Abstract
Escherichia coli phage N15 encodes the slightly acidic, 630-residue protein of 72.2 kDa called protelomerase (TelN). TelN is a component of the N15 replication system proposed to be involved in the generation of the linear prophage DNA. This linear DNA molecule has covalently closed ends. The reaction converting circular plasmids into linear molecules was catalyzed in vitro. We demonstrate that the product of telN functions as the protelomerase in the absence of other N15-encoded factors. Purified TelN processes circular and linear plasmid DNA containing the proposed target site telRL to produce linear double-stranded DNA with covalently closed ends. The 56-bp telRL target site consists of a central telO palindrome of 22 bp and two 14-bp flanking sequences comprising inverted repeats. telO is separated from these repeats by 3 bp on each side. The telRL sequence is sufficient for TelN-mediated processing. The ends of the DNA molecules generated in vitro have the same configuration as do those observed in vivo. TelN exerts its activity as cleaving-joining enzyme in a concerted action.
Keywords: replication, prophage, TelN protein
The temperate lambdoid Escherichia coli phage N15 was discovered by Victor Ravin in 1964. The total N15 nucleotide sequence has been determined recently (46,375 bp; GenBank accession no. AF064539). Interpretation of the DNA sequence combined with the fact that the prophage is a linear plasmid molecule with covalently closed ends (1) suggested that N15 replication must differ considerably from that of phage λ. A large replication protein, RepA, of 1,324 aa residues has been predicted that is supposed to have at least primase and helicase activity but probably also origin-recognition ability (GenBank accession no. AF064539 and ref. 2). In principle, the repA gene product resembles the multifunctional replication protein α of the E. coli satellite phage P4. Replication of P4 is independent of chromosome-encoded initiation functions of the host (3, 4). Currently it is known that N15 replication is independent of at least dnaA, dnaJ, dnaK, grpE, and recA (5, 6). However, its mode of replication still is not understood.
The N15 prophage structure is unique in E. coli, but similar structures have been found in the pathogenic organism Borrelia burgdorferi. The chromosome and many plasmids in this organism exist as linear molecules with covalently closed ends (7, 8). In both cases, N15 and B. burgdorferi gene products were predicted that share weak similarity with each other and with members of the family of site-specific recombinases known as integrases (9). These integrase candidates are the products of gene 29 and gene BBB03, respectively. The corresponding proteins were thought to be responsible for the generation of the covalently closed ends of the DNA molecules. Thus, N15 may serve as a model system to study replication of linear chromosomes of Borrelia causing Lyme disease. In N15, the target sequence for the enzyme was called tos (telomerase occupancy site; Fig. 3A; ref. 2). tos contains a series of inverted repeats centered on a large palindromic sequence. This palindrome, or part of it, could form a potential cruciform structure that may function as the real substrate for the enzyme. The predicted enzyme thought to act on tos was named protelomerase (gene 29 ≡ telN), a prokaryotic telomerase (2). Unlike eukaryotic telomerases, the N15 protelomerase was predicted to be a purely proteic enzyme devoid of any RNA component.
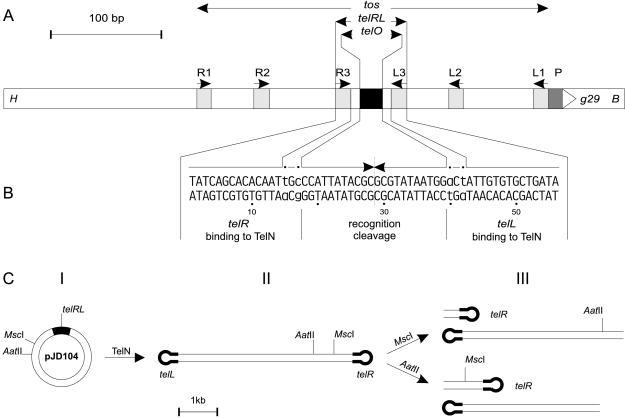
Figure 3.
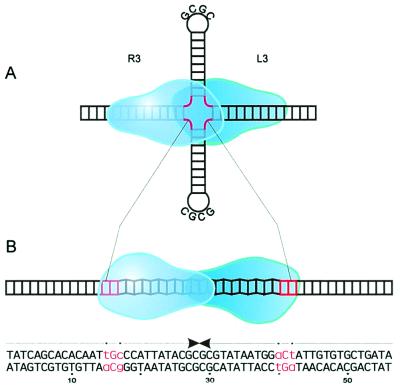
(A) Schematic representation of tos and adjacent regions. tos (telomerase occupancy site; ref 2) contains six inverted repeats (R1/L1 and R2/L2, 10 bp each, and R3/L3, 14 bp each, light gray boxes) and the 22-bp palindrome telO (black box). The pairs of repeats differ in sequence. telRL is composed of telO and the repeats R3 and L3. P indicates the potential promoter (dark gray box) for gene 29 (g29), encoding the protelomerase. The 573-bp fragment (positions 24,474 to 25,037 of the N15 phage genome, GenBank accession no. AF064539) is flanked by a HindIII site (H) and a BamHI site (B). (B) The 56-bp sequence of telRL is shown. The arrows indicate the inverted sequence repetition that is interrupted by 2 bp on either side, which are printed in lowercase and marked by dots. The 28-bp sequences telL and telR designate the ends of the left arm and the right arm of the N15 prophage. (C) Schematic presentation of the processing of the tos substrate pJD104. Plasmid pJD104 (I, the black box represents the tos region) was incubated with TelN protein, resulting in a double-stranded linear product with covalently closed ends (II). The thick lines represent the covalently closed left and right halves of the tos region. Product II was digested with MscI or AatII to yield products schematized as in III.
To identify the protelomerase gene, we have isolated the N15 telN gene by molecular cloning, overexpressed the gene, and shown that the purified protein is indeed responsible for its predicted action of generating covalently closed ends on suitable recombinant substrates in vitro.
Materials and Methods
Bacterial Strains, Plasmids, and Media.
The following E. coli strains were used in this study. SCS1 (recA1, endA1, gyrA96, thi-1, hsdR17(rK− mK+), supE44, relA1; Stratagene) was used as the host for the telN overexpression plasmid pJD101. SURE 2 {e14−(McrA−), Δ(mcrCB-hsdSMR-mrr)171, endA1, supE44, thi-1, gyrA96, relA1, lac, recB, recJ, sbcC, umuC∷Tn5(Kmr), uvrC, [F′ proAB, lacIqZΔ(M15Tn10(Tcr)), Amy+, Cmr]; Stratagene} was used for the construction of substrates containing the TelN target sequences. Vectors used for molecular cloning were pMS119EH (Apr) (4) and pBR329 (Apr, Cmr, Tcr) (10). Cells were grown in YT medium (0.5% yeast extract/1% tryptone/0.5% NaCl) (11), buffered with 25 mM 3-(N-morpholino)propane sulfonic acid (sodium salt, pH 8.0) and supplemented with 25 μg of thiamine hydrochloride per ml. When appropriate, antibiotics were added as follows: ampicillin (sodium salt, 100 μg/ml), tetracycline hydrochloride (10 μg/ml), and kanamycin sulfate (30 μg/ml).
DNA Techniques.
Standard molecular cloning techniques were performed as described (12). DNA was sequenced by the dideoxynucleotide chain termination method (13).
Electron Microscopy.
For electron microscopy, pJD104 DNA (4,375 bp) was processed with TelN and the reaction product was purified by electrophoresis on 0.7% agarose gels and by subsequent extraction. The isolated product was mixed with 50% formamide/20 mM sodium carbonate/2 mM EDTA/0.03% cytochrome C and was spread on a water surface as described (14). For denaturation, the product was boiled for 2 min in 100 mM potassium phosphate buffer, pH 7.0, containing 50% formamide, 0.5% formaldehyde, and 0.5% glyoxal, then chilled in ice water and spread as above. For size determination, double-stranded RSF1010 DNA (8,684 bp) or single-stranded φX174 phage DNA (5,386 nt) was added as internal length standard before spreading.
Construction of Plasmids.
By using N15 phage-DNA as template, the gene 29 (GenBank accession no. AF064539; telN, GenPept accession no. AAB81106.1) was obtained by PCR with the following primers: coding strand, 5′-ATCGGATCCCGATATCCAGAGACTTAGAAACGGG-3′, and noncoding strand, 5′-ATATAAAGCTTCTTTTAGCTGTAGTACGTTTCCCATGCG-3′. A BamHI site at position −34 to the start codon was introduced at the 5′ end of the gene, and a HindIII site was introduced at its 3′ end. Non-N15 nucleotides are italicized. The 1.9-kb fragment (N15 positions 24,961 to 26,890), including the original SD-sequence of gene 29, was digested with BamHI and HindIII and inserted into the corresponding sites in pMS119EH, resulting in pJD101.
The regions tos, telRL, and telO were all placed as HindIII-BamHI fragments in pBR329 (3.8 kb) resulting in plasmids pJD104 (tos), pJD105 (telRL), and pJD106 (telO). The nucleotide sequence of each of the constructions was verified. Fragments were obtained as follows.
The tos region of N15 was isolated by PCR, using primers 5′-CTTACTTAAGCTTAAGCGCAACGGTATTACTTACGTTGG-3′ (HindIII) and 5′-GCGGGATCCTCACAAGCGTGTTGATCAACTCACC-3′ (BamHI) with N15 phage-DNA as template. The resulting 573-bp fragment contained N15 positions 24,474 to 25,037.
Two complementary oligonucleotides were annealed to form the telRL region, 5′-AGCTTTATCAGCACACAATTGCCCATTATACGCGCGTATAATGGACTATTGTGTGCTGATAG-3′ and 5′-GATCCTATCAGCACACAATAGTCCATTATACGCGCGTATAATGGGCAATTGTGTGCTGATAA-3′. The DNA contained N15 positions 24,775 to 24,830.
To form the telO region, two complementary oligonucleotides were annealed, 5′-AGCTTCCATTATACGCGCGTATAATGG-3′ and 5′-GATCCCCATTATACGCGCGTATAATGG-3′. The DNA contained N15 positions 24,792 to 24,813.
Assay for Cleaving-Joining Activity of TelN Protein.
In a total volume of 20 μl of 20 mM Tris⋅HCl (pH 7.6), 5 mM CaCl2, 50 mM potassium glutamate, 0.1 mM EDTA, and 1 mM DTT, 0.15 pmol of DNA substrate and 0.3 pmol of TelN (23 units) were incubated at 30°C for 30 min. One unit was defined as the amount of enzyme required to process 1 pmol of substrate per h at 30°C in a volume of 20 μl. The reaction was stopped by adding SDS to a final concentration of 0.1% (wt/vol). The products were separated by electrophoresis on a 0.7% agarose gel [TBE buffer (90 mM Tris/90 mM boric acid/2 mM EDTA, pH 8.3), 10 V/cm (12)], stained with ethidium bromide, and quantified and documented by using a Molecular Dynamics FluorImager 575.
Results
Molecular Cloning and Purification of TelN Protein.
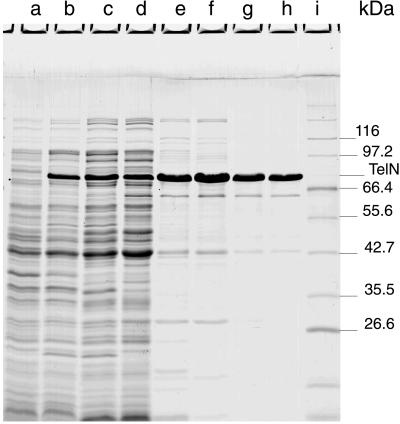
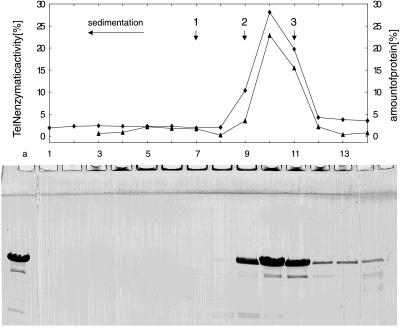
Molecular cloning of N15 gene 29 in a Ptac/lacI-based expression vector resulted in plasmid pJD101. On induction of SCS1(pJD101) with isopropyl β-d-thiogalactopyranoside, a soluble protein of 75 kDa was overproduced, which is close to the expected size of 72.2 kDa (630 aa) (Fig. 1). The N-terminal sequence of this protein matched that of the predicted telN product (SKVKIGE). The N-terminal methionine was missing. Purification of the TelN protein by a four-column procedure resulted in a nearly homogenous protein preparation. A protein of 65 kDa still present in the preparation (Fig. 1, lane h; and Table 1, fraction V) corresponds to a truncated TelN product, because its N-terminal sequence is identical to that of TelN. Probably degradation has occurred at the C terminus. A second product of 42 kDa also was apparent. The nature of this protein is still unknown. Fraction V of the purification was subjected to glycerol gradient centrifugation (Fig. 2). The sedimentation coefficient of TelN (5.1 S20,W) is slightly above that expected for a monomeric protein of 72.2 kDa, suggesting that TelN behaves as a monomer in solution. Analysis of each of the fractions for TelN activity showed that the peak of the enzymatic activity coincided with the presence of TelN protein (Fig. 2). The 42-kDa protein sedimented faster in glycerol gradient centrifugation than did TelN (6.2 S20,W versus 5.1 S20,W), indicating that this protein oligomerizes. Because TelN is monomeric and the N-terminal sequence of the 42-kDa polypeptide does not match any TelN sequence, the 42-kDa protein is most likely an impurity.
Figure 1.
Purification of TelN protein. Aliquots of extracts and pooled peak fractions were resolved with an SDS/PAGE (15%) and stained with Serva Blue R after electrophoresis. Expression of telN in SCS1(pJD101) was chemically induced in the presence of 1 mM isopropyl β-d-thiogalactopyranoside. Cells were lysed by either SDS or Brij-58/lysozyme. The amount of total protein loaded onto the gel is given in parentheses. Lanes a and b, SDS extract of noninduced and isopropyl β-d-thiogalactopyranoside-induced cells, respectively (31 μg, 34 μg); lane c, Brij-58/lysozyme extract of induced cells (51 μg); lane d, fraction I (46 μg); lane e, fraction II (9.2 μg); lane f, fraction III (14.5 μg); lane g, fraction IV (4.1 μg); lane h, fraction V (3.9 μg); lane i, molecular mass standards.
Table 1.
Purification of TelN
| Fraction | Purification step | Protein, mg | Yield, % | Purity, % |
|---|---|---|---|---|
| I | Precipitation with (NH4)2SO4 | 2394 | 100 | 7 |
| II | Heparin-Sepharose CL-6B | 201 | 83 | 70 |
| III | Hydroxylapatite Bio-Gel HT | 186 | 77 | 70 |
| IV | DEAE-Sephacel | 61.9 | 32 | 90 |
| V | Phosphocellulose P11 | 11.4 | 7 | 96 |
The purification of N15 TelN was followed by SDS/PAGE before its activity was discovered. Cultures (4 × 1.2 liters) of SCS1(pJD101) were grown at 37°C with shaking. At an A600 of 0.5, isopropyl β-d-thiogalactopyranoside was added to 1 mM. Shaking was continued for 5 h. The cells were harvested by centrifugation at 4,000 × g for 10 min, resuspended in 1 mM spermidine Tris hydrochloride/200 mM NaCl/2 mM EDTA, pH 7.5, and frozen in liquid nitrogen. The following steps were performed at 0–4°C. Frozen cells (36 g in 100 ml) were thawed and adjusted to 40 mM Tris⋅HCl, pH 7.6/3.5% sucrose/0.13% Brij-58/1 M NaCl/0.3 mg/ml lysozyme. After incubation for 1 h, the lysate was centrifuged at 90,000 × g for 90 min. To the supernatant, solid ammonium sulfate was added to a saturation of 60% and stirred for 30 min. The precipitate was collected by centrifugation at 90,000 × g for 30 min, dissolved in buffer A [20 mM Tris⋅HCl, pH 7.6/1 mM DTT/0.1 mM EDTA/10% (wt/vol) glycerol] containing 50 mM NaCl, and dialyzed three times against this buffer (Fraction I, 100 ml; Fig. 1, lane d). Fraction I was loaded onto a heparin-Sepharose CL-6B column (2.6 × 15 cm) equilibrated with buffer A containing 50 mM NaCl, and then was washed with 250 ml of this buffer. Proteins were eluted with a 750-ml linear gradient of 50–600 mM NaCl in buffer A. TelN eluted at 430 mM NaCl. Peak fractions were pooled (Fraction II, 175 ml; Fig. 1, lane e). Fraction II was loaded onto a hydroxylapatite Bio-Gel HT column (2.6 × 5 cm) equilibrated with buffer B [20 mM potassium phosphate, pH 6.8/50 mM KCl/1 mM DTT/0.1 mM EDTA/10% (wt/vol) glycerol]. The column was washed with 100 ml of buffer B. Proteins were eluted with a 250-ml linear gradient of 20–500 mM potassium phosphate. TelN eluted at 180 mM phosphate. Peak fractions were pooled (Fraction III, 75 ml; Fig. 1, lane f). Fraction III was diluted with 125 ml of buffer A containing 50 mM NaCl and loaded onto a DEAE-Sephacel column (2.6 × 5 cm) equilibrated with buffer A containing 50 mM NaCl, and then was washed with 60 ml of the same buffer. Proteins were eluted with a 250-ml linear gradient of 50–600 mM NaCl in buffer A. TelN eluted at 160 mM NaCl. Peak fractions were pooled (Fraction IV, 58 ml; Fig. 1, lane g). Fraction IV was diluted with buffer C [50 mM Tris⋅H3PO4/1 mM DTT/0.1 mM EDTA/10% (wt/vol) glycerol] containing 50 mM NaCl and loaded onto a phosphocellulose P11 column (1.6 × 9 cm) equilibrated with the same buffer. The column was washed with 80 ml of buffer C containing 50 mM NaCl. Proteins were eluted with a 200-ml linear gradient of 50–600 mM NaCl. TelN eluted at 340 mM NaCl. The peak fraction was concentrated by dialysis against 20% (wt/vol) polyethylene glycol 20,000 in buffer A and than dialyzed against 50% glycerol in buffer A and stored at −20°C (Fraction V, 4.8 ml; Fig. 1, lane h). Under these conditions, the enzymatic activity was stable for at least 1 yr.
Figure 2.
Glycerol gradient centrifugation of TelN protein. A 3.8-ml linear gradient from 15% to 35% (wt/vol) glycerol was overlaid with a TelN protein solution (Table 1, fraction V; 120 μl containing 400 μg of protein). Centrifugation was carried out at 100,000 × g for 15 h at 4°C. Fourteen fractions of 270 μl each were collected; 15 μl of each fraction and a 3-μl aliquot of the TelN protein loaded onto the gradient were separated by using a 15% SDS/PAGE gel and stained with Serva Blue R after electrophoresis. The fractions were assayed for TelN activity, as described in Materials and Methods. The amount of protein contained within each fraction and the TelN activity of each fraction (relative to the total amount and activity applied) are shown in the diagram (▴, relative enzymatic activity; ♦, relative amount of protein). The direction of sedimentation is right to left. Arrows indicate the positions of peaks of the reference proteins [1, catalase (11.3); 2, aldolase (7.8); and 3, BSA (4.4)]. The sedimentation coefficient is given in parenthesis in Svedberg units (S20,W).
The Target for TelN Is telRL.
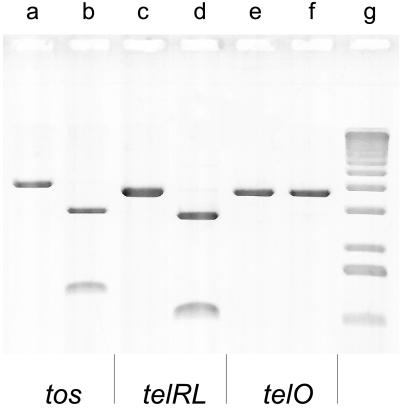
The proposed TelN activity is thought to lead to linearization of the N15 circular phage DNA in vivo, each end constituted by either telR or telL, respectively (2). To assay for TelN′s in vitro activity, three recombinant substrates were constructed containing the site of its proposed action (Fig. 3B). tos, telRL, and telO were inserted into plasmid pBR329 as described in Material and Methods. The tos derivative can be maintained only in a recBJ strain, whereas telO and telRL seem to be stable also in recBJ+ strains (data not shown). In vitro TelN-derived DNA products were analyzed by agarose gel electrophoresis. Prelinearized substrates, obtained by AatII cleavage, were processed by TelN into two fragments, the combined length of which corresponded to the full-length form FIII (linear) substrates applied (Fig. 4, lanes a–d). TelO plasmid DNA remained almost inert to TelN in either form (Fig. 4, lanes e and f). Because tos and telRL are readily processed in vitro by TelN but telO is not, the reaction requires telO and at least the repeats L3 and R3 to proceed efficiently (Fig. 3A). Negatively supercoiled circular substrate DNA containing tos or telRL also was processed by TelN to yield linear DNA (data not shown).
Figure 4.
The recognition sequence for TelN lies within telRL. tos (in pJD104), telRL (in pJD105), and telO (in pJD106) were linearized with AatII and then incubated with 1 pmol of TelN protein (70 units); 0.15 pmol of DNA was applied per assay. Lanes a, c, and e, TelN omitted; lanes b, d, and f, in the presence of TelN; lane g, size marker (1-kb DNA ladder).
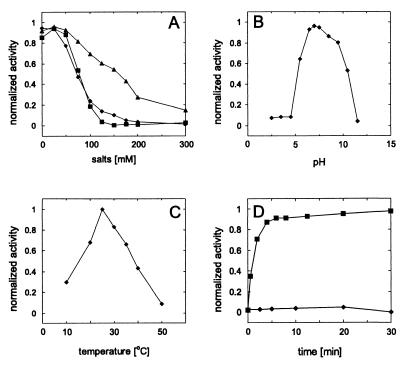
Optimization of the assay conditions on the circular tos DNA substrate pJD104 demonstrated that the TelN-mediated reaction is salt-sensitive, because NaCl and KCl inhibit the reaction almost completely at 200 mM (Fig. 5A). Because such a strong inhibition was not observed in the presence of potassium glutamate, the effect might be caused by chloride ions. The optimal pH for the reaction is approximately 7 and the temperature optimum lies at around 25°C (Fig. 5 B and C). As indicated by the time course, the TelN reaction proceeded rapidly and was almost completed after 10 min, without a recognizable lag phase (Fig. 5D). A requirement for divalent cations of the reaction seems to exist, because at higher concentrations of EDTA (>10 mM) the TelN-mediated reaction was inhibited. This finding is not surprising, because a binding motif for divalent cations was predicted for the TelN sequence (amino acid positions 541 to 563, . . . idepdDESQDDELDEDEIeldeg . . .) (15).
Figure 5.
Optimization of reaction conditions for TelN enzymatic activity. The reactions were performed as described in Materials and Methods, with 0.3 pmol of TelN protein and 0.15 pmol of pJD104 per assay. (A) Effect of NaCl (■), KCl (♦), and potassium glutamate (▴). (B) Effect of pH. (C) Effect of temperature. (D) Time dependency of pJD104 (■) [pBR329 (♦)] processing by TelN.
TelN Is a Cleaving-Joining Enzyme.
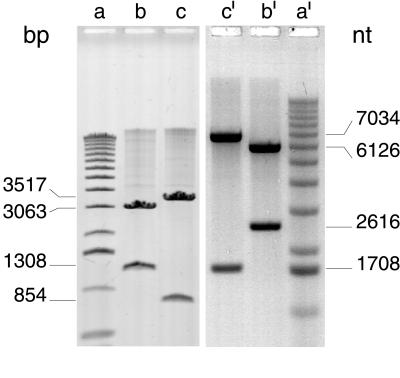
To analyze the linear DNA products generated by TelN, three different methods were used: alkaline agarose gel electrophoresis, electron microscopy, and nucleotide sequencing. The purpose of this extensive analysis was to unravel the configuration of the linear tos product obtained by TelN treatment (Fig. 3C, II). The circular tos substrate pJD104 was converted by TelN to form FIII DNA. Subsequent digestions with restriction endonucleases MscI or AatII, which cut the linear product only once, as expected, generated two fragments of different sizes (Fig. 3C, III). The fragment sizes observed on a nondenaturing agarose gel (Fig. 6, lanes b and c) coincided with those sizes predicted from the nucleotide sequence. Analysis of these fragments by alkaline gel electrophoresis showed that they migrated in the form of single-stranded DNA, with twice the size of that expected for the two single strands of the double-stranded DNA fragments (Fig. 6, lanes b′ and c′). This result demonstrates that the fragments contain one additional covalent linkage per fragment, resembling a hairpin-like structure.
Figure 6.
Product analysis of TelN enzymatic activity. Products of the reaction outlined in the legend to Fig. 4 were analyzed on a 0.7% and a 1% agarose gel under nondenaturing and alkaline conditions, respectively. One microgram of DNA per lane was applied. Electrophoresis was carried out under nondenaturing conditions [45 mM Tris-borate, pH 8.0/1 mM EDTA (10 V/cm for 90 min)] and under alkaline conditions [1 mM EDTA/50 mM NaOH (6 V/cm for 4.5 h)] (16, 17). Gels were stained with ethidium bromide and a FluorImager scan is shown. Lanes a and a′, size marker (1-kb DNA ladder); lanes b and b′, pJD104 after reaction with AatII and TelN; lanes c and c′, pJD104 after reaction with MscI and TelN, both shown in Fig. 3C, III.
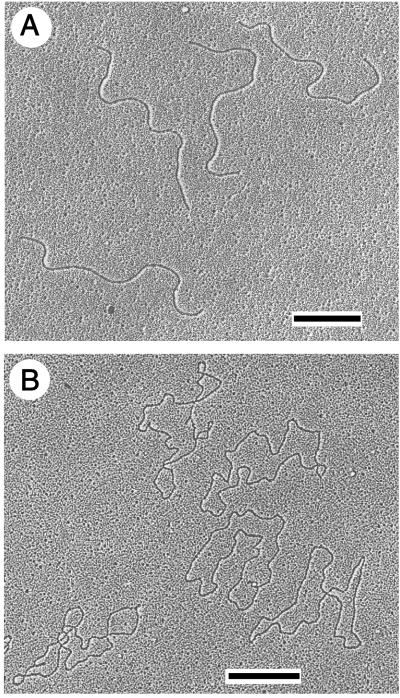
By electron microscopy it was shown that the TelN-derived DNA product of pJD104 (tos) is indeed linearized and shows the expected length (Fig. 7A). Irreversible denaturation by formaldehyde of this molecule resulted in single-stranded circular DNA (Fig. 7B), twice the length of the linear DNA, demonstrating that both ends of the TelN-generated product are covalently closed.
Figure 7.
Electron micrographs of products of pJD104 after reaction with TelN (Fig. 3C, II). The sample was prepared as described in Materials and Methods. Micrographs were taken and the DNA length was determined. (A) DNA under nondenaturing conditions; 63 molecules were measured (size 4.30 kb ± 150 bp, SD = 3.4%). (B) Denatured DNA; 81 molecules were measured (size 8.85 knt ± 460 nt, SD = 5.3%). [Bars indicate 1 kb (A) and 1 knt (B), respectively.]
In addition, the 854-bp and the 3,517-bp DNA fragments (Fig. 3C, III) generated by TelN processing and by subsequent cleavage by MscI were isolated and sequenced. One end of the small fragment is constituted by telR, the left-hand arm of the twofold rotationally symmetrical telRL sequence (Fig. 3B). telR differs from telL in only two nucleotide positions located between telO and the two flanking 14-bp inverted repeats R3 and L3, respectively (Fig. 3B). The nucleotide sequence obtained consists of a huge inverted repeat containing the sequence of the upper and the lower strands of telR in the center (Fig. 3B). Accordingly, one end of the larger fragment was formed by telL. This result was expected for a linear DNA-fragment with a covalently closed end and demonstrates that a hairpin-like structure was formed. Because DNA does not permit a 180° turn between two bases, we propose that a small loop evolves at the junction of the upper and the lower strands. Each of the results of these analyses demonstrates that the TelN-generated linear DNA product has joined ends. These ends must have been the consequence of a cleaving-joining reaction catalyzed by TelN.
These data show that the product of telN is indeed the hypothesized protelomerase that generates linear DNA molecules from telRL-containing DNA in the absence of any further N15-encoded protein, chromosome-encoded protein, or RNA. Both form FI DNA and form FIII DNA function as substrates for TelN, indicating that negative supercoiling of the DNA is not required for the processing reaction.
Discussion
The target sequence for the TelN-mediated reaction is contained within telRL, because tos and telRL were processed into linear products with covalently closed ends with similar efficiency, but telO was nearly inert to TelN. The processing efficiency of TelN substrates shorter than 40 bp, and lacking the same number of base pairs on either side of telRL, decreases rapidly. Preliminary data indicate that the 36-bp derivative did not function as a TelN substrate anymore. The 56-bp telRL sequence contains 2 bp flanking the central 22-bp telO palindrome on each side, which interrupt the twofold rotational symmetry (Fig. 3B). This asymmetry allows differentiation between telL and telR, the left- and right-end arms of the linear prophage. It is conceivable that processing must occur within the 22 bp of telO because the 2 bp disrupting the symmetry appear in telL and telR, respectively. Which of the phosphodiester bonds are cleaved and joined remains an open question that may be answered by a mutagenesis study. However, cleavage in telO probably occurs either in a staggered way or directly in the middle of the 22-bp sequence (Fig. 3B), the latter option being much less probable because of sterical reasons. Theoretically telRL has the potential to form a cruciform structure by extruding telO as hairpins (Fig. 8A). Thus, the secondary structure of the TelN recognition site also may play an important role in the reaction. The telO region has been predicted to provide B–Z DNA junctions, which might facilitate processing by TelN (Fig. 8B and ref. 2). Nuclease S1 cleavage may be used to differentiate between the two possibilities.
Figure 8.
Proposed models for TelN-telRL interaction. (A) Hairpin model. The palindrome telO is extruded as hairpins. The 3-bp telRL region interrupting the palindrome (see Fig. 3B) is marked in red. (B) B–Z form model. The region proposed to contain DNA in Z-conformation is shown in zigzag.
TelN has an excess of 13 acidic aa residues and, accordingly, a calculated isoelectric point of pI = 5.60. Despite this slight acidity of TelN, the high precision reaction takes place. DNA binding studies by fragment retention in gel electrophoresis on the telRL mutant substrate (vide supra) and on telO indicated that TelN has a strong non-sequence-specific DNA binding ability and, in addition, a sequence-specific binding ability to both telRL and telO (our unpublished results). Preliminary data indicate that base pair changes A20T and T21A (counted from the left end of the sequence shown in Fig. 3B), which destroy the symmetry of telO within telRL, abolish processing by TelN. However, TelN retained the specific DNA binding ability for this mutant substrate. Thus, telO might function not only as the cleavage region but also as the recognition site for TelN.
Subsequent to recognition of telRL by TelN and complex formation, the generation of the covalently closed ends might occur by cleaving-joining events. In a two-step reaction, cleavage would be required first on each of the complementary DNA strands. A transient covalent intermediate between TelN and the DNA might be generated by nucleophilic substitution by the hydroxyl group of an amino acid side chain. Secondly, the new phosphodiester bond with the complementary strand would be constituted in a transesterification catalyzed by the DNA-TelN protein adduct. Two cleaving steps, one on each strand, and two joining steps are required to form the two hairpin structures telL and telR. Therefore, TelN could possess two active sites, or more likely, two TelN molecules could bind to the substrate and function in a concerted action.
Sequence alignments suggested a relationship between TelN and certain integrases (Int), i.e., phage HK022 Int, phage P2 Int, Haemophilus influenzae HP1 Int, Bacillus subtilis CodV Int, B. subtilis RipX Int, and Staphylococcus aureus TnpA Int (2, 9). The overall similarity between the sequences is rather low. However, one motif (bxxaxxxxxahLGHxxxxTxxxY; b, basic residue; a, acidic residue; h, hydrophobic residue) seems to be conserved in all of the proteins mentioned. The motif localizes toward TelN′s C terminus and may be part of the enzyme's active center. The hydroxyl group of tyrosine 424 is a likely candidate to provide the nucleophile needed to hydrolyze the phosphodiester bond. Site-directed mutagenesis of TelN supports this idea. Replacement of Y424 by phenylalanine resulted in a complete inactivation of TelN, indicating that the hydroxyl of the aromatic side chain is essential (data not shown). Replacement of the same tyrosine by alanine inactivates the protein, as does the mutation H415A. The residues H415 and Y424 are highly conserved in a block of 12 residues in the proteins mentioned. On the basis of their importance, we hypothesize that these residues are likely to belong to the active center of TelN.
Acknowledgments
We thank Hans Lehrach for generous support. E.L. is indebted to the late Valentin Rybchin for the introduction to the fascinating phage N15 system and for many provocative and entertaining discussions. The expert technical assistance of Marianne Schlicht is greatly appreciated. We thank Gerhild Lüder for preparing the electron micrographs and Gianni Dehò for kindly providing phage N15. We are indebted to Ellen Zechner for critically reviewing the manuscript. This work was supported by Grant LA 672 of the Deutsche Forschungsgemeinschaft and Grant 96–1492 of the International Association of the Promotion of Cooperation with Scientists from the New Independent States of the Former Soviet Union (INTAS) to E.L.
Abbreviations
- TelN
protelomerase
- Int
integrase
References
- 1.Svarchevsky A N, Rybchin V N. Mol Genet (Moscow) 1984;N10:16–22. [Google Scholar]
- 2.Rybchin V N, Svarchevsky A N. Mol Microbiol. 1999;33:895–903. doi: 10.1046/j.1365-2958.1999.01533.x. [DOI] [PubMed] [Google Scholar]
- 3.Ziegelin G, Lanka E. FEMS Microbiol Rev. 1995;17:99–107. doi: 10.1111/j.1574-6976.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 4.Strack B, Lessl M, Calendar R, Lanka E. J Biol Chem. 1992;267:13062–13072. [PubMed] [Google Scholar]
- 5.Svarchevsky A N. Ph.D. thesis. Leningrad, U.S.S.R.: Leningrad State University; 1986. [Google Scholar]
- 6.Vostrov A A, Malinin A Y, Rybchin V N, Svarchevsky A N. Genetika. 1992;28:186–188. [PubMed] [Google Scholar]
- 7.Casjens S, Murphy M, DeLange M, Sampson L, van Vugt R, Huang W M. Mol Microbiol. 1997;26:581–596. doi: 10.1046/j.1365-2958.1997.6051963.x. [DOI] [PubMed] [Google Scholar]
- 8.Barbour A, Garon C. Science. 1987;237:409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- 9.Esposito D, Scocca J J. Nucleic Acids Res. 1997;25:3605–3614. doi: 10.1093/nar/25.18.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covarrubias L, Bolivar F. Gene. 1982;17:79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- 11.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 12.Sambrook J, Fritsch E T, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 13.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiess E, Lurz R. Methods Microbiol. 1988;20:293–323. [Google Scholar]
- 15.Kretsinger R H. Cold Spring Habor Symp Quant Biol. 1987;52:499–510. doi: 10.1101/sqb.1987.052.01.057. [DOI] [PubMed] [Google Scholar]
- 16.McDonnel M W, Simon M N, Studier F W. J Mol Biol. 1977;110:119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- 17.Fürste J P, Pansegrau W, Ziegelin G, Kröger M, Lanka E. Proc Natl Acad Sci USA. 1989;86:1771–1775. doi: 10.1073/pnas.86.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]