Abstract
Halocidin is a heterodimer antimicrobial peptide previously isolated from the tunicate Halocynthia aurantium. Based on the larger monomer (18Hc) of halocidin, nine halocidin congeners, including a series of 6 peptides truncated successively from the carboxyl-terminal end of 18Hc and 3 analogs (18HcKK, K19Hc, and K19HcKK), which have lysine residues in place of two internal histidines or have a lysine added to the amino terminus of the 18Hc molecule, were prepared. Each peptide was also converted into a homodimeric version. The antimicrobial activities of halocidin congeners truncated from the C terminus were dramatically decreased, suggesting that the full length of 18Hc is required for maintaining its maximum antimicrobial activity. Dimer forms of halocidin congeners exhibited stronger antimicrobial activities than the monomer of the corresponding peptide. Four dimer peptides (di-18Hc, di-18HcKK, di-K19Hc, and di-K19HcKK) were analyzed for antimicrobial activities against 10 clinically isolated antibiotic-resistant bacteria in elevated concentrations of NaCl or MgCl2. Of the peptides studied here, di-K19Hc retained invariably strong activity against all bacteria in diverse conditions and also showed much reduced hemolytic activity against human erythrocytes.
Over the last two decades, cationic antimicrobial peptides have been identified from a wide variety of organisms, including bacteria, plants, vertebrates and invertebrates (2, 22). They can be classified into four structural groups: amphipathic α-helical peptides, disulfide-bonded β-sheet peptides, extended peptides with one or two amino acids predominating, and loop-structured peptides (6, 9). Because of their rapid microbicidal effects and generally good selectivity for microbial targets, some of these peptides provide leads for developing novel antibiotics for bacteria resistant to conventional antibiotics. Novel antibiotics that simulate native antimicrobial peptides include IB-367 (17), a protegrin analog, and MSI-78 (4), a magainin analog. Others are hybrid molecules that combine domains of two natural peptides, e.g., CEME (20), a cecropin-melittin hybrid, and P18 (23), a cecropin-magainin hybrid. The chain length of peptides and positioning of their nonpolar and cationic residues profoundly influence their activities, although finding exact relationships between structure and function is often difficult.
Halocidin, an antimicrobial peptide, was recently purified from the hemocytes of a marine invertebrate, the tunicate Halocynthia aurantium (10). The peptide was active against two antibiotic-resistant bacteria, Staphylococcus aureus and Pseudomonas aeruginosa. In the present work, the antimicrobial activities of diverse synthetic halocidin congeners were examined and compared to those of other standard peptides, including P18 and melittin, a toxic peptide from bee venom, against 10 clinically isolated antibiotic-resistant bacteria under a variety of conditions. One of these halocidin congeners manifested much decreased hemolysis of human erythrocytes while still retaining strong antimicrobial activity.
MATERIALS AND METHODS
Synthetic peptides.
Halocidin is a heterodimeric peptide consisting of two monomers with 18 and 15 amino acid residues. Since the larger monomer (18Hc) was more effective than the smaller one in a previous study (10), we designed a variety of congeners based on the 18Hc structure. A set of truncated peptides (17Hc, 16Hc, 15Hc, 14Hc, 13Hc, and 12Hc) were prepared by successively removing residues from the carboxyl terminus of 18Hc. Replacing His7 and His8 of 18Hc with lysines gave a peptide we call 18HcKK. K19Hc and K19HcKK, were designed by adding a lysine residue to the amino terminus of 18Hc and 18HcKK, respectively. All halocidin congeners were synthesized by an automated solid-phase peptide synthesizer (Pioneer Applied Biosystems, Foster, Calif.) at the Korea Basic Science Institute and repurified by C18 reverse-phase high-pressure liquid chromatography. To prepare dimer molecules of halocidin congeners, each monomer was incubated in 0.1 M ammonium bicarbonate (1 mg/ml) at room temperature for 72 h. The resulting homodimer peptides were purified by reverse-phase high-pressure liquid chromatography as described above. As standards in our biological assays, P18 was synthesized at the Korea Basic Science Institute and melittin was purchased from Sigma (St. Louis, Mo.). Table 1 shows the primary sequence of the halocidin congeners and standard peptides. Peptide concentrations were determined by the bicinchoninic acid method (Pierce, Rockford, Ill.).
TABLE 1.
Primary structures of halocidin congeners and standard peptidesa
| Peptide | Amino acid sequence |
|---|---|
| 12Hc | WLNAL LHHGL NC* |
| 13Hc | WLNAL LHHGL NCA* |
| 14Hc | WLNAL LHHGL NCAK* |
| 15Hc | WLNAL LHHGL NCAKG* |
| 16Hc | WLNAL LHHGL NCAKG V* |
| 17Hc | WLNAL LHHGL NCAKG VL* |
| 18Hc | WLNAL LHHGL NCAKG VLA* |
| 18HcKK | WLNAL LKKGL NCAKG VLA* |
| K19Hc | KWLNAL LHHGL NCAKG VLA* |
| K19HcKK | KWLNAL LKKGL NCAKG VLA* |
| P18 | KWKLF KKIPK FLHLA KKF* |
| Melittin | GIGAV LKVLT TGLPA LISWI KRKRQ Q* |
Asterisks signify C-terminal amidation. Lysine residues substituted or added are underlined.
Bacterial strains.
For determining the antimicrobial activities of peptides against antibiotic-resistant bacteria, we used clinically isolated, multidrug-resistant bacterial strains from the Culture Collection of Antibiotic-Resistant Microbes (CCARM) at Seoul Women's University in Korea. These included 4 strains of multidrug-resistant P. aeruginosa (MDRPA) (CCARM2002, CCARM2092, CCARM2095, and CCARM2109), 4 strains of methicillin-resistant S. aureus (MRSA) (CCARM3001, CCARM3543, CCARM3570, and CCARM3572), and 2 strains of vancomycin-resistant Enterococcus faecium (VRE) (CCARM5028 and CCARM5029). Alternatively, antimicrobial activities of the truncated congeners of halocidin were tested against Escherichia coli KCCM11569 and Bacillus subtilis KCCM11495 obtained from the Korean Culture Center of Microorganisms.
Antimicrobial and hemolytic activities.
The antimicrobial activity of each peptide was tested by a broth microdilution assay slightly modified from the procedure recommended by the National Committee for Clinical Laboratory Standards (18). Bacteria were grown overnight in Mueller-Hilton broth (MHB) (50 ml in a 250-ml Byrex flask) at 200 rpm and 37°C to the stationary phase. The cultures were diluted in fresh MHB to a final concentration of 2 × 104 to 2 × 105 CFU/ml. A stock solution of each peptide was prepared in 0.01% acetic acid at 640 μg/ml in a polypropylene microtube. The peptide solution was then serially twofold diluted in 0.01% acetic acid to 10 μg/ml. After 100-μl aliquots of the microbial suspension were dispensed into each well of a 96-well polypropylene microtiter plate (Costar 3790; Corning, Corning, N.Y.), 11 μl of peptide solution was added. The antibacterial activities of peptides were assessed by visible turbidity in each well of the plate after 18 h of incubation at 37°C. MICs were expressed as an interval (a to b), where a is the highest concentration tested at which bacteria are still growing and b is the lowest concentration that causes complete growth inhibition. In experiments to test the effects of salt and Mg2+ on antimicrobial activities of peptides, we used MHB supplemented with the predetermined concentration of NaCl (100, 200, and 300 mM) or MgCl2 (1, 3, and 5 mM).
In addition, antibacterial activities of peptides were tested against MDRPA CCARM2109 and VRE CCARM5028 in an ultrasensitive radial diffusion assay (10, 14). Washed mid-logarithmic-phase bacteria were trapped in thin underlay gels, which contained 9 mM sodium phosphate, 1 mM sodium citrate buffer, 1% (wt/vol) agarose (A 6013; Sigma), and 0.3 mg of tryptic soy broth (TSB; Difco, Augsburg, Md.)/ml. The gel solution was adjusted to pH 7.4 prior to sterilization by autoclaving. In some experiments, the underlay agars were supplemented with up to 300 mM NaCl. Stock peptide solutions and serial twofold dilutions, ranging in concentration from 3.12 to 200 μg/ml, were prepared in 0.01% acetic acid. Peptide samples (5 μl) were loaded into 3-mm-diameter wells that had been punched in underlay gels. After incubation at 37°C for 3 h, a 10-ml overlay gel of 1% agarose and 6% TSB was poured on the underlay gel. After the plates were incubated overnight at 37°C, the clear zone diameters were measured to the nearest 0.1 mm and graphed against the log10 of the peptide concentration. Zone diameters were expressed in units (0.1 mm = 1 U).
Hemolysis of human red blood cells was performed as previously described (10, 13) for the dimers of four halocidin congeners: 18Hc, 18HcKK, K19Hc, and K19HcKK. Melittin, P18, and magainin 1 were used as standard peptides. All biological assays in the present work were repeated three times.
CD spectra.
Circular dichroism (CD) spectra of halocidin congeners (di-18Hc, di-18HcKK, di-K19Hc, and di-K19HcKK) were measured with a J-715 spectropolarimeter (Jasco, Tokyo, Japan). Three hundred micrograms of each peptide was dried by vacuum centrifugation (Centra Evaporator; Bioneer, Daejon, Korea) and resuspended in 1 ml of 10 mM sodium phosphate buffer (pH 7.0) (i) without other additives, (ii) with 10% sodium dodecyl sulfate (SDS) or (iii) with 50% trifluoroethanol (TFE) (a known helix-inducing solvent). The spectra were measured at room temperature in a 1-mm-path-length quartz cell. The scanning speed was 10 nm/min (190 to 260 nm), and each spectrum was the average of five scans.
RESULTS
Relative activity of truncated forms and analogs of 18Hc.
We prepared 18Hc and nine halocidin congeners, including a series of 6 peptides truncated successively from the carboxyl-terminal end of 18Hc and 3 analogs (18HcKK, K19Hc, and K19HcKK) of the 18Hc molecule. Each peptide was also converted into a homodimeric version by air oxidation to form a connecting cysteine-disulfide bridge. Table 2 shows the antimicrobial activities (MICs) of monomeric and dimeric peptides against E. coli KCCM11569 and B. subtilis KCCM11495. These tests were done in medium with a low content of NaCl. The antimicrobial activities of the peptides decreased dramatically as they were shortened. Although dimeric 17Hc maintained activity against both bacteria, further truncation (16Hc to 12Hc) eliminated the activity almost entirely. When we compared the dimeric and monomeric forms of six truncated halocidin congeners (16Hc, 17Hc, 18Hc, 18HcKK, K19Hc, and K19HcKK), the dimeric congener had stronger antimicrobial activities than the corresponding monomer (10).
TABLE 2.
MICs of halocidin congeners, P18, and melittin for E. coli and B. subtilis
| Peptide | MIC (μg/ml)
|
|
|---|---|---|
| E. coli | B. subtilis | |
| 12Hc | >64 | >64 |
| 13Hc | >64 | >64 |
| 14Hc | >64 | >64 |
| 15Hc | >64 | >64 |
| 16Hc | >64 | >64 |
| 17Hc | 16-32 | 32-64 |
| 18Hc | 8-16 | 8-16 |
| 18HcKK | 8-16 | 8-16 |
| K19Hc | 4-8 | 8-16 |
| K19HcKK | 4-8 | 8-16 |
| di-12Hc | >64 | >64 |
| di-13Hc | >64 | >64 |
| di-14Hc | >64 | >64 |
| di-15Hc | >64 | >64 |
| di-16Hc | 32-64 | 8-16 |
| di-17Hc | 2-4 | <1 |
| di-18Hc | 2-4 | <1 |
| di-18HcKK | 2-4 | 4-8 |
| di-K19Hc | 2-4 | <1 |
| di-K19HcKK | 2-4 | <1 |
| P18 | <1 | 1-2 |
| Melittin | 1-2 | 2-4 |
Relative activity in the presence of salts and divalent ions.
To assess the effects of salinity, dimers (di-18Hc, di-18HcKK, di-K19Hc, and di-K19HcKK) of four congeners were tested for their activities in various concentrations of NaCl against 10 different antibiotic-resistant bacteria. The MICs of peptides under different NaCl concentrations are shown in Table 3. Among the halocidins and standards, di-K19Hc invariably exhibited the lowest MIC for all bacteria over the whole range of NaCl concentrations examined in our assay.
TABLE 3.
Effects of salinity on MICs of halocidin congeners, P18, and melittin for antibiotic-resistant bacteriaa
| NaCl concn (mM) | MIC (μg/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSA strain:
|
MDRPA strain:
|
VRE strain:
|
||||||||
| 3001 | 3543 | 3570 | 3572 | 3002 | 2092 | 2095 | 2109 | 5028 | 5029 | |
| di-18Hc | ||||||||||
| 0 | 4-8 | 2-4 | 2-4 | 4-8 | 2-4 | 2-4 | 8-16 | 2-4 | 2-4 | 2-4 |
| 100 | 4-8 | 8-16 | 2-4 | 4-8 | 8-16 | 2-4 | 8-16 | 4-8 | 2-4 | 2-4 |
| 200 | 4-8 | 8-16 | 2-4 | 4-8 | 8-16 | 2-4 | 8-16 | 8-16 | 2-4 | 2-4 |
| 300 | 8-16 | 8-16 | 2-4 | 4-8 | 16-32 | 2-4 | 8-16 | 8-16 | 2-4 | 2-4 |
| di-18HcKK | ||||||||||
| 0 | 8-16 | 8-16 | 4-8 | 4-8 | 2-4 | 2-4 | 4-8 | 2-4 | 1-2 | 1-2 |
| 100 | 16-32 | 16-32 | 8-16 | 8-16 | 8-16 | 4-8 | 4-8 | 4-8 | 1-2 | 1-2 |
| 200 | 32-64 | 32-64 | 16-32 | 16-32 | 16-32 | 8-16 | 8-16 | 8-16 | 2-4 | 2-4 |
| 300 | >64 | >64 | 16-32 | 32-64 | 32-64 | 8-16 | 16-32 | 16-32 | 2-4 | 2-4 |
| di-K19Hc | ||||||||||
| 0 | 2-4 | 2-4 | 2-4 | 4-8 | 2-4 | 1-2 | 4-8 | 2-4 | 1-2 | 1-2 |
| 100 | 2-4 | 2-4 | 2-4 | 4-8 | 2-4 | 1-2 | 4-8 | 2-4 | 1-2 | 1-2 |
| 200 | 2-4 | 2-4 | 2-4 | 4-8 | 4-8 | 1-2 | 4-8 | 2-4 | 2-4 | 2-4 |
| 300 | 4-8 | 2-4 | 2-4 | 4-8 | 4-8 | 2-4 | 4-8 | 2-4 | 2-4 | 2-4 |
| di-K19HcKK | ||||||||||
| 0 | 4-8 | 8-16 | 2-4 | 8-16 | 2-4 | 2-4 | 4-8 | 2-4 | 1-2 | 1-2 |
| 100 | 16-32 | 16-32 | 8-16 | 16-32 | 2-4 | 2-4 | 4-8 | 4-8 | 2-4 | 1-2 |
| 200 | 32-64 | 32-64 | 16-32 | >64 | 4-8 | 2-4 | 4-8 | 4-8 | 2-4 | 2-4 |
| 300 | >64 | 32-64 | 32-64 | >64 | 8-16 | 2-4 | 4-8 | 4-8 | 2-4 | 2-4 |
| P18 | ||||||||||
| 0 | 4-8 | 16-32 | 16-32 | 8-16 | 4-8 | 8-16 | 2-4 | 1-2 | 2-4 | 4-8 |
| 100 | 32-64 | 16-32 | 16-32 | >64 | 4-8 | 8-16 | 4-8 | 4-8 | 4-8 | 4-8 |
| 200 | >64 | 16-32 | >64 | >64 | 8-16 | >64 | 8-16 | 16-32 | >64 | >64 |
| 300 | >64 | 16-32 | >64 | >64 | 16-32 | >64 | 32-64 | >64 | >64 | >64 |
| Melittin | ||||||||||
| 0 | 1-2 | 1-2 | 1-2 | 1-2 | 4-8 | 4-8 | 4-8 | 2-4 | 1-2 | 1-2 |
| 100 | 2-4 | 1-2 | 1-2 | 1-2 | 4-8 | 4-8 | 4-8 | 4-8 | 1-2 | 1-2 |
| 200 | 2-4 | 1-2 | 1-2 | 1-2 | 8-16 | 8-16 | 8-16 | 8-16 | 1-2 | 1-2 |
| 300 | 2-4 | 2-4 | 1-2 | 1-2 | 16-32 | 16-32 | 16-32 | 16-32 | 1-2 | 1-2 |
Bacterial strains were clinically isolated at the Culture Collection of Antibiotic-Resistant Microbes.
Dimeric forms of 18HcKK and K19HcKK (congeners that contained lysine substitutions for two internal histidines) were relatively less potent than dimers of 18Hc and K19Hc against four strains of MRSA and four strains of MDRPA, although there was no significant difference in antimicrobial activities against two VRE strains. In addition, di-K19Hc and di-K19HcKK, having an extra Lys residue at the amino terminus, showed generally stronger antimicrobial activities against all bacteria than di-18Hc and di-18HcKK, respectively. However, di-K19HcKK showed substantially less activity than di-18HcKK only against MRSA CCARM3572.
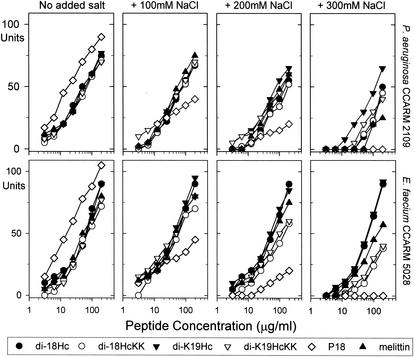
We tested the effects of NaCl on peptide activity against MDRPA and VRE in radial diffusion assays (Fig. 1). These revealed that the antimicrobial activities of P18 were highly sensitive to the presence of salt in the test media and that melittin maintained its activity only against VRE under elevated NaCl concentrations. In contrast, di-18Hc and di-K19Hc remained active at NaCl concentrations up to 300 mM and activities of di-18HcKK and di-K19HcKK decreased but were not abolished as NaCl concentrations increased. Under all conditions, di-K19Hc exhibited highest antimicrobial activities against two bacteria, which was consistent with data shown in Table 3.
FIG. 1.
Effects of salinity on antimicrobial activities of halocidin congeners, P18, and melittin against P. aeruginosa (MDRPA) and E. faecium (VRE) in radial diffusion assay. Different concentrations of NaCl were added to underlay agars which contained 9 mM sodium phosphate, 1 mM sodium citrate buffer, 1% (wt/vol) agarose, and 0.3 mg of TSB/ml. The diameters of the clear zones are displayed in units (10 U = 1 mm). The antimicrobial activities were graphed against log concentration of peptides. Mean values were obtained from tests repeated three times.
We performed additional antimicrobial testing with MRSA and MDRPA in media containing different concentrations of MgCl2 to ascertain whether the antimicrobial activities of halocidin congeners were affected by this divalent cation (Table 4). As in the case of NaCl effects, activities of di-K19Hc were minimally influenced by MgCl2 concentration. The MICs of di-K19Hc for MRSA and MDRPA in the presence of 5 mM MgCl2 were measured to be below 8 μg/ml and 16 μg/μl, respectively. In contrast, antimicrobial activities of other peptides were dramatically decreased in the elevated concentrations of MgCl2.
TABLE 4.
Effects of MgCl2 on MICs of halocidin congeners, P18, and melittin for MRSA and MDRPA
| Peptide | MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MRSAa at MgCl2 concn (mM) of:
|
MDRPAb at MgCl2 concn (mM) of:
|
|||||||
| 0 | 1 | 3 | 5 | 0 | 1 | 3 | 5 | |
| di-18Hc | 2-4 | 8-16 | 16-32 | 16-32 | 2-4 | 16-32 | >64 | >64 |
| di-18HcKK | 4-8 | 4-8 | 16-32 | 16-32 | 2-4 | 4-8 | >64 | >64 |
| di-K19Hc | 2-4 | 2-4 | 4-8 | 4-8 | 2-4 | 4-8 | 8-16 | 8-16 |
| di-K19HcKK | 2-4 | 4-8 | 8-16 | 8-16 | 2-4 | 2-4 | 8-16 | 16-32 |
| P18 | 16-32 | 16-32 | 32-64 | >64 | 1-2 | 1-2 | 8-16 | 16-32 |
| Melittin | 1-2 | 16-32 | 16-32 | 16-32 | 2-4 | 8-16 | >64 | >64 |
S. aureus CCARM3570.
P. aeruginosa CCARM2109.
CD spectrometry.
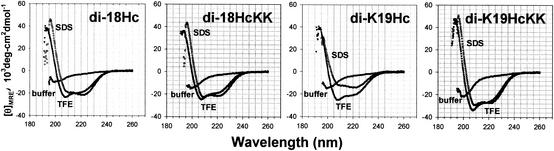
CD spectra of four dimer peptides were measured in 10 mM sodium phosphate buffer and in membrane-mimicking environments (10% SDS or 50% TFE in the buffer) (Fig. 2). In additive-free buffer, all peptide spectra were characteristic of unordered structure. However, in the presence of SDS or TFE, the four spectra had the typical appearance of α-helix-rich structures, with dichroic minimal values at 208 and 222 nm and a maximum near 194 nm (1).
FIG. 2.
CD spectra of halocidin congeners. These studies were performed with 25 μM concentrations of each peptide in various buffers: phosphate buffer (•), 10% SDS in phosphate buffer (○), and 50% TFE in phosphate buffer (▾).
Hemolysis activity of 18Hc congeners.
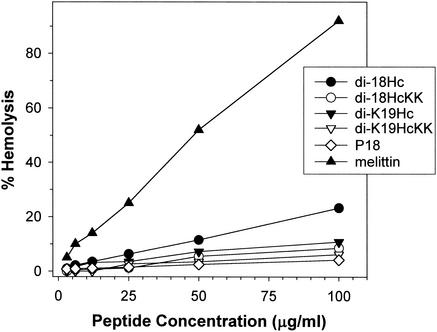
The hemolysis activity of 18Hc congeners against human erythrocytes was significantly reduced by adding a lysine residue (K) to the N terminus or substituting lysines for the internal histidine residues (Fig. 3). As reported in a previous paper (10), the present work revealed that di-18Hc had nearly 20% hemolysis activity at 100 μg/ml. In contrast, the hemolytic activity of di-K19Hc was less than half that of di-18Hc. Moreover, the hemolytic activities of di-K19HcKK and di-18HcKK were equivalent to that of P18, previously described as nonhemolytic peptide (23).
FIG. 3.
Hemolytic activities for halocidin congeners were tested against human erythrocytes. Triton X-100 (1%) was used as the control for 100% hemolysis, and 0.01% acetic acid was used as the peptide-free control. P18 and melittin were used as standard peptides. The percent hemolysis was calculated with the following equation: hemolysis (%) = (A540 of sample − A540 of peptide-free control)/(A540 of 100% control − A540 of peptide-free control) × 100.
DISCUSSION
Many studies have been done to identify or design new peptide antibiotics effective against antibiotic-resistant bacteria. Amphipathic α-helical peptides, such as cecropin, magainin, and melittin, have often been regarded as good models because synthetic versions are easily made. To improve their activities, many natural antimicrobial peptides have been changed by amino acid substitution, truncation, or elongation (5, 15). Additionally, several new synthetic peptides have been constructed by a combinatorial rearrangement of critical parts required for the activities of the original peptides (3, 21). Recently Hara et al. (7) reported that the disulfide-dimerized analogues of magainin 2 form larger pores in a membrane with a threefold-longer lifetime than the monomeric ones do. It was also confirmed that magainin 2 dimerizes upon binding to phospholipid bilayers (25). In addition, dimerized melittin analogues were designed to facilitate the self-association and improve their biological activities (24). These works strongly suggest that the dimerization of α-helical peptides via interdisulfide bond between the substituted Cys residues might be a powerful modification to strengthen the activity of a monomeric antimicrobial peptide.
In a previous work (10) and in the present work, it has been shown that native halocidin has a dimeric α-helical structure and that halocidin dimer congeners have stronger antimicrobial activities than the monomer forms. We inferred from these results that the halocidin monomer, 18Hc, could be a valuable prototype for developing a new peptide antibiotic with striking biological activities.
Because the antimicrobial activities of the C-terminally truncated congeners of 18Hc were significantly decreased (Table 2), it was suggested that the entire length of 18Hc is required for maintaining maximum activity. For most antimicrobial peptides, a net positive charge imparted by basic amino acid residues has been considered to be essential for interactions with anionic microbial surface components and for exerting their antimicrobial activities. The primary structure of 18Hc contains a lysine and two histidines as basic amino acid residues. Compared to Lys and Arg residues, histidine has a low pKa of approximately 6.5. Therefore, it was assumed that the substitution of two Lys residues for His residues of 18Hc would increase its cationicity at pH 7.4 and may result in the augmentation of its antimicrobial activity as in the case of clavanin A, which is a histidine-rich antimicrobial peptide isolated from the other tunicate, Styela clava (11, 12). However, dimer peptides (di-18HcKK and di-K18HcKK) of two isoforms with two Lys residue substitutions showed unexpectedly lower antimicrobial activities than dimers of 18Hc and K19Hc in all antimicrobial tests. Therefore, we concluded that two internal adjacent His residues (7His and 8His) are critical parts for halocidin to maintain its potent antimicrobial activity even in elevated concentrations of NaCl and MgCl2. In a work elucidating the relationship between structure and function, it was described that a tryptophan residue (Trp) located in the N-terminal region of certain antimicrobial peptides plays an essential part in killing bacteria. Tryptophan is also the N-terminal residue of 18Hc, and its deletion from 18Hc notably decreased antimicrobial activity. Cecropins are well-known antimicrobial peptides with a Trp in the N-terminal region (19). While the amino acid sequence of cecropin D starts with a Trp, cecropins A and B have an N-terminal lysine followed by a Trp. Comparison of the antimicrobial activities of cecropins A and D revealed that cecropin A retained much stronger potency against gram-positive bacteria than cecropin D (8, 16). Accordingly, we prepared K19Hc and K19HcKK to enhance the activity of the 18Hc congener. Compared to the biological activities of di-18Hc and di-18HcKK, di-K19Hc and di-K19HcKK not only showed improved antimicrobial activities in all but also reduced hemolytic activity against human erythrocytes.
We have described the antimicrobial activities of several congeners based on the 18Hc of halocidin. Of the congeners studied here, di-K19Hc had the best biological activities against a panel of antibiotic-resistant bacteria. It worked well in elevated concentrations of salt and had significantly decreased hemolytic activity. Although much remains to be done, the present studies suggest that dimeric α-helical peptides deserve consideration in the design of antimicrobial peptides.
Acknowledgments
We thank Robert I. Lehrer (University of California—Los Angeles Medical School) for providing valuable discussions and comments.
This work was financially supported by grant R05-2001-000-00349-0 from the Korea Science and Engineering Foundation (KOSEF).
REFERENCES
- 1.Bruch, M. D., M. M. Dhingra, and L. M. Gierasch. 1991. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an alpha-helical region of carboxypeptidase A. Proteins 10:130-139. [DOI] [PubMed] [Google Scholar]
- 2.Devine, D. A., and R. E. Hancock. 2002. Cationic peptides: distribution and mechanisms of resistance. Curr. Pharm. Des. 8:703-714. [DOI] [PubMed] [Google Scholar]
- 3.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs, P. C., A. L. Barry, and S. D. Brown. 1998. In vitro antimicrobial activity of MSI-78, a magainin analog. Antimicrob. Agents Chemother. 42:1213-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacometti, A., O. Cirioni, F. Barchiesi, M. Fortuna, and G. Scalise. 1999. In vitro anticryptosporidial activity of ranalexin alone and in combination with other peptides and with hydrophobic antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 18:827-829. [DOI] [PubMed] [Google Scholar]
- 6.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara, T., H. Kodama, M. Kondo, K. Wakamatsu, A. Takeda, T. Tachi, and K. Matsuzaki. 2001. Effects of peptide dimerization on pore formation: antiparallel disulfide-dimerized magainin 2 analogue. Biopolymers 58:437-446. [DOI] [PubMed] [Google Scholar]
- 8.Hultmark, D., A. Engstrom, H. Bennich, R. Kapur, and H. G. Boman. 1982. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur. J. Biochem. 127:207-217. [DOI] [PubMed] [Google Scholar]
- 9.Hwang, P. M., and H. J. Vogel. 1998. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 76:235-246. [DOI] [PubMed] [Google Scholar]
- 10.Jang, W. S., K. N. Kim, Y. S. Lee, M. H. Nam, and I. H. Lee. 2002. Halocidin: a new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett. 521:81-86. [DOI] [PubMed] [Google Scholar]
- 11.Lee, I. H., C. Zhao, Y. Cho, S. S. Harwig, E. L. Cooper, and R. I. Lehrer. 1997. Clavanins, alpha-helical antimicrobial peptides from tunicate hemocytes. FEBS Lett. 400:158-162. [DOI] [PubMed] [Google Scholar]
- 12.Lee, I. H., Y. Cho, and R. I. Lehrer. 1997. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 65:2898-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, I. H., Y. S. Lee, C. H. Kim, C. R. Kim, T. Hong, L. Menzel, L. M. Boo, J. Pohl, M. A. Sherman, A. Waring, and R. I. Lehrer. 2001. Dicynthaurin: an antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. Biochim. Biophys. Acta 1527:141-148. [DOI] [PubMed] [Google Scholar]
- 14.Lehrer, R. I., M. Rosenman, S. S. Harwig, R. Jackson, and P. Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167-173. [DOI] [PubMed] [Google Scholar]
- 15.Merrifield, R. B., E. L. Merrifield, P. Juvvadi, D. Andreu, and H. G. Boman. 1994. Design and synthesis of antimicrobial peptides. Ciba Found. Symp. 186:5-26. [PubMed] [Google Scholar]
- 16.Moore, A. J., W. D. Beazley, M. C. Bibby, and D. A. Devine. 1996. Antimicrobial activity of cecropins. J. Antimicrob. Chemother. 37:1077-1089. [DOI] [PubMed] [Google Scholar]
- 17.Mosca, D. A., M. A. Hurst, W. So, B. S. Viajar, C. A. Fujii, and T. J. Falla. 2000. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 1993. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 19.Otvos, L., Jr. 2000. Antimicrobial peptides isolated from insects. J. Pept. Sci. 6:497-511. [DOI] [PubMed] [Google Scholar]
- 20.Piers, K. L., and R. E. Hancock. 1994. The interaction of a recombinant cecropin/melittin hybrid peptide with the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 12:951-958. [DOI] [PubMed] [Google Scholar]
- 21.Saugar, J. M., T. Alarcon, S. Lopez-Hernandez, M. Lopez-Brea, D. Andreu, and L. Rivas. 2002. Activities of polymyxin B and cecropin A-, melittin peptide CA(1-8)M(1-18) against a multiresistant strain of Acinetobacter baumannii. Antimicrob. Agents Chemother. 46:875-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott, M. G., and R. E. Hancock. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407-431. [PubMed] [Google Scholar]
- 23.Shin, S. Y., S. T. Yang, E. J. Park, S. H. Eom, W. K. Song, Y. Kim, K. S. Hahm, and J. I. Kim. 2002. Salt resistance and synergistic effect with vancomycin of alpha-helical antimicrobial peptide P18. Biochem. Biophys. Res. Commun. 290:558-562. [DOI] [PubMed] [Google Scholar]
- 24.Takei, J., A. Remenyi, A. R. Clarke, and C. E. Dempsey. 1998. Self-association of disulfide-dimerized melittin analogues. Biochemistry 37:5699-5708. [DOI] [PubMed] [Google Scholar]
- 25.Wakamatsu, K., A. Takeda, T. Tachi, and K. Matsuzaki. 2002. Dimer structure of magainin 2 bound to phospholipid vesicles. Biopolymers 64:314-327. [DOI] [PubMed] [Google Scholar]