 (IL-8) and related chemokines in human neutrophils. J. Biol. Chem. 271:20540-20544. [DOI] [PubMed] [Google Scholar]
- 5.Entschladen, F., M. Gunzer, C. M. Scheuffele, B. Niggemann, and K. S. Zanker. 2000. T lymphocytes and neutrophil granulocytes differ in regulatory signaling and migratory dynamics with regard to spontaneous locomotion and chemotaxis. Cell. Immunol. 199:104-114. [DOI] [PubMed] [Google Scholar]
- 6.Grutkoski, P. S., R. D'Amico, A. Ayala, and H. H. Simms. 2002. TNF-α stimulated PMN suppress migration and bactericidal activity of PMN in a paracrine manner. Crit. Care Med. 30:591-597. [DOI] [PubMed] [Google Scholar]
- 7.Hall, D. A., I. J. M. Beresford, C. Browning, and H. Giles. 1999. Signalling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: a comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPγS binding induced by IL-8 and GROα. Br. J. Pharmacol. 126:810-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane, M. P., and R. M. Strieter. 2000. Chemokine signaling in inflammation. Crit. Care Med. 28:N13-N26. [DOI] [PubMed] [Google Scholar]
- 9.Kew, R. R., T. M. Hyers, and R. O. Webster. 1990. Human C-reactive protein inhibits neutrophil chemotaxis in vitro: possible implications for the adult respiratory distress syndrome. J. Lab. Clin. Med. 115:339-345. [PubMed] [Google Scholar]
- 10.Laudanna, C., D. Mochly-Rosen, T. Liron, G. Constantin, and E. C. Butcher. 1998. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J. Biol. Chem. 273:30306-30315. [DOI] [PubMed] [Google Scholar]
- 11.Risso, A. 2000. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J. Leukoc. Biol. 68:785-792. [PubMed] [Google Scholar]
- 12.Schagat, T. L., J. A. Wofford, K. E. Greene, and J. R. Wright. 2003. Surfactant protein A differentially regulates peripheral and inflammatory neutrophil chemotaxis. Am. J. Physiol. Lung Cell Mol. Physiol. 284:L140-L147. [DOI] [PubMed] [Google Scholar]
- 13.Stanton, K. J., M. B. Frewin, and P. W. Gudewicz. 1999. Heterologous desensitization of IL-8-mediated chemotaxis in human neutrophils by a cell-binding fragment of fibronectin. J. Leukoc. Biol. 65:515-522. [DOI] [PubMed] [Google Scholar]
- 14.Territo, M. C., T. Ganz, M. E. Selsted, and R. Lehrer. 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Investig. 84:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldkamp, K. E., H. C. J. M. Heezius, J. Verhoef, J. A. G. van Strijp, and K. P. M. van Kessel. 2000. Modulation of neutrophil chemokine receptors by Staphylococcus aureus supernate. Infect. Immun. 68:5908-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner, J. G., and R. A. Roth. 2000. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol. Rev. 52:349-374. [PubMed] [Google Scholar]
- 17.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
- 18.Yomogida, S., I. Nagaoka, K. Saito, and T. Yamashita. 1996. Evaluation of the effects of defensins on neutrophil functions. Inflamm. Res. 45:62-67. [DOI] [PubMed] [Google Scholar]