Abstract
Organisms producing extended-spectrum β-lactamases (ESBLs) have been reported in many countries, but there is no information on the prevalence of ESBL-producing members of the family Enterobacteriaceae in Ireland. A total of 925 isolates of ampicillin-resistant members of the Enterobacteriaceae were received from six hospitals in Ireland over a 3-year period from September 1996 to September 1999. Isolates were screened for ESBL production by the double-disk diffusion (DDD) method. DDD-positive isolates that were (i) confirmed as ESBL producers by National Committee for Clinical Laboratory Standards (NCCLS) confirmatory testing and (ii) susceptible to cefoxitin by disk diffusion were considered ESBL producers. By these criteria, 27 (3%) of the ampicillin-resistant members of the Enterobacteriaceae studied were categorized as ESBL producers. Molecular typing suggested that some intra- and interhospital spread of ESBL-producing isolates had occurred. DNA sequencing of amplified blaTEM and blaSHV genes resulted in the detection of a novel blaTEM ESBL gene, blaTEM-102 in two isolates (Klebsiella pneumoniae and Enterobacter cloacae) received from the same hospital but isolated from different patients. The study suggests dissemination of ESBL-producing bacteria within the health care system in Ireland and emphasizes the need for measures to control such spread.
The most significant mechanism of resistance to the β-lactam antimicrobial agents is the production of β-lactamase enzymes. Extended-spectrum β-lactamases (ESBLs) hydrolyze the expanded-spectrum cephalosporins (e.g., cefotaxime and ceftazidime) and monobactams, do not hydrolyze the cephamycins (e.g., cefoxitin), and are inhibited by the β-lactamase inhibitors (e.g., clavulanic acid). The first ESBL, derived from SHV-1 was reported in Germany in 1983 and was designated SHV-2 (17). At this time, more than 50 TEM-derived and 30 SHV-derived ESBLs have been characterized (http:www.lahey.org/studies/webt.htm). These ESBLs are primarily produced by members of the family Enterobacteriaceae, but they have also been reported in other families of gram-negative bacteria. In the Enterobacteriaceae, the predominant host species for ESBLs are Escherichia coli, Klebsiella pneumoniae, and to a lesser extent, Klebsiella oxytoca (25, 27). The first outbreak of a TEM-derived ESBL, TEM-3, was recorded in France (28). Outbreaks of ESBL-producing organisms have since been reported worldwide (3, 4, 9, 18, 22, 29, 35). Prior to this study, there had been no detailed investigation of the prevalence of ESBLs in Ireland, although one institution had reported an outbreak of ESBL-producing K. pneumoniae (11).
In the absence of specific surveillance, the prevalence of ESBLs in a country or region may be underrecognized, as many routine susceptibility test methods employed in clinical laboratories may not detect the production of ESBLs. Throughout the period of this study, routine antimicrobial susceptibility testing in most clinical laboratories in Ireland was performed by a modification of the same-plate comparative method developed by Stokes (31). The Stokes method is a nonstandardized, disk diffusion method in which susceptibility is assessed by evaluation of the diameter of the zone of inhibition of growth of the test strain relative to the diameter of the zone for the control strain (in this case, E. coli ATCC 25922). Both test and control organisms are plated on a single plate, and the antimicrobial disks are placed at the boundary between the test and control strains. The Stokes method is now being progressively replaced by standardized antimicrobial susceptibility testing. The performance of the modified Stokes method for detection of ESBL-mediated resistance to broad-spectrum cephalosporins has not been defined, although experience in our laboratory indicates that ESBL-producing isolates may be considered sensitive to cefotaxime and ceftazidime by the Stokes method (unpublished observations). Routine susceptibility testing also does not differentiate between resistance to extended-spectrum cephalosporins mediated by ESBL or by other types of β-lactamase production. In particular, high-level production of chromosomal AmpC β-lactamases can also confer resistance to the extended-spectrum cephalosporins and aztreonam, and it is not possible to distinguish between these mechanisms of resistance on the basis of routine susceptibility testing (6).
Susceptibility to extended-spectrum cephalosporins in the presence of clavulanic acid is the phenotypic characteristic of ESBL-mediated resistance that differentiates it from resistance due to AmpC β-lactamases (6, 32, 33). A variety of methods for detection of ESBL production based on this characteristic have been developed. These methods include the double-disk diffusion (DDD) test, the ESBL Etest, the Vitek ESBL test, and the National Committee for Clinical Laboratory Standards (NCCLS) screening and confirmatory tests for ESBL production (for isolates of E. coli, K. pneumoniae, and K. oxytoca). Susceptibility testing to cephamycins (e.g., cefoxitin) may also help to distinguish between these two types of β-lactamase production, given that AmpC β-lactamases confer resistance to the cephamycins and ESBLs do not.
K. oxytoca possesses a chromosomal K1 β-lactamase which has the properties of an ESBL. Hyperproduction of this enzyme confers high-level resistance resistance to the penicillins, aztreonam, and cefuroxime, moderate resistance to ceftriaxone and cefotaxime, and susceptibility to ceftazidime (33, 34).
A number of hospitals throughout Ireland were invited to participate in a study to determine to what extent ESBL-producing bacteria were present in Ireland. All ESBL-producing strains are ampicillin resistant (6). β-Lactamase-mediated ampicillin resistance in Enterobacteriaceae is generally readily identified by the Stokes methods; therefore, participating hospitals were invited to submit isolates of Enterobacteriaceae identified as ampicillin resistant by routine testing for evaluation for the presence of ESBLs.
MATERIALS AND METHODS
Bacterial strains.
A total of 925 isolates of ampicillin-resistant members of the family Enterobacteriaceae were collected from six hospitals throughout Ireland (Table 1). Isolates were collected over a 3-year period (September 1996 to September 1999) from blood, sputum, urine, and other clinical specimens. The identity of all isolates was confirmed by using API 20E (BioMérieux, Marcy l'Etoile, France).
TABLE 1.
Species distribution of the 925 isolates of ampicillin-resistant members of the family Enterobacteriaceae collected in Ireland and breakdown of isolates
| Species | Total no. of isolates collected | No. of ESBL-producing isolatesa by:
|
No. of β-lactamase-producing isolates by:
|
||
|---|---|---|---|---|---|
| DDD test | NCCLS MIC confirm test | IEFb | PCR | ||
| Escherichia coli | 442 | 15 | 9 | 11 | 7 |
| Klebsiella pneumoniae | 70 | 7 | 7 | 7 | 7 |
| Klebsiella oxytoca | 14 | 3 | 3 | 3 | 1 |
| Other species of Klebsiella | |||||
| Klebsiella ornithinolytica | 4 | 1 | 0 | 1 | 1 |
| Klebsiella ozaenae | 4 | 3 | 3 | 3 | 3 |
| Klebsiella spp. | 20 | 0 | 0 | 0 | 0 |
| Other species of Entero- bacteriaceae | |||||
| Enterobacter aerogenes | 3 | 2 | 2 | 0 | 0 |
| Enterobacter agglomerans | 5 | 2 | 2 | 1 | 1 |
| Enterobacter cloacae | 36 | 15 | 12 | 14 | 7 |
| Enterobacter fergusonii | 1 | 0 | 0 | 0 | 0 |
| Enterobacter sakazakii | 5 | 1 | 1 | 1 | 1 |
| Enterobacter spp. | 4 | 0 | 0 | 0 | 0 |
| Hafnia alvei | 3 | 1 | 0 | 1 | 0 |
| Kluyvera spp. | 10 | 3 | 3 | 2 | 2 |
| Morganella morganii | 3 | 0 | 0 | 0 | 0 |
| Proteus mirabilis | 7 | 0 | 0 | 0 | 0 |
| Proteus spp. | 3 | 0 | 0 | 0 | 0 |
| Proteus vulgaris | 1 | 0 | 0 | 0 | 0 |
| Salmonella entericac | 280 | 0 | 0 | 0 | 0 |
| Serratia liquefaciens | 2 | 1 | 1 | 1 | 0 |
| Serratia marcescens | 7 | 1 | 0 | 1 | 1 |
| Yersinia enterocolitica | 1 | 1 | 0 | 0 | 0 |
Number of isolates identified as suspect ESBL producers based on the DDD test and number of isolates with positive results by the NCCLS MIC broth microdilution confirmatory test for ESBL production.
IEF, isoelectric focusing.
These 280 isolates of Salmonella enterica incorporate various serotypes.
Antimicrobial susceptibility testing.
The DDD method (15) using the modifications developed by Coudron et al. (7) was used as the primary screening method for ESBL production in this study. Antimicrobial disks were obtained from Oxoid (Basingstoke, United Kingdom) or Difco (Surrey, United Kingdom). Aztreonam (30 μg), ceftriaxone (30 μg), and cefpodoxime (10 μg) were placed edge to edge 15 mm from a central amoxicillin-clavulanic acid disk (20 μg of amoxicillin and 10 μg of clavulanic acid) on a Mueller-Hinton agar plate inoculated with a suspension of test organism equal to a 0.5 McFarland standard. Enhancement of the zone of inhibition between any one of the β-lactam disks and the clavulanic acid-containing disk was presumed positive for ESBL production. The NCCLS broth microdilution screening and confirmatory tests for ESBL production were performed in triplicate on isolates identified as suspect ESBL producers by the DDD method (23). E. coli ATCC 25922 and K. pneumoniae ATCC 700603 were used as control strains for all phenotypic testing.
Isoelectric focusing.
Isoelectric focusing was performed in duplicate on all isolates identified as suspect ESBL producers by the DDD method. Isolates were cultured as previously described (20), and cells were lysed by freeze-thawing (27). Electrophoresis was performed on precast polyacrylamide gels, pH 3.5 to 9.5 (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Enzyme standards from E. coli C600 transconjugant strains harboring plasmids encoding a β-lactamase (TEM-3 [pI 6.3], TEM-4 [pI 5.9], TEM-12 [pI 5.25], SHV-1 [pI 7.6], SHV-3 [pI 7.0], and SHV-5 [pI 8.2]) were used as pI markers for all analyses. Enzyme activity was detected by placing filter paper soaked in nitrocefin (500 μg/ml) (Becton Dickinson) over the focused gel.
Amplification and sequencing of β-lactamase genes.
Isolates suspected of ESBL production based on results with the DDD method were examined for the presence of blaTEM and blaSHV by PCR using specific primers. The primers (5′-GTATGGATCCTCAACATTTCCGTGTCG-3′, starting at position 205, and 5′-ACCAAAGCTTAATCAGTGAGGCA-3′, starting at position 1067) and amplification conditions for detection of blaTEM were as described previously (30). The expected product was 862 bp. Primers (5′-TCGGGCCGCGTAGGCATGAT-3′ and 5′-AGCAGGGCGCAATCCCGCG-3′) for amplification of blaSHV were designed from published sequence data. Amplification was performed in a 50-μl volume in a solution of 1.25 U of Taq polymerase (Promega), 10× MgCl2-free reaction buffer (Promega), 2.0 mM MgCl2 (Promega), 200 μM deoxynucleoside triphosphates (Promega), and 250 mM (each) primer (Amersham Pharmacia Biotech). Cycling conditions were as follows: (i) an initial denaturation step of 4 min at 94°C; (ii) 34 cycles, with 1 cycle consisting of 45 s at 94°C, 45 s at 62°C, and 1 min at 72°C; and (iii) a final extension step of 2 min at 72°C. The expected product was 812 bp. E. coli C600 transconjugant strains harboring plasmids incorporating blaTEM or blaSHV were used as positive controls. To ensure that all DNA could be amplified, primers specific for the 16S/23S rRNA intergenic spacer region were used as described previously to amplify 250- to 600-bp fragments of the spacer region from the DNA prepared from all bacteria (2). PCR products were electrophoresed in a 1% agarose gel and visualized with ethidium bromide (0.5 μg/ml). DNA molecular weight marker XIV (Boehringer Mannheim) was used as a size standard. All PCR analyses were performed in duplicate.
PCR products were ligated to pCR2.1 vector (Invitrogen, Groningen, The Netherlands) and transformed into One Shot INVαF′ cells (Invitrogen) in accordance with the manufacturer's instructions. Positive clones were harvested, and plasmid DNA was extracted by using the Qiagen spin miniprep kit (Qiagen, Crawley, United Kingdom). EcoRI sites flank the position on the vector at which the bla gene is inserted. The presence of the bla gene was confirmed by digestion with EcoRI (Boehringer Mannheim) and sequenced using M13 forward and reverse primers, which flank the location of the gene in the plasmid.
Molecular typing of strains.
Plasmid DNA was isolated using a modified version of the method of Kado and Liu (16). Plasmids were visualized by electrophoresis on a 1% agarose gel at 70 V for 4 h, followed by staining with a 1-mg/ml solution of ethidium bromide. The supercoiled DNA marker (2 to 10 kb) and the laboratory strain E. coli J53 R1, which harbors a plasmid of 93 kb, were used as size standards.
For pulsed-field gel electrophoresis (PFGE) analysis, whole chromosomal DNA was prepared from bacteria embedded in agarose and digested with XbaI as previously described (24). DNA fragments were resolved on 1.2% (wt/vol) agarose gels run on a Pharmacia LKB gene navigator at 6 V/cm with pulse times ramped at 5 to 60 s for 3 h and then at 5 to 20 s for 20 h. Pulse markers (50 to 1,000 kb; Sigma) consisting of concatemers of lambda DNA were used as size standards. Gels were stained in ethidium bromide (5 μg/ml) and visualized under UV light.
Randomly amplified polymorphic DNA (RAPD) PCR was performed using a combination of three primers, primers 4, 5, and 6 from the Ready To Go RAPD analysis kit (Amersham Pharmacia Biotech). The reaction was set up in accordance with the manufacturer's instructions. Cycling conditions were as follows: (i) an initial denaturation step of 3 min at 94°C; (ii) 35 cycles, with 1 cycle consisting of denaturation (20 s at 94°C), annealing (20 s at 40°C), and extension (45 s at 72°C); and (iii) a final extension step of 5 min at 72°C. Electrophoresis was performed in a 1% agarose-1% NuSieve agarose gel in 1× Tris-borate-EDTA (TBE) at 2 V/cm and 146 mA for 4.5 h (12). DNA was visualized by staining the gel with 5 μg of ethidium bromide per ml in 0.5× TBE. DNA molecular weight marker XIV (Boehringer Mannheim) was used as a size standard.
TIFF images of the PFGE and RAPD PCR gels were scanned into the Bionumerics software (Applied Maths, Kortrijk, Belgium) and normalized by aligning the molecular size marker with the reference standard for the database. Analysis of banding patterns generated by PFGE and RAPD PCR was performed using the Dice coefficient at band migration tolerances of 3 and 1.2%, respectively. Clustering of patterns was performed by unweighted pair group with arithmetic averages (UPGMA).
Nucleotide sequence accession number.
The sequence of the novel β-lactamase, TEM-102, has been deposited in GenBank and assigned accession number AY029354.
RESULTS
A total of 925 ampicillin-resistant members of the family Enterobacteriaceae were collected from six hospitals in Ireland (Table 1). Fifty-six (6%) isolates were identified as suspect ESBL producers by the DDD method of Coudron et al. (7). The isolate collection comprised 442 E. coli (15 [3.4%] DDD positive), 70 K. pneumoniae (7 [10%] DDD positive), 14 K. oxytoca (3 [21%] DDD positive), 28 Klebsiella spp. (species other than K. pneumoniae or K. oxytoca) (4 [14%] DDD positive), 54 Enterobacter spp. (20 [37%] DDD positive), and 317 other species of Enterobacteriaceae (7 [2%] DDD positive) (Table 1).
The NCCLS broth microdilution confirmatory test criteria (23) confirmed ESBL production (>sixfold reduction in MIC of cefotaxime or ceftazidime in the presence of clavulanic acid) in 43 isolates, namely, 9 E. coli, 7 K. pneumoniae, 3 K. oxytoca, 3 Klebsiella ozaenae, 2 Enterobacter aerogenes, 2 Enterobacter agglomerans, 12 Enterobacter cloacae, 1 Enterobacter sakazakii, 3 Kluyvera spp., and 1 Serratia liquefaciens. The NCCLS broth microdilution confirmatory test criteria for ESBL production are specified only for isolates of E. coli, K. pneumoniae, and K. oxytoca. Twenty-seven isolates with positive results with NCCLS ESBL confirmatory test criteria were susceptible to cefoxitin; these 27 isolates comprised 7 E. coli, 7 K. pneumoniae, 2 K. oxytoca, 3 K. ozaenae, 2 E. agglomerans, 2 E. cloacae, 3 Kluyvera spp., and 1 S. liquefaciens.
β-Lactamase enzymes with pI values in the range 5.2 to 6.5 (consistent with TEM-like β-lactamase) were identified in 33 (59%) isolates, enzymes with pI values in the range 7.0 to 8.2 (consistent with SHV-like enzymes) were detected in 14 (25%) isolates, and no β-lactamase was visualized in 9 (16%) cases (4 E. coli, 2 E. aerogenes, 1 E. agglomerans, 1 E. cloacae, 1 Yersinia enterocolitica). Sixteen isolates (28%) (2 E. coli, 4 K. pneumoniae, 7 E. cloacae, 1 E. sakazakii, 1 Kluyvera species, and 1 S. liquefaciens) produced β-lactamase enzymes with pI values greater than 8.2 consistent with AmpC-like β-lactamase. More than one β-lactamase was identified in 14 (25%) isolates (4 K. pneumoniae, 2 K. oxytoca, 1 K. ozaenae, 6 E. cloacae, and 1 E. sakazakii).
The blaTEM gene alone was detected in 25 isolates (7 E. coli, 2 K. pneumoniae, 1 K. oxytoca, 1 Klebsiella ornithinolytica, 2 K. ozaenae, 1 E. agglomerans, 7 E. cloacae, 1 E. sakazakii, 2 Kluyvera spp., and 1 S. marcescens). The blaSHV gene alone was detected in two isolates (one K. pneumoniae and one K. ozaenae). Both the blaTEM and blaSHV genes were detected in four K. pneumoniae isolates. Neither the blaTEM gene nor the blaSHV gene was detected in 25 isolates (8 E. coli, 2 K. oxytoca, 2 E. aerogenes, 1 E. agglomerans, 8 E. cloacae, 1 Hafnia alvei, 1 Kluyvera species, 1 S. liquefaciens, and 1 Y. enterocolitica). The nucleotide sequences and corresponding derived amino acid sequences were elucidated for all partial blaTEM and blaSHV genes detected by PCR. Of these, 14 corresponded to TEM-1, 7 corresponded to TEM-2, 3 corresponded to TEM-12, 1 corresponded to TEM-33, 2 corresponded to TEM-35, 3 corresponded to SHV-1, 2 corresponded to SHV-11, and 1 corresponded to SHV-5 (Tables 2 and 3). The derived amino acid sequences of the blaTEM genes from two isolates (one K. pneumoniae isolate and one E. cloacae isolate) revealed substitutions not previously observed. Both had a phenylalanine residue at position 21, a serine residue at position 164, and a methionine residue at position 265. This sequence has been designated TEM-102 (Table 2) (http:www.lahey.org/studies/webt.htm). Various silent substitutions were also observed in the amino acid sequences of the TEM- and SHV-derived amino acid sequences.
TABLE 2.
Sequence analysis of the 29 isolates found to harbor the blaTEM gene by PCRa
| Speciesa (no. of isolates) | Amino acid at positionb:
|
β-Lacta- mase | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 21 | 39 | 42 | 51 | 69 | 92 | 104 | 115 | 127 | 130 | 153 | 164 | 165 | 182 | 196 | 204 | 218 | 237 | 238 | 240 | 244 | 262 | 265 | 268 | 275 | 276 | ||
| EC (5) | S | I | Q | L | Q | A | L | M | G | E | D | I | S | H | R | W | M | G | R | G | A | G | E | R | V | T | S | R | N | TEM-1 |
| KP (5) | ||||||||||||||||||||||||||||||
| KO (1) | ||||||||||||||||||||||||||||||
| OK (1) | ||||||||||||||||||||||||||||||
| OE (2) | ||||||||||||||||||||||||||||||
| EC (1) | K | TEM-2 | ||||||||||||||||||||||||||||
| OE (6) | ||||||||||||||||||||||||||||||
| OK (2) | S | TEM-12 | ||||||||||||||||||||||||||||
| OE (1) | ||||||||||||||||||||||||||||||
| EC (1) | L | TEM-33 | ||||||||||||||||||||||||||||
| OE (2) | L | D | TEM-35 | |||||||||||||||||||||||||||
| KP (1) | F | S | M | TEM-102 | ||||||||||||||||||||||||||
| OE (1) | ||||||||||||||||||||||||||||||
EC, E. coli; KP, K. pneumoniae; KO, K. oxytoca; OK, other species of Klebsiella; OE, other species of Enterobacteriaceae.
Amino acid residues are numbered according to the numbering system of Ambler et al. (1). The one-letter amino acid code is used as follows: A, alanine; C, cysteine; D, aspartic acid; E, glutamic acid; F, phenylalanine; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; M, methionine; N, asparagine; P, proline; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine; W, tryptophan.
TABLE 3.
Derived amino acid sequences of the six isolates in which blaSHV genes were detected by PCRa
| Speciesa (no. of isolates) | Amino acid at positionb:
|
β-Lactamase | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 8 | 35 | 43 | 48 | 54 | 75 | 80 | 89 | 122 | 129 | 130 | 140 | 156 | 158 | 173 | 179 | 187 | 188 | 192 | 193 | 205 | 238 | 240 | ||
| KP (3) | F | I | L | R | E | G | V | V | E | L | M | S | A | G | N | N | D | A | A | K | L | R | G | E | SHV-1 |
| KP (2) | Q | SHV-11 | |||||||||||||||||||||||
| OK (1) | S | K | SHV-5 | ||||||||||||||||||||||
KP, K. pneumoniae; OK, other species of Klebsiella.
Amino acid residues are numbered in according to the numbering system of Ambler et al. (1). The one-letter amino acid code is used as follows: A, alanine; C, cysteine; D, aspartic acid; E, glutamic acid; F, phenylalanine; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; M, methionine; N, asparagine; P, proline; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine; W, tryptophan.
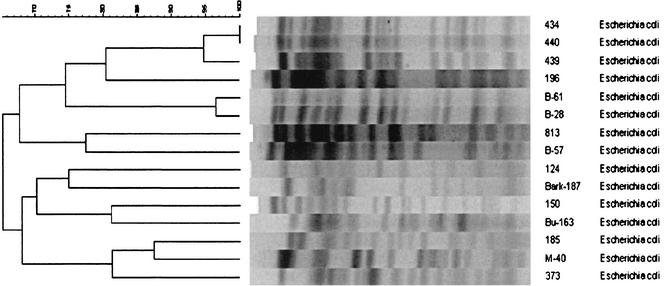
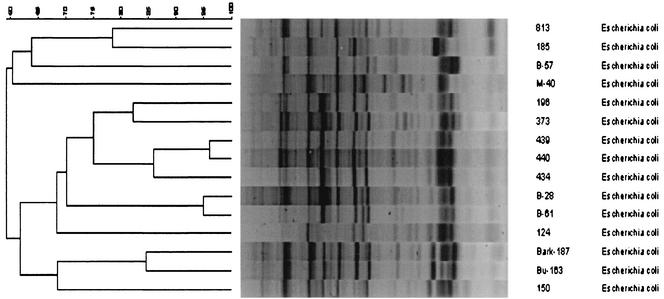
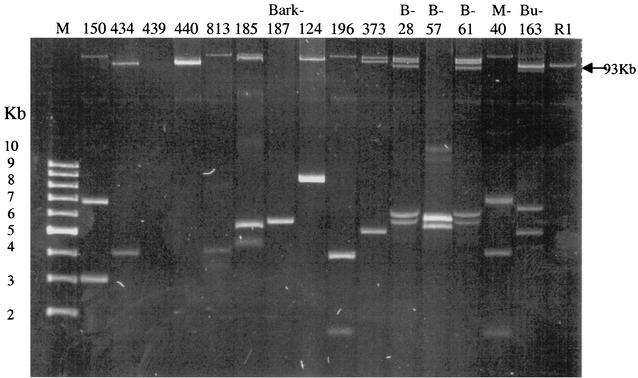
PFGE of the 15 E. coli isolates using XbaI generated 12 major patterns of 12 to 20 fragments from approximately 40 to 500 kb (Fig. 1). Analogous to PFGE, 12 major patterns were also recognized among the 15 isolates of E. coli analyzed by RAPD PCR (Fig. 2). Thirteen different plasmid profiles, each including one to five plasmids with molecular sizes ranging from 3 to 105 kb, were found among the 15 isolates of E. coli (Fig. 3). Three consecutive isolates from a single patient were identified as having similarity values of 96 to 100% by PFGE and RAPD analysis, but the isolates had different plasmid profiles. Two E. coli isolates from different patients in one hospital were 96% similar and had identical plasmid profiles.
FIG. 1.
Dendrogram (generated by the Bionumerics software) showing the relationship of the 15 E. coli isolates digested with XbaI by PFGE. The bands generated were analyzed using the Dice coefficient and UPGMA. The numbers over the dendrogram are percent similarity values.
FIG. 2.
Dendrogram (generated by the Bionumerics software) showing the relationship of the 15 E. coli isolates by RAPD PCR using a combination of three primers. The bands generated were analyzed using the Dice coefficient and UPGMA. The numbers over the dendrogram are percent similarity values.
FIG. 3.
Plasmid profile analysis of the 15 isolates of E. coli. Lanes: M, supercoiled DNA molecular size markers (2 to 10 kb); R1, size marker (laboratory strain E. coli J53 R1 containing a 93-kb plasmid).
PFGE of the seven isolates of K. pneumoniae yielded five major patterns of 12 to 18 fragments, ranging in size from approximately 35 to 400 kb. RAPD PCR analysis generated seven major patterns. Seven different plasmid profiles, each including two to eight plasmids with molecular sizes ranging from 1.8 to 115 kb, were identified. Two of these isolates were consecutive isolates from the same patient, had a similarity value of 100% by PFGE analysis (88% by RAPD PCR), and had similar plasmid profiles. A third isolate from a different patient and sent by a separate institution was identified as 88% similar to these two isolates based on PFGE analysis.
PFGE and RAPD PCR both yielded two major patterns among the three isolates of K. oxytoca examined. PFGE yielded two patterns and RAPD PCR yielded three distinct patterns among the three isolates of K. ozaenae. Two of the K. ozaenae isolates from different patients in one hospital were 98% similar by PFGE analysis (88% by RAPD PCR) and had identical plasmid profiles.
PFGE generated 14 major patterns of 14 to 21 fragments ranging in size from 30 to 500 kb from the 15 isolates of E. cloacae analyzed. Thirteen major patterns were recognized by RAPD PCR. Fourteen different plasmid profiles, each including one to four plasmids with molecular sizes ranging from 3.5 to 125 kb, were recognized. Two of these isolates, received from the same hospital but isolated from different patients, were 95% similar and had identical plasmid profiles.
DISCUSSION
This is the first extensive, multicenter study to prospectively look for ESBL-producing bacteria in Ireland. Screening ampicillin-resistant members of the family Enterobacteriaceae from six hospitals in Ireland by the DDD method of Coudron et al. (7) identified 56 suspect ESBL producers among 925 isolates (6%). NCCLS broth microdilution ESBL confirmatory test was positive for 43 (77%) of these isolates of which 19 were isolates of species for which the confirmatory test is recommended (E. coli, K. pneumoniae, and K. oxytoca) and 24 were other species of Enterobacteriaceae (3 K. ozaenae, 2 E. aerogenes, 2 E. agglomerans, 12 E. cloacae, 1 E. sakazakii, 3 Kluyvera spp., and 1 S. liquefaciens). Resistance to cefoxitin was noted in two NCCLS ESBL confirmatory test-positive E. coli isolates, 1 NCCLS ESBL confirmatory test-positive K. oxytoca isolate, and 13 (all Enterobacter spp.) NCCLS ESBL confirmatory test-positive isolates of Enterobacteriaceae other than E. coli or Klebsiella species. AmpC β-lactamase production is associated with resistance to cefoxitin, and this may account for the observed cefoxitin resistance in these 16 isolates.
AmpC β-lactamases are chromosomally encoded in several species of gram-negative bacteria, including Enterobacter spp., E. coli, Morganella morganii, Citrobacter freundii, and Serratia marcescens among others (14, 24). These enzymes are generally inducible, with the exception of the AmpC β-lactamase of E. coli, which is produced constitutively at low levels (8, 13). Cefoxitin is a potent inducer of AmpC production (21). AmpC β-lactamases generally have pIs of greater than 8 (6). Of these 16 cefoxitin-resistant isolates, 12 produced β-lactamases with pIs of greater than 8; however, AmpC is not inhibited by clavulanic acid and does not explain a positive result by DDD and NCCLS ESBL confirmatory test. Constitutive production of ESBL combined with cefoxitin-inducible high-level AmpC production is one possible explanation for these findings. Multiple β-lactamases were not identified by isoelectric focusing; however, isoelectric focusing was performed on isolates that were not subjected to induction prior to analysis.
Cefoxitin-resistant ESBL producers have been reported previously (33). Loss or alteration of the porin(s) which allows entry of the cephamycins (cefoxitin) to the cell may have resulted in the observed cefoxitin resistance (26). Martínez-Martínez et al. (19) recently reported that the loss of porins OmpK36 and OmpK35 resulted in increased resistance to cefoxitin in ESBL-producing isolates of K. pneumoniae. It is also possible that the ESBL confirmatory test is not entirely specific even among isolates of those three species for which it is recommended. If we consider the status of ESBL production in these 16 isolates as uncertain, the most conservative estimate (ESBL positive, cefoxitin susceptible) of the rate of ESBL production among ampicillin-resistant isolates in Ireland is 0.017% for E. coli, 10% for K. pneumoniae, and 14% for K. oxytoca.
TEM and/or SHV β-lactamase enzymes were detected by analytical isoelectric focusing in 33 of the 43 isolates that gave positive results by the NCCLS ESBL confirmatory test. The presence of the blaTEM gene and/or blaSHV gene was confirmed by PCR in 25 of these isolates (4 isolates harbored both blaTEM and blaSHV genes). Repeated failure of amplification was not due to inhibition of amplification, as the 16S/23S rRNA intergenic spacer region was amplified in each case. Sequencing of amplicons indicated blaTEM-1, blaTEM-2, or blaSHV-1 in 22 isolates as follows: 12 isolates had blaTEM-1 (5 E. coli, 3 K. pneumoniae, 1 K. oxytoca, 1 K. ornithinolytica, 1 E. cloacae, and 1 Kluyvera species), 7 isolates had blaTEM-2 (1 E. coli, 5 E. cloacae, and 1 E. sakazakii), 1 isolate had blaSHV-1 (K. pneumoniae), and 2 isolates had both blaTEM-1 and blaSHV-1 (K. pneumoniae). blaSHV-11 was detected in two isolates of K. pneumoniae. First described in Switzerland, SHV-11 is not considered an ESBL, although it is acknowledged that MICs of ceftriaxone, cefotaxime, cefepime, and ceftazidime are raised slightly (23a). The failure to identify ESBL sequences by amplification and sequencing from these isolates confirmed as ESBL producers by the NCCLS ESBL broth microdilution confirmatory method suggests that multiple blaTEM and/or blaSHV sequences may be present in these isolates and amplification methods may not detect the specific gene that encodes the ESBL. Alternatively, these isolates may produce non-TEM and non-SHV ESBLs (5).
ESBL sequences were identified in six isolates. Three isolates harbored blaTEM-12 (2 K. ozaenae and 1 E. agglomerans), and one isolate (K. ozaenae) harbored blaSHV-5. The TEM-12-producing K. ozaenae isolates were indistinguishable by PFGE and plasmid profile. The three TEM-12-producing isolates were isolated from different patients in a single hospital, which is consistent with dissemination of an ESBL-producing K. ozaenae strain and interspecies transfer of an ESBL gene.
Two isolates (1 K. pneumoniae and 1 E. cloacae) harbored the same novel TEM ESBL, TEM-102. The amino sequence of this novel ESBL differed from the amino acid sequence of TEM-1 by three amino acid substitutions: phenylalanine for leucine at position 21, serine for arginine at position 164, and methionine for threonine at position 265 (Table 2). These isolates were isolated from different patients in one hospital, which is consistent with dissemination of an ESBL-encoding gene.
Three isolates produced inhibitor-resistant β-lactamases; 2 produced TEM-35 (1 Kluyvera species and 1 S. marcescens), and 1 produced TEM-33 (E. coli). The inhibitor resistance phenotype was noted in each of the isolates. The two TEM-35-producing isolates were isolated from different patients in one hospital.
Three isolates of K. oxytoca were positive by the NCCLS ESBL confirmatory test. By isoelectric focusing, TEM-like β-lactamase was detected in two of these isolates, and SHV-like β-lactamase was detected in all three of these isolates. The presence of blaTEM-1 was confirmed by PCR and sequencing analysis in one of these isolates. All K. oxytoca characteristically produce a chromosomally encoded β-lactamase, K1, belonging to one of two groups, OXY-1 or OXY-2 (10). The OXY-1 β-lactamases of pI 7.5 to 8.2 and OXY-2 β-lactamases of pI 5.2 to 8 have been characterized (1a, 10). Hyperproduction of these enzymes confers high-level resistance to aztreonam, ceftriaxone, and cefuroxime, moderate resistance to cefotaxime, and low-level resistance to ceftazidime and can be mistaken for ESBL production. Overall, results with these three isolates appear most consistent with K1 hyperproduction.
This is the first extensive multicenter study to prospectively look for ESBL-producing bacteria in Ireland. The results indicate that ESBL-producing bacteria are present at significant levels and that intrahospital spread may have occurred.
Acknowledgments
We gratefully acknowledge Ron Jones, University of Iowa, for providing transconjugant E. coli C600 ESBL-positive strains.
We thank Wyeth Lederle Ireland, the Health Research Board of Ireland, and Enterprise Ireland for financial support.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Arakawa, Y., M. Ohta, N. Kido, M. Mori, H. Ito, and T. Komatsu. 1989. Chromosomal β-lactamases of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob. Agents Chemother. 33:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, T., G. Colleran, M. Glennon, L. K. Dunican, and F. Gannon. 1991. The 16S-23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1:51-56. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind, A., and G. Hori. 1987. Novel R-factor borne β-lactamase of Escherichia coli conferring resistance to cephalosporins. Infection 15:257-259. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet, R., J. L. M. Sampaio, C. Chanal, D. Sirot, C. De Champs, J. L. Viallard, R. Labia, and J. Sirot. 2000. A novel class A extended-spectrum β-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob. Agents Chemother. 44:3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coudron, P. E., E. S. Moland, and C. Sanders. 1997. Occurrence and detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J. Clin. Microbiol. 35:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullman, W., A. Dalkoff, and W. Dick. 1984. Nonspecific induction of β-lactamase in Enterobacter cloacae. J. Gen. Microbiol. 130:1781-1786. [DOI] [PubMed] [Google Scholar]
- 9.Essack, S. Y., L. M. C. Hall, D. G. Pillay, M. L. McFadyen, and D. M. Livermore. 2001. Complexity and diversity of Klebsiella pneumoniae strains with extended-spectrum β-lactamases isolated in 1994 and 1996 at a teaching hospital in Durban, South Africa. Antimicrob. Agents Chemother. 45:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier, B., P. H. Roy, P. H. Lagrange, and A. Philippon. 1996. Chromosomal β-lactamase genes of Klebsiella oxytoca are divided into two main groups, blaOXY-1 and blaOXY-2. Antimicrob. Agents Chemother. 40:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grogan, J., H. Murphy, and K. Butler. 1998. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a Dublin paediatric hospital. Br. J. Biomed. Sci. 55:111-117. [PubMed] [Google Scholar]
- 12.Grundmann, H. J., K. J. Towner, L. Dijkshoorn, P. Gerner-Smidt, M. Maher, H. Seifert, and M. Vaneechoutte. 1997. Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J. Clin. Microbiol. 35:3071-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honoré, N., M. H. Nicolas, and S. T. Cole. 1986. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been detected from Escherichia coli. EMBO J. 5:3709-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 16.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knothe, H., P. Shah, V. Kromery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 18.Ling, J. M., I. C. Koo, K. M. Kam, and A. F. Cheng. 1998. Antimicrobial susceptibilities and molecular epidemiology of Salmonella enterica serotype Enteritidis strains isolated in Hong Kong from 1986 to 1996. J. Clin. Microbiol. 36:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Martínez, L., S. Hernández-Alléz, S. Albetí, J. M. Tomás, V. J. Benedi, and G. A. Jacoby. 1996. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 40:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthew, M., A. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 21.Minami, S., N. Matsubara, and A. Yotsuji. 1983. Induction of cephalosporinase production by various penicillins in Enterobacteriaceae. J. Antibiot. 36:1387-1395. [DOI] [PubMed] [Google Scholar]
- 22.M'Zali, F. H., J. Heritage, D. M. Gascoyne-Binzi, M. Denton, N. J. Todd, and P. M. Hawkey. 1997. Transcontinental importation into the United Kingdom of Escherichia coli expressing a plasmid-mediated AmpC-type β-lactamase exposed during an outbreak of SHV-5 extended-spectrum β-lactamase in a Leeds hospital. J. Antimicrob. Chemother. 40:823-831. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, fifth edition M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23a.Nüesch-Inderbinen, M. T., F. H. Kayser, and H. Hächler. 1997.. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob. Agents Chemother. 41:943-949. [DOI] [PMC free article] [PubMed]
- 24.Pfaller, M. A., R. N. Jones, S. A. Marshall, S. L. Coffman, R. J. Hollis, M. B. Edmond, and R. P. Wenzel. 1997. Inducible AmpC β-lactamase producing Gram-negative bacilli from blood stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE). Diagn. Microbiol. Infect. Dis. 28:211-219. [DOI] [PubMed] [Google Scholar]
- 25.Philippon, A., R. Labia, and G. A. Jacoby. 1989. Extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 33:1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders, C. C., and W. E. Sanders, Jr. 1992. β-Lactam resistance in Gram-negative bacteria: global trends and clinical impact. Clin. Infect. Dis. 15:824-839. [DOI] [PubMed] [Google Scholar]
- 27.Shen, D., P. Winokur, and R. N. Jones. 2001. Characterisation of extended-spectrum β-lactamase producing K. pneumoniae from Beijing, China. Int. J. Antimicrob. Agents 18:185-188. [DOI] [PubMed] [Google Scholar]
- 28.Sirot, D., J. Sirot, R. Labia, A. Morand, P. Courvalin, A. Dorfeuille-Michaud, R. Derroux, and R. Cluzel. 1987. Transferable resistance to third generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. J. Antimicrob. Chemother. 20:323-334. [DOI] [PubMed] [Google Scholar]
- 29.Spencer, R. C., P. F. Wheat, T. G. Winstanley, D. M. Cox, and S. J. Plested. 1987. Novel β-lactamase in a clinical isolate of Klebsiella pneumoniae conferring unusual resistance to β-lactam antibiotics. J. Antimicrob. Chemother. 20:919-921. [DOI] [PubMed] [Google Scholar]
- 30.Stapleton, P., P. J. Wu, K. Shannon, G. French, and I. Phillips. 1995. Incidence and mechanisms of resistance to the combination of amoxicillin and clavulanic acid in Escherichia coli. Antimicrob. Agents Chemother. 39:2478-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes, E. J., G. L. Ridgway, and M. W. D. Wren. 1993. Clinical microbiology, vol. 7. Edward Arnold, London, United Kingdom.
- 32.Tenover, F. C., M. J. Mohammed, T. S. Gorton, and Z. F. Dembek. 1999. Detection and reporting of organisms producing extended-spectrum β-lactamases: survey of laboratories in Connecticut. J. Clin. Microbiol. 37:4065-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson, K. S., C. C. Sanders, and E. S. Moland. 1999. Use of microdilution panels with and without β-lactamase inhibitors as a phenotypic test for β-lactamase production among Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter freundii, and Serratia marcescens. Antimicrob. Agents Chemother. 43:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson, K. S., and E. S. Moland. 2000. Version 2000: the new β-lactamases of Gram-negative bacteria at the dawn of the new millennium. Microb. Infect. 2:1225-1235. [DOI] [PubMed] [Google Scholar]
- 35.Yan, J. J., S. M. Wu, S. H. Tsai, J. J. Wu, and I. J. Su. 2000. Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae producing extended-spectrum β-lactamases and identification of a novel AmpC enzyme (CMY-8) in southern Taiwan. Antimicrob. Agents Chemother. 44:1438-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]