Abstract
Background
Cancer of the oral tongue is the second most common cancer among males in various parts of India. Despite advances in diagnosis and treatment the failure rates in cancer of the oral tongue are high and survival poor. Majority of these failures occur in untreated neck.
Method
A retrospective review of the records of 75 patients undergoing surgery for the treatment of squamous cell carcinoma of the oral tongue was carried out to ascertain the pattern of metastasis in the neck and to evaluate the sensitivity of clinical examination in predicting nodal spread.
Results
All the patients underwent primary surgery. Cervical lymph node metastasis was found in 35.6% of T1 and T2 tumours and 62.35% of T3 and T4 tumours. Sensitivity of clinical examination was found to be 54.5% and specificity of 61.9%. Level II was the most commonly involved (63.6%). Isolated level IV involvement was never found in clinically negative neck. Tumour stage and node status were found to have a significant impact on disease free survival in both univariate and multivariate analysis.
Conclusions
As the sensitivity and specificity of the clinical examination is low we suggest that methods like ultrasound or CT Scan of the neck should be regularly employed to improve the sensitivity and specificity of the examination. Furthermore as isolated level IV involvement is never found in our series, we suggest that a prophylactic supraomohyoid neck dissection should be carried out in all patients with a clinically node negative neck with cancer of oral tongue, to achieve a better disease free survival.
Keywords: Oral Cancer, tongue, cancer, lymph node, metastasis, survival
Introduction
Squamous cell carcinoma of the tongue is a common malignancy treated by surgeons. Incidence of tongue cancer in India is second highest in the world. Among males the age adjusted incidence rate is as high as 14/100,000 per/year in Ahmedabad while among females it is 7.4/ 100,000 in Mumbai [1]. In Trivandrum the incidence of tongue cancer is 5.2/ 100,000 among males and 2.4/100,000 among females [2].
Tongue is a complex anatomical site and its form and function are crucial for efficient swallowing, speech and appreciation of taste. In past different methods of treatment have been employed but tongue has remained a difficult area to assess and treat. The survival in carcinoma of the tongue is poor compared to other subsites in oral cavity.
The distribution of cervical lymph node metastasis from squamous cell carcinoma at different subsites in the oral cavity has been described before [3-5]. However, there are very few studies from India, where the incidence of tongue cancer is high. The distribution of metastases suggests skip lesions and peppering [3,5,6]. Predicting the lymphatic spread from cancer of the oral tongue can help in choosing the appropriate surgical procedure and may also help in predicting the outcome. The purpose of the present study was to assess the pattern of cervical node metastasis from carcinoma of the oral tongue and to evaluate the impact of tumour status on survival.
Patients and Methods
A retrospective analysis of 75 patients undergoing primary surgical resection for squamous cell carcinoma of oral tongue between January 1997 and December 1998, in a single surgical unit, was carried out to evaluate the pattern of nodal metastasis and survival. The variables like age, sex, tumour size, grade, clinical TNM, pathological TNM and histopathological levels of involvement were extracted from case records. All the patients underwent evaluation under general anaesthesia before the surgery and clinical TNM was modified if indicated. The types of surgical procedures performed are detailed in table 1. Adjuvant radiotherapy (45 Gy/15 fraction) was given if the primary tumour was greater than 2 cm, had close margins or nodal involvement was present or there was extranodal involvement. Minimum duration of follow-up was two years following surgery. Survival analysis was carried by Kaplan-Meier method and the curves were compared using log rank test. Multivariate analysis was carried out using Cox proportional hazard model.
Table 1.
Surgical management of neck by pathological node status.
| pN0 | pN1 | pN2a | pN2b | pN2c | pN3 | Total | Percentage | |
| Level I Clearance* | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1.3 |
| SOHND | 15 | 4 | 0 | 3 | 0 | 0 | 22 | 29.3 |
| MND | 21 | 7 | 1 | 8 | 0 | 1 | 38 | 50.6 |
| RND | 0 | 1 | 1 | 4 | 0 | 0 | 6 | 8 |
| B/L SOHND | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1.3 |
| I/L MND C/L SOHND | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 4 |
| B/L MND | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 2.6 |
| I/L MND C/L SOHND | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1.3 |
SOHND – Supra omohyoid neck dissection; MND – Modified neck dissection; RND – Radical neck dissection; B/L – Bilateral; I/L – Ipsilateral; C/L-Contralateral. * patient had a cardiac arrest during surgery and the procedure was abandoned, this patient went on to develop neck node recurrence and was successfully salvaged by second surgery.
Results
Age of the patients ranged from 22 to 75 years with a mean age of 52.6 years. The male, female ratio was 1.5:1 (Table 2). All the patients had undergone primary surgical resection.
Table 2.
Frequency distribution of various parameters studied.
| Variable | Frequency | Percentage |
| Sex | ||
| Male | 45 | 60 |
| Female | 30 | 40 |
| Tumour Status: | ||
| T1 | 13 | 17.3 |
| T2 | 33 | 44 |
| T3 | 15 | 20 |
| T4 | 14 | 18.7 |
| Clinical node status: | ||
| cN0 | 41 | 54.7 |
| cN1 | 26 | 34.7 |
| cN2b | 6 | 8 |
| cN2c | 2 | 2.7 |
| Pathological node status: | ||
| pN0 | 42 | 56 |
| pN1 | 12 | 16 |
| pN2a | 3 | 4 |
| pN2b | 16 | 21.3 |
| pN2c | 1 | 1.3 |
| pN3 | 1 | 1.3 |
| Pathological node distribution in the neck (33 cases) | ||
| I | 6 | 18.2 |
| II | 12 | 36.4 |
| III | 2 | 6.1 |
| I, II | 7 | 21.2 |
| I, III | 1 | 1.3 |
| I, IV | 1 | 1.3 |
| II, III | 0 | 0 |
| II, IV | 1 | 1.3 |
| III, IV | 2 | 6.1 |
| I, II, III, IV | 1 | 1.3 |
cN – clinical node status, pN – pathological node status.
At presentation, 44% of patients had T2 tumour followed by T3 in 20% (Table 2). Clinically 34 patients were found to be neck node positive of which 26 (34.7%) were cN1, 6 (8%) were cN2b and 2 (2.7%) were cN2c. Histologically 33 cases were found to have positive neck nodes, of which majority (16; 21.3%) were pN2b. The T and N stage distribution is detailed in table 2. Cervical lymph node metastasis was present in 47.1% of T1 tumours, 24.13% of T2 tumours, 63.6% of T3 tumours and 61.1% of T4 tumours (Table 3). Histologically 18 cases were true positive for nodal metastasis while 26 cases were true negative giving a sensitivity of 54.5% and specificity of 61.9% for clinical examination of the neck (Table 4). Synchronous discontinuous operations were performed in all cases. None of the patients had a positive resection margin, however the margin was close (2–5 mm) in 11 cases. Neck was managed by vein preserving neck dissection in 27 cases (36%) and supraomohyoid neck dissection in 22 cases (29.3%) (Table 1). It was observed that the nodal involvement in ipsilateral level II was highest (63.6%), followed by the involvement of level I (51.5%), level III (18.2%) and level IV (15.2%) (Table 1). Table 2 shows the distribution of nodes at different levels of the neck in varying combination. Skip metastasis to level III / III and IV occurred in 5 cases. On histopathological examination, only one patient had bilateral positive nodes. These nodes were located at level I bilaterally and the patient had undergone bilateral vein preserving neck dissection (Table 1). A total of 59 patients received adjuvant radiotherapy, radiotherapy was given to 32/34 patients with N+ disease, or extranodal spread. Nine patients with T1N0 disease and 5 patients with T2N0 disease and one patient each with T1N1 and T2N1 disease did not receive adjuvant radiotherapy.
Table 3.
Nodal metastasis by tumour stage.
| pN0 | pN1 | pN2a | pN2b | pN2c | pN3 | % of positive nodes | |
| T1 | 9 | 4 | 1 | 2 | 1 | 0 | 47.05 |
| T2 | 22 | 2 | 0 | 4 | 0 | 1 | 24.13 |
| T3 | 4 | 2 | 2 | 3 | 0 | 0 | 63.6 |
| T4 | 7 | 4 | 0 | 7 | 0 | 0 | 61.1 |
Table 4.
Sensitivity and Specificity of clinical examination in predicting nodal metastasis from cancer of the oral tongue.
| Pathological | |||
| +ve | -ve | ||
| Clinical | -ve | 15 | 26 |
| +ve | 18 | 16 | |
| Sensitivity : | 54.5% | ||
| Specificity : | 61.9% | ||
Over the two-year follow-up, 19 cases failed, the failure was nodal in 8, primary in 8, and both primary and nodal in 3 patients. The clinical TNM, pathological TNM and level of positive node are illustrated in additional table 1. The overall disease free survival (DFS) was found to be 78.4% at 1 – year and 68.4% at 2 – years (Table 5) (fig 1). Patients with T4 tumours were found to have a significantly poor survival, with the disease free survival at the end of 2 – years being 36.6%. T1 and T2 tumours showed better prognosis with disease free survival of 76.2% and 83.5% respectively at the end of 2 – years (Table 5) (figure 2). Histopathological node negative cases had a DFS of 79% at the end of 2 – years whereas pathological N1 (pN1) had survival of 72.7% and pN2 of 39.5% (P = 0.03) (Table 5) (figure 3). No other parameter including use of adjuvant radiotherapy was found to have significant effect on disease free survival. On multivariate analysis the tumour size and nodal metastasis were found to be significant independent prognostic factors. The hazard ratio for failure was 1.0 for T2, 1.7 for T1, 1.72 for T3 and 1.84 for T4 tumours. Similarly hazard ratio for failure in N0 disease was 1.0, it was 9.9 in N1, 14.9 in N2 and 21.2 in N3 disease. Sex was also found to be an independent prognostic factor with hazard ratio for males being 1.4 however this difference was statistically not significant.
Table 5.
Disease free survival (DFS) among patients with cancer of the oral tongue by various parameters
| Variable | Total No. of cases | Survival 1 year | Survival 2 years | |
| Overall DFS | 75 | 78.4% | 68.42% | |
| Tumour status: | ||||
| T1 | 13 | 85.71% | 76.2% | |
| T2 | 33 | 88.7% | 83.5% | |
| T3 | 15 | 51.4% | 51.4% | |
| T4 | 14 | 68.6% | 36.6% | P = .03 |
| Nodal status: | ||||
| pN0 | 42 | 87% | 79% | |
| pN1 | 12 | 72.7% | 72.7% | |
| pN2 | 20 | 61.4% | 39.5% | |
| pN3 | 1 | - | - | P = .03 |
Figure 1.
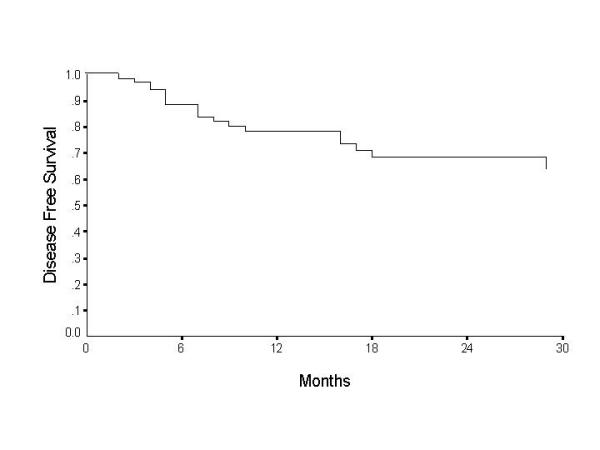
Overall disease free survival for carcinoma oral tongue
Figure 2.
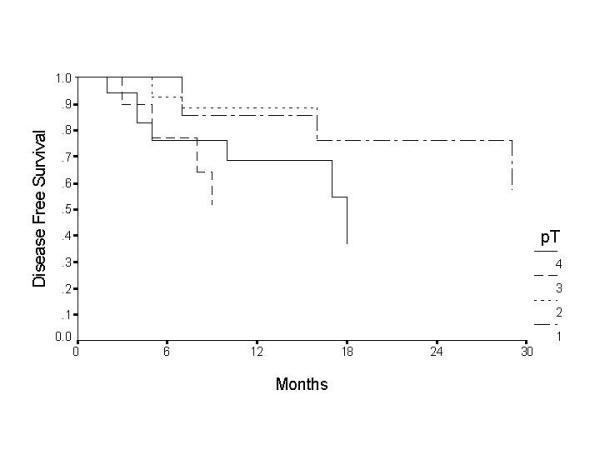
Disease free survival by tumour stage for patients with cancer of the oral tongue.
Figure 3.
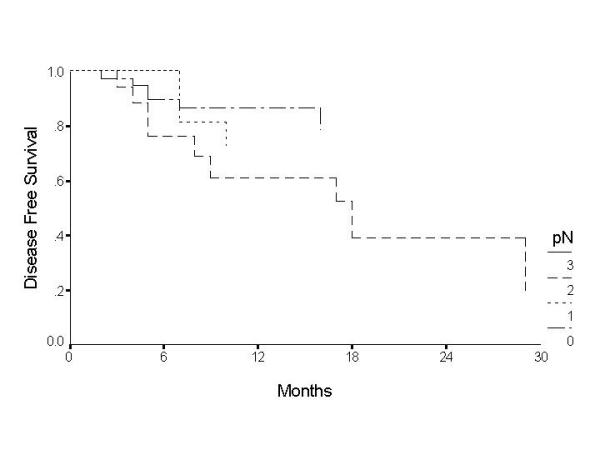
Disease free survival by nodal status for patients with cancer of the oral tongue.
Discussion
Squamous cell carcinoma arising in oral tongue is curable if assessed and treated appropriately. Clinically undetectable nodal metastasis is the commonest cause of treatment failure. Incidence of neck metastasis in oral SCC is reported to be 34% to 50% [7,8]. In our series, level II was found to be the most commonly involved site (63.6%) and level IV involvement was seen in 15.2 % patients. Isolated level IV involvement in absence of involvement of one of the higher levels (I, II or III) was not documented in any patient in the present study. Further, clinically node negative cases never showed level IV involvement. Shah et al (1990) [4] had documented similar results. Hence, a supraomohyoid neck dissection is justified when the neck is clinically negative. On the contrary Byers et al (1997) [3], found 16% of patients with oral cancer, to have metastasis in level IV without nodes in level I, II or III.
It was seen that only 35.6% of T1 and T2 lesions showed nodal metastasis where as 62.3% of the larger tumours showed metastasis (Table 3). Previous studies [9,6] support our finding that tumour size is a predictor of lymph node metastasis though they propose that tumour thickness is a more reliable factor. This is further explained by DiTroia (1972) [10], who points to difficulty for the tumour emboli to form in small calibre lymphatics of the superficial areas, compared with wider lymphatic of deeper tissues. However, tumour thickness is a radiological or histological parameter, which cannot be assessed preoperatively by clinical examination or biopsy.
In our study, sensitivity and specificity of clinical examination was poor suggesting that clinical examination is of limited use in predicting cervical nodal metastasis. This is in accord with previous studies [5,6]. One option would be to look for a more reliable methods to asses the neck. Sajeeda et al (2000) [11] documented that ultrasonography is not only useful in detecting neck nodes but is also useful in assessing the nodal characteristic and the degree of vascular invasion. Takashima et al (1997) [12] demonstrated ultrasound guided fine needle aspiration to have 93.7% diagnostic accuracy. Computed tomographic (CT) scanning too is very useful for the purpose of early detection of neck metastasis [13]. Magnetic resonance imaging (MRI) on the other hand has been found to have little advantage over clinical examination as the soft tissue contrast resolution reported by MRI is inadequate to detect minimal morphological changes in lymph nodes [14].
The technique of sentinel node biopsy is gaining popularity. Surgery on the primary tumour often modifies lymphatic drainage, so sentinel node biopsy is useful when primary tumour and neck are operated at the same time [15]. If the treatment decision has to be based on the present sensitivity and specificity of clinical examination, then a minimum selective neck dissection should be carried out for all node negative necks where primary is being treated by surgery as primary modality. Studies have documented that ipsilateral level I, II and III neck dissection is an adequate diagnostic procedure for staging of the clinically node negative neck in early oral tongue cancer [16,17]. Its diagnostic role cannot be replaced by preoperative radiological screening and intraoperative frozen section sampling.
T1 and T2 tumours show a similar, significantly higher disease free survival compared to T3 and T4 tumours, even though the number in the present study is small the conclusions can be drawn with conviction from these results as shown in earlier studies on smaller sample size [18]. Brown et al (1989) [19] too have shown similar results. Nodal metastases too significantly correlate with disease free survival as also documented by Woolgar et al (1999)[6]. Similar results were seen in the present study as well, where tumour size, nodal status and surgery as primary modality were found to be a significant predictor of disease free survival.
In the light of the present results showing a poor sensitivity and specificity of clinical examination in predicting nodal metastasis and infrequent involvement of level IV in the absence of involvement of higher levels, we suggest that a supraomohyoid neck dissection should be carried out in all patients of the oral tongue cancer with clinically node negative neck to achieve a better disease free survival.
Contributor Information
CS Nithya, Email: oncosurgery@hotmail.com.
Manoj Pandey, Email: manojpandey@rcctvm.org.
BR Naik, Email: oncosurgery@hotmail.com.
Iqbal M Ahamed, Email: oncosurgery@rcctvm.org.
References
- Perkin DM, Whelan SL, Ferlay J, Raymond L, Young J, Eds Cancer incidence in five continents IARC Sci Pub no 143, Lyon, France. 1995.
- Varghese C, Vijayprasad B. Population based cancer registry, Trivandrum, 1991–1995. Regional Cancer Centre, Trivandrum; 1999. [Google Scholar]
- Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of "skip metastasis" in the neck from squamous carcinoma of the tongue. Head Neck. 1997;19:14–19. doi: 10.1002/(SICI)1097-0347(199701)19:1<14::AID-HED3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Shah JP, Candela FC, Poddar AK. The pattern of cervical lymph node metastases from squamous carcinoma of the oral cavity. Cancer. 1990;66:109–113. doi: 10.1002/1097-0142(19900701)66:1<109::aid-cncr2820660120>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Woolgar JA, Scott J. Prediction of cervical lymph node metastasis in squamous cell carcinoma of the tongue/floor of mouth. Head Neck. 1995;17:463–472. doi: 10.1002/hed.2880170603. [DOI] [PubMed] [Google Scholar]
- Woolgar JA. T2 carcinoma of the tongue the histopathologist's perspective. Br J of Oral Maxillofacial Surg. 1999;37:187–193. doi: 10.1054/bjom.1999.0034. [DOI] [PubMed] [Google Scholar]
- Lee JG, Krause CJ. Radical neck dissection: elective, therapeutic and secondary. Arch Otolaryngol. 1975;101:656–659. doi: 10.1001/archotol.1975.00780400014004. [DOI] [PubMed] [Google Scholar]
- Woolgar JA, Rogers S, West CR, Errington RD, Brown JS, Vaughan ED. Survival and patterns of recurrence in 200 oral cancer patients treated by radical surgery and neck dissection. Oral Oncol. 1999;35:257–265. doi: 10.1016/S1368-8375(98)00113-4. [DOI] [PubMed] [Google Scholar]
- Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19:205–210. doi: 10.1002/(SICI)1097-0347(199705)19:3<205::AID-HED7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- DiTroia JF. Nodal metastases and prognosis in carcinoma of the oral cavity. Otolaryngol Clin N Am. 1972;5:333–342. [PubMed] [Google Scholar]
- Sajeeda S, Panda N, Mann SB, Katariya S, Kalagara S. The role of ultrasonography in the management of tumours of the neck. Ear Nose Throat J. 2000;79:586–589. [PubMed] [Google Scholar]
- Takashima S, Sone S, Nomura N, Tomiyama N, Kobayashi T, Nakamura H. Nonpalpable lymph nodes of the neck. Assessment with US and US-guided Fine Needle Aspiration Biopsy. J Clin Ultrasound. 1997;25:283–292. doi: 10.1002/(SICI)1097-0096(199707)25:6<283::AID-JCU1>3.3.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Umeda M, Nishimatsu N, Teranobu O, Shimada K. Criteria for diagnosing lymph node metastasis form squamous cell carcinoma of the oral cavity: A study of the relationship between computed tomographic and histologic findings and outcome. J Oral Maxillofac Surg. 1998;56:585–593. doi: 10.1016/s0278-2391(98)90457-8. [DOI] [PubMed] [Google Scholar]
- Feinmesser R, Freeman JL, Noyek AM, Brit D, Gullane P, Mullen JB. MRI and neck metastases: a clinical, radiological, pathological correlative study. J Otolaryngol. 1990;19:136–140. [PubMed] [Google Scholar]
- Chiesa F, Mauri S, Grana C, Tradati N, Calabrese L, Ansarin M, et al. Is there a role for sentinel node biopsy in early N0 tongue tumours? Surgery. 2000;128:16–21. doi: 10.1067/msy.2000.106809. [DOI] [PubMed] [Google Scholar]
- Byers RM, Clayman GL, McGill D, Andrews T, Kare RP, Roberts DB, Gopefert H. Selective neck dissections for squamous carcinoma of the upper aerodigestive tract: Patterns of regional failure. Head Neck. 1999;21:499–505. doi: 10.1002/(SICI)1097-0347(199909)21:6<499::AID-HED1>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK, Ho CM. Clinico-pathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg. 1999;177:90–92. doi: 10.1016/S0002-9610(98)00294-3. [DOI] [PubMed] [Google Scholar]
- Mathew A, Pandey M, Murthy NS. Survival analysis: Caveats and pitfall. Eur J Surg Oncol. 1999;25:321–329. doi: 10.1053/ejso.1998.0650. [DOI] [PubMed] [Google Scholar]
- Brown B, Barnes L, Mazariegos J, Taylor F, Johnson J, Wagner RL. Prognostic factors in mobile tongue and floor of mouth carcinoma. Cancer. 1989;64:1195–1202. doi: 10.1002/1097-0142(19890915)64:6<1195::aid-cncr2820640606>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]


