Abstract
GABAergic activation of substantia nigra pars reticulata (SNR) at postnatal day (PN) 15 has sex-specific features on seizure control in vivo and electrophysiological responses in vitro. In males, the GABAA-receptor agonist muscimol has proconvulsant effects and induces depolarizing responses. In females, muscimol has no effect on seizures and evokes hyperpolarizing responses. We determined the time period during which sex hormones must be present to produce the sex-specific muscimol effects on seizures and their influence on SNR GABAA receptor-mediated postsynaptic currents. Exposure to testosterone or its metabolites (estrogen or dihydrotestosterone) during PN0-2 in females or males castrated at PN0 was sufficient to produce proconvulsant muscimol effects but did not affect the in vitro GABA responses, which remained hyperpolarizing. The data suggest that the PN0-2 period is critical for the development of the seizure-controlling SNR system; the hormonal effect on seizure control is independent from their effect on GABA conductance.
Keywords: substantia nigra, sex hormones, muscimol infusions, seizure, GABA, patch clamp
During development, circulating sex hormones have organizational effects leading to permanent differences between males and females in distinct brain regions (Gorski, 1984). The presence or absence of testosterone, acting via its metabolites, dihydrotestosterone and estrogen, determines the future “male” or “female” brain phenotype (McEwen, 1992). In humans, the incidence of epilepsy is higher in the youngest age groups and population studies have shown that males have a higher incidence of seizures than females (Hauser, 1997). These epidemiological data strengthen the need to understand mechanisms underlying the sex-dependent differentiation of the CNS, especially in relation to structures involved in seizure control. Understanding of how the sex hormones can modulate seizure-controlling substrates during development is important for the identification of possible targets for prevention or treatments of seizure disorders.
The substantia nigra pars reticulata (SNR), a midbrain structure populated largely by GABAergic neurons, serves as a main output of the basal ganglia. The SNR is involved in the control of seizures in an age- and sex-specific fashion (Velíšková and Moshé, 2001; Velíšková and Moshé, 2006). In immature rats [postnatal day (PN) 15], localized bilateral SNR microinfusions of muscimol, an agonist at GABAA receptors, have proconvulsant effects on flurothyl-induced clonic seizures in males but not in females (Moshé et al., 1994; Velíšková and Moshé, 2001). Additionally, in vitro gramicidin patch clamp recordings in slices from PN14-17 males and females show sex-specific responses of the SNR GABAergic neurons to bath application of muscimol (Galanopoulou et al., 2003b) and to synaptically released GABA (Kyrozis et al., 2006). In males, both have depolarizing effects on the membrane potential of the SNR GABAergic neurons, while in females the response is hyperpolarizing.
The majority of sex differences in the brain develop under the influence of perinatal testosterone (Arnold and Gorski, 1984). In rats during postnatal development, the brain is especially sensitive to the organizational effects of testosterone during the first six days of life (Goy and McEwen, 1980). We have previously shown that the presence of postnatal testosterone is responsible for the male proconvulsant phenotype of SNR muscimol effects on clonic seizures (Velíšková and Moshé, 2001). Testosterone depletion by castration of males at PN0 leads to female-like responses of SNR muscimol on clonic flurothyl-induced seizures at PN15. Daily administration of testosterone (from PN0 to PN14) to PN0 castrated males or to intact female rats results in the male proconvulsant phenotype (Velíšková and Moshé, 2001). These data show that postnatal testosterone has pivotal organizing effects on the SNR seizure-controlling network but do not define the critical period during which the presence of testosterone leads to the formation of the muscimol-sensitive proconvulsant SNR region in PN15 rats. Further, as the testosterone affects the brain via its metabolites (Arnold and Gorski, 1984), the question remains, whether estrogen or dihydrotestosterone mediates the sexual differentiation of the SNR.
The main aims of this study were to determine in vivo, 1) the critical postnatal period necessary for the testosterone exposure to induce the proconvulsant phenotype of SNR muscimol effects on clonic seizures and 2) whether exposure to one of testosterone metabolites (dihydrotestosterone or estrogen) or both is necessary to achieve this effect. We also tested, using in vitro gramicidin perforated patch clamp recordings, whether the SNR GABAA receptor-mediated postsynaptic currents (PSCs) are changing as the result of the hormonal exposure during the critical period.
Methods
Sprague-Dawley rats (Taconic Farms, New York) were housed under standard conditions in our animal facility, which is accredited by the American Association for Accreditation of Laboratory Animal Care. The pups were kept with their dams. The day of birth was considered PN0.
In vivo studies
Neonatal castration
Castration in males was performed as described previously (Velíšková and Moshé, 2001). Briefly, a sufficient level of general anesthesia was reached by lowering the body temperature with crushed ice. Under aseptic conditions, two small incisions were performed on the lower abdomen between the hindlimb insertion point and genital prominentia and the testes were carefully extracted. The wounds were sutured and treated with disinfectant. The pups were warmed for 2 h at 30°C and returned to their respective dams. The rats were castrated at PN0 to prevent any postnatal testosterone surge (Pfeiffer, 1936). In additional rats, the natural testosterone surge was permitted during the early neonatal period by performing the castration at a later date. In a pilot experiment we found that in males castrated at PN5, SNR muscimol infusions at PN15 had proconvulsant effects compared to saline controls. This effect is similar to that observed in intact male rats with undisturbed testosterone surge (Velíšková and Moshé, 2001). Thus, to further narrow the early postnatal period of testosterone exposure, necessary to induce the muscimol-sensitive proconvulsant SNR region, we used males castrated at PN3 (n=15).
Administration of testosterone
Female rats (n=26) and males castrated at PN0 (prior to their natural testosterone surge; n=14) were injected daily with testosterone propionate (TP; 0.1 mg/0.02ml s.c.) from PN0 to PN2. This dose of TP, when administered daily for 15 days, induced the proconvulsant male type SNR muscimol effect on the clonic seizure threshold in females and in PN0 castrated males (Velíšková and Moshé, 2001).
Administration of testosterone metabolites
Female rats (n=14) or males castrated at PN0 (n=17) were injected with diethylstilbestrol (DES; 2 μg/0.02ml s.c.) or 5α-androstan-17b-ol-3-one (5α-dihydrotestosterone, DHT; 100 μg/0.02 ml s.c.; females: n=12; PN0 castrated males: n=12) from PN0 to PN2. DES is a synthetic estrogen, which in contrast to β-estradiol does not bind to α-fetoprotein and thus produces stable estrogen blood levels (Savu et al., 1979). At this daily dose, DES produces sexual differentiating effects in other brain regions (McAbee and Doncarlos, 1999). DHT is a non-aromatizable androgen, which has been shown to exert sexual differentiating effects (van der Schoot, 1980).
Stereotaxic surgery and seizure testing
Bilateral cannulae (Plastics One, Roanoke, VA, USA) were implanted in the SNR at PN13 under ketamine (70 mg/kg)-xylazine (10 mg/kg) anesthesia as described previously (Velíšková and Moshé, 2001). With the incisor bar positioned 3.5 mm below the interaural bar, the stereotaxic coordinates for the SNR were: anterior-posterior = 5.3 mm from bregma, medial-lateral = 3.5 mm from midline, dorsal-ventral = 6.0 mm below the skull. The cannulae were inclined at an angle of 15° from the sagittal plane. After surgery, rats were allowed to recover for 2 days with their dams.
At PN15, 30 min prior to seizure testing, muscimol (100 ng/0.25 μl saline) or saline (0.25 μl) were injected in the SNR bilaterally (Velíšková and Moshé, 2001).
For seizure testing, rats were exposed to flurothyl as described previously (Velíšková and Moshé, 2001). The flurothyl seizure threshold was determined as the amount of flurothyl (in μl) necessary to elicit the first clonic seizure characterized by facial and forelimb clonus while the righting reflex is preserved.
After completion of the seizure testing, the animals were sacrificed, their brains were removed and frozen in 2-methylbutane at -35°C. Coronal sections were stained with thionin for histological examination of the cannulae placements. Only rats with bilateral symmetrical placements of both cannulae in the SNR were used. We have found previously that drug infusions above the SNR have no effect on flurothyl seizure threshold (Xu et al., 1991; Velíšková et al., 1998).
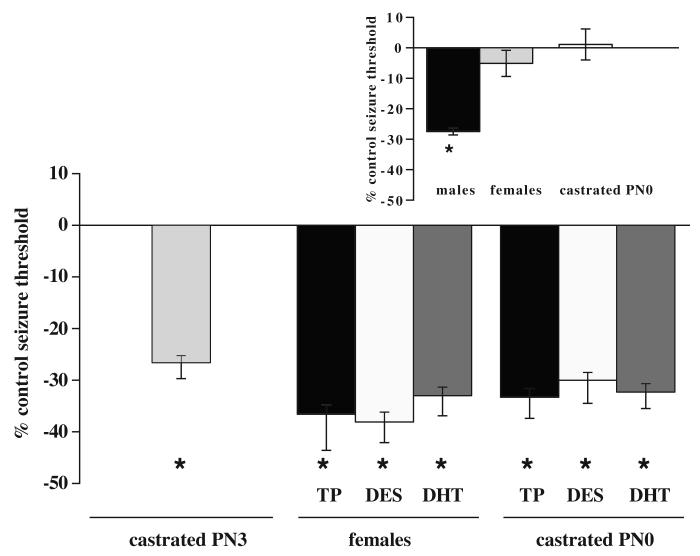
For clarity of the comparisons in the graphic presentation of the data, we added in Figure 2, an inset showing the original, already published, data (Velíšková and Moshé, 2001) of the effects of SNR muscimol infusions on flurothyl seizure threshold at PN15 males, PN15 females, and PN15 neonatally castrated males.
Figure 2.
Effects of administration of exogenous testosterone or its metabolites. The flurothyl seizure threshold was determined for the first clonic seizure in muscimol-infused (normalized to saline-infused) rats at PN15. Muscimol infusions in the SNR had proconvulsant effects in all groups compared to saline infused controls suggesting that exposure to testosterone or its metabolites during the early postnatal period (irrespective to the sex) is responsible for the male proconvulsant phenotype of seizure-controlling SNR effects. The groups included male rats with early life natural testosterone exposure (castrated at PN3; taken from Figure 1), females and males castrated at PN0, which were hormonally treated with TP, DES, or DHT between PN0-PN2.
Inset: For clarity, we included the already published data on SNR muscimol effects on flurothyl-induced seizures in intact males, females, and males castrated at PN0 (Velíšková and Moshé, 2001). These data show that SNR muscimol had a proconvulsant effect in intact males but not in intact females or castrated males at PN0.
An asterisk indicates significant differences (P < 0.05) from respective sex- and treatment specific saline controls.
In vitro studies
For the in vitro gramicidin perforated patch clamp studies we used the following groups of rats: intact males and females, males castrated at PN0, females treated with TP, DHT or DES between PN0-PN2, and males castrated at PN3 (which were thus exposed to natural testosterone for three days).
Coronal 300 μm thick slices including the SNR were cut from ketamine-anesthetized PN13-16 rats with a vibratome (OTS 4000, Electron Microscopy Sciences, Hatfield, PA). Dissection solution contained (in mM): sucrose 210, KCl 3.5, CaCl2 1, MgCl2 4, NaHCO3 26, NaH2PO4 1.25, D-glucose 10.
Slices were incubated for at least one hour in a chamber with artificial cerebrospinal fluid, which contained (in mM): NaCl 126, KCl 4, NaH2PO4 1.25, MgCl2 2, CaCl2 2, NaHCO3 26, D-glucose 10. Perforated patch clamp recordings from SNR neurons were performed with the cation-permeable ionophore gramicidin (0.5 μg/ml; DMSO 0.1% v/v), to avoid alteration of intracellular chloride. The pipette solution contained (in mM): K2SO4 77, NaCl 5, MgCl2 2, CaCl2 0.5, EGTA 5, HEPES 10, pH=7.3, 295 mOsm. The junction potential (measured -10 mV) was electronically compensated. All bath solutions were bubbled with 95% O2/5% CO2 gas mixture and supplemented with ascorbic acid (100 μM), and pyruvate (500 μM); pH=7.3-7.4; 300-310 mOsm. Recordings were performed at room temperature.
Only presumed SNR GABAergic neurons were included in the study. Their distinction from the infrequently encountered SNR dopaminergic cells was made on the basis of their narrow action potentials (<1ms halfwidth), biphasic after hyper polarization, firing rate and lack of the voltage sag in response to hyperpolarizing currents (Richards et al., 1997).
To obtain PSCs generated by GABAA receptor activation, we delivered rectangular electrical pulses of 100 μsec duration using a stainless steel bipolar stimulating electrode (Frederick Haer Co., Bowdoingham, ME) inserted in the slice at a distance of 200-400 μm from the recorded neuron. 6-cyano-7-nitroquinoxalene-2,3,-dione (CNQX; 10 μM) and kynurenic acid (1mM) were present in the bath solution to block PSCs generated by synaptic glutamate release.
The resting membrane potential was determined as an average of the action potential threshold and the potential of the through of the afterhyperpolarization as described previously (Kyrozis et al., 2006). To assess the reversal potential (EGABA) of GABAergic PSCs, 10 mV voltage steps between -110 and -30 mV were applied from a holding potential of -70 mV and stimulations were delivered at each voltage. Peak current of every PSC was measured. A straight line was fitted to the points of the current-voltage curve and its intersection with zero current was taken as the reversal potential. The electrical driving force was defined as EGABA and its difference from resting membrane potential (EGABA-Vr).
Table 1 summarizes the experimental design and hormonal treatments for both the in vivo and in vitro experiments.
Table 1.
Summary of the experimental design and hormonal treatments for both the in vivo and in vitro experiments.
| Sex | Castration | Hormonal status | Testing in vivo | In vitro patch clamp | ||
|---|---|---|---|---|---|---|
| Testosterone (natural or exogenous) | DES(exogenous) | DHT(exogenous) | ||||
| Male | Natural PN0-15 | PN15* | PN13-16 | |||
| Female | PN15* | PN13-16 | ||||
| Male | PN0 | PN15* | PN13-16 | |||
| Male | PN3 | Natural PN0-3 | PN15 | PN13-16 | ||
| Female | Exogenous daily PN0-2 | PN15 | PN13-16 | |||
| Female | Daily PN0-2 | PN15 | PN13-16 | |||
| Female | Daily PN0-2 | PN15 | PN13-16 | |||
| Male | PN0 | Exogenous daily PN0-2 | PN15 | NT | ||
| Male | PN0 | Daily PN0-2 | PN15 | NT | ||
| Male | PN0 | Daily PN0-2 | PN15 | NT | ||
NT = not tested.
Asterisks represent the groups, which were previously reported (Velíšková and Moshé, 2001).
Data Analysis
We compared the flurothyl seizure threshold between the SNR muscimol- and saline-infused rats at PN15 using the Student’s t-test. We used one-way ANOVA followed by a post-hoc Fisher Protected Least Significant Difference Test to determine whether the individual hormone treatments had equal effects in both sexes.
In the electrophysiological evaluation, GABAergic responses were compared by one-way ANOVA, followed by a Tukey-Kramer Multiple Comparison Test.
The significance level for all statistical comparisons was preset to P<0.05. The data are presented as mean ± SEM.
Results
In vivo SNR muscimol effects on clonic seizure threshold in PN15 rats
Determination of the critical period in males
In our previous study we found that the lack of postnatal testosterone by neonatal castration in males at PN0 (Pfeiffer, 1936) resulted in loss of the proconvulsant SNR muscimol effects on seizures (Velíšková and Moshé, 2001). In the present study, rats were castrated at PN3 to allow for exposure to testosterone for the first three days of life. In these PN3 castrated rats (Figure 1), muscimol (n= 7) infusions into the SNR had a proconvulsant effect compared to respective saline-infused (n=8) controls (Student’s t-test t=2.6; P<0.05).
Figure 1.
Determination of the critical period in males. The flurothyl seizure threshold for the first clonic seizure was determined at PN15 in male rats castrated at PN3. The rats were thus exposed to natural testosterone for three days. Muscimol infusions in the SNR had proconvulsant effects compared to saline-infused controls suggesting that exposure to natural testosterone during the early postnatal period is responsible for the male proconvulsant phenotype of seizure-controlling SNR effects. The asterisk indicates a significant difference (P < 0.05) from saline-infused control.
Effects of exogenous testosterone administration during PN0-2
In intact females, administration of testosterone at PN0-2 resulted in the proconvulsant effects of SNR muscimol (n=13) on flurothyl seizures compared to their respective saline-infused (n=13) controls (Student’s t-test: t=4.9; P<0.05; Figure 2). Similarly, in males castrated at PN0 with exogenous testosterone for three days (PN0-2), SNR muscimol (n=6) had a proconvulsant effect on flurothyl seizures compared to saline-infused (n=8) controls (Student’s t-test: t=4.9; P<0.05; Figure 2).
Effects of administration of testosterone metabolites during PN0-2
We determined whether estrogenic or androgenic metabolites of testosterone are responsible for the occurrence of the proconvulsant effects of SNR muscimol infusions (Figure 2). In females, administration of either DES or DHT resulted in proconvulsant SNR muscimol effects on seizures compared to SNR saline-infused controls (DES: muscimol, n=7; saline, n=7; Student’s t-test: t=4.6; DHT: muscimol, n=6; saline, n=6; Student’s t-test: t=4.0; P<0.05). In castrated males at PN0, administration of both testosterone metabolites also resulted in proconvulsant effects of SNR muscimol infusions on flurothyl seizures compared to saline-infused controls (DES: muscimol, n=9; saline, n=8; Student’s t-test: t=4.9; DHT: muscimol, n=6; saline, n=6; Student’s t-test: t=4.7; P<0.05).
In vitro perforated-patch clamp recording
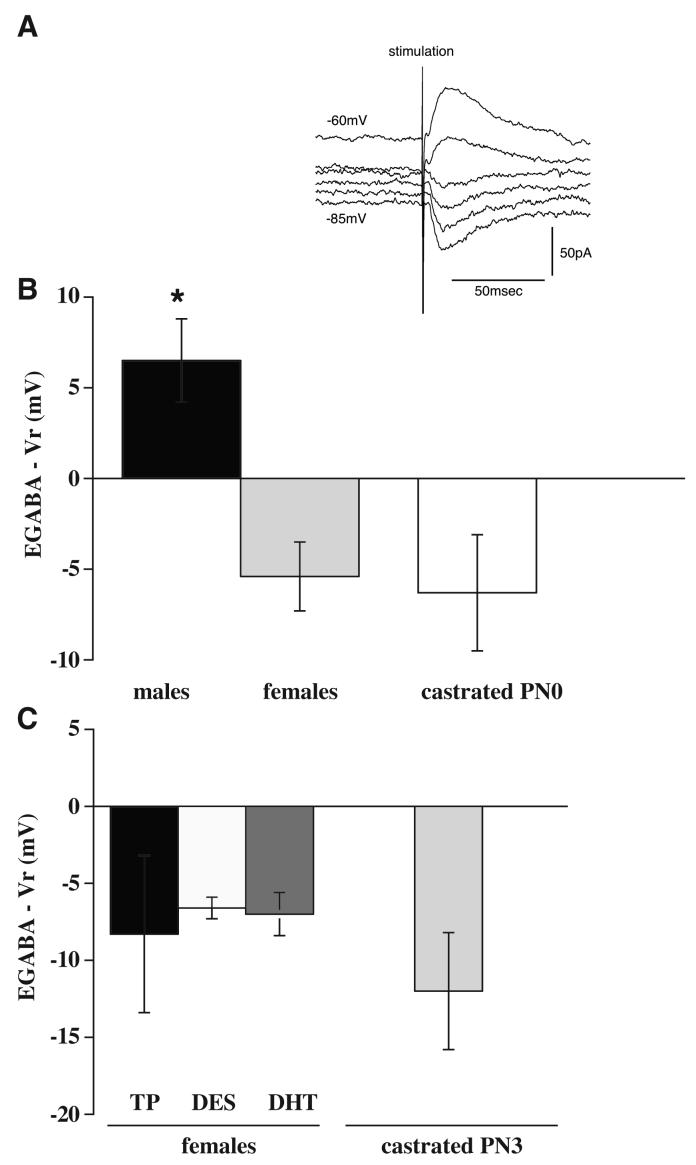
Only GABAergic neurons were studied. The resting membrane potential (Vr) did not differ significantly among the groups (males castrated PN0, n=6: -63.2 ± 1.0 mV; intact females, n=7: -66.7 ± 1.3 mV; intact males, n=4: -61.0 ± 3.1 mV; females TP, n=4: -63.0 ± 2.4 mV; females DES, n=17: -64.6 ± 1.1 mV; females DHT, n=14: -66.9 ± 1.6 mV; males castrated PN3, n=8: -61.0 ± 2.2 mV P=0.18).
Effects associated with castration in males at PN0
We first tested the GABA responses of the SNR GABAergic neurons in male rats castrated at PN0 (Figure 3B) since this had not been determined in our previous studies. In neurons from neonatally castrated males at PN0, the driving force was hyperpolarizing, a -6.3 ± 3.2 mV change from resting potential (EGABA was -69.5 ± 3.1 mV). A similar effect was observed in intact females: the driving force was also hyperpolarizing, a -5.4 ± 1.9 mV change from resting potential (EGABA was -72.1 ± 2.2 mV). On the other hand in intact males, we found a depolarizing effect: The driving force was a +6.5 ± 2.3 mV change from resting potential (EGABA was -55.8 ± 2.2 mV).
Figure 3.
In vitro perforated patch clamp recordings. Changes in driving forces (EGABA -Vr; Vr = resting potential) of GABAA receptor mediated currents in SNR GABAergic neurons in slices from PN13-16 intact males, females, castrated males at PN0, females with TP, DES, or DHT administered at PN0-2 and males castrated at PN3. A. GABAA receptor mediated PSCs in a GABAergic neuron from the SNR of a male castrated at PN0. Raw traces of current are shown in voltage clamp mode, holding at a series of voltages (-85 to -60mV at intervals of 5mV). In this neuron, EGABA = -68mV. B. In intact males, the driving force is depolarizing (positive); in females or neonatally castrated males it is hyperpolarizing (negative; mean + SEM). C. In female rats treated with exogenous testosterone or its metabolites during PN0-2, the driving force is hyperpolarizing. In PN3 castrated male rats, which were exposed to natural testosterone (and its metabolites) for three days, the driving force is also hyperpolarizing.
Effects of exogenous testosterone administration during PN0-2
In females, administration of testosterone at PN0-2 did not change the female-like hyperpolarizing GABA responses. Figure 3C shows that the driving force was a -8.3 ± 5.1 mV change from resting potential (EGABA was 71.3 ± 6.0 mV).
Effects of administration of testosterone metabolites during PN0-2
In females, administration of either testosterone metabolite did not affect the hyperpolarizing GABA responses (Figure 3C): DES: the driving force was a -6.6 ± 0.7 mV change from resting potential (EGABA was -71.2 ± 1.3 mV); DHT: the driving force was a -7.0 ± 1.4 mV change from resting potential (EGABA was -73.9 ± 1.6 mV).
Effect of endogenous testosterone during the first three days of life
In males, castration at PN3 allowed exposure to natural testosterone for three days of life. This short exposure to natural testosterone was not sufficient to induce depolarizing effects and in contrast to intact males, the GABA responses were hyperpolarizing. The driving force was a -10.8 ± 1.9 mV change from resting potential (EGABA was -71.8 ± 1.5 mV).
The driving force and the EGABA in intact males were significantly different from any other group tested as revealed by ANOVA with Tukey-Kramer Multiple Comparisons Test. There were no other statistically significant differences among the hormone-treated groups or between the intact females and castrated males at PN0 or PN3 in any parameter tested.
Discussion
In vivo studies
The SNR controls seizures in a sex-specific manner. In PN15 rats, bilateral muscimol infusions in the SNR have a proconvulsant effect in male rats but no effect in female rats on clonic seizures induced by flurothyl (Velíšková and Moshé, 2001). The proconvulsant muscimol effect is lost in males by castration at PN0 but not in sham operated males. These data imply a role of postnatal testosterone exposure in the formation of the proconvulsant effects but not a role of perinatal stress (Velíšková and Moshé, 2001). In the current study, we identified a critical period necessary for testosterone exposure to induce the proconvulsant type of SNR muscimol effects on seizures in either sex. The data show that the testosterone surge in males during the first three postnatal days (Weisz et al., 1980) or administration of exogenous testosterone during PN0-2 in females and neonatally castrated males (at PN0) is sufficient to imprint the proconvulsant phenotype at PN15.
We further show that the sex differences in SNR responses to muscimol at PN15 can be induced by administration of both testosterone metabolites, DES and DHT from PN0-2. Our study corroborates data regarding the important role of both estrogens and androgens in sexual differentiation of distinct brain regions (Cooke et al., 1998). It was previously thought that the masculinization of the brain is mainly mediated by the action of estrogen (Kelly, 1991), while the role of androgens was restricted to spinal cord structures, such as nucleus bulbocavernosus (Breedlove et al., 1982). However, recent reports suggest that androgens are also important for the sexual differentiation in the CNS. Studies using male rats with testicular feminization mutation (tfm) revealed the significant role of androgen receptors in the masculinization of several forebrain regions, including amygdala, hippocampus, and locus coeruleus (Garcia-Falgueras et al., 2005; Jones and Watson, 2005; Morris et al., 2005). The important role of androgen receptor function in sexual behavior has been also shown using androgen receptor knockout mice, stressing a role for both androgen and estrogen receptors (Sato et al., 2004). Thus, both androgen and estrogen receptor signaling seems to be important for masculinizing effects (Shughrue and Dorsa, 1994; Breedlove, 1997; Han and De Vries, 2003).
In vitro studies
The sex difference in the effects of SNR muscimol infusions on seizures at PN15 could potentially be, at least in part, due to sex differences in the local responses of SNR neurons to GABAA receptor activation. In previous studies, we found that the male PN15 SNR expressed lower mRNA levels of the GABAA receptor α1 subunit and neuronal specific potassium chloride cotransporter KCC2 compared to the female SNR (Galanopoulou et al., 2003b; Ravizza et al., 2003). The in vitro recordings showed that male SNR neurons were depolarized in response to muscimol application or synaptically released GABA, while female neurons were hyperpolarized (Galanopoulou et al., 2003b; Kyrozis et al., 2006). In the present study, we examined whether perinatal hormonal manipulations that affected the SNR-mediated seizure control had an analogous effect on the electrical direction of GABAA receptor responses (Galanopoulou et al., 2003b; Kyrozis et al., 2006). Similarly to the demasculinizing effect observed in vivo (Velíšková and Moshé, 2001), the present data show that castration in males at PN0 is associated with hyperpolarizing GABA responses, which are characteristic for females at this age. A three-day exogenous administration of testosterone or its metabolites between PN0-2 in female rats or the natural presence of testosterone and its metabolites for 3 days in male rats prior to castration at PN3 is not sufficient to produce the depolarizing GABA responses characteristic for males around PN14. It should be pointed out that we did not perform any studies following the administration of testosterone or its metabolites in male rats castrated at PN0. This is because male rats castrated at PN3 are exposed to natural testosterone (and its metabolites) for 3 days, a condition that is modeled in females treated with testosterone PN0-2.
The data suggest that the SNR GABA responses may require longer exposure period than 3 days or continuous testosterone administration to masculinize male rats and defeminize female rats in term of local SNR GABA responses at PN15. Alternatively, exposure at later time points may be more capable of altering the responses. One of the main regulators of the direction of GABA responses is the neuronal specific potassium chloride co-transporter KCC2, which is essential for lowering the intracellular Cl- (Rivera et al., 1999). The depolarizing GABA responses in the SNR of male rats are associated with low expression of the KCC2, while the hyperpolarizing GABA responses in females correspond to higher expression of KCC2 in the SNR (Galanopoulou et al., 2003b). The SNR expression of KCC2 can be acutely modulated by exogenous administration of testosterone or its metabolites (Galanopoulou and Moshé, 2003a) at a later age (PN13-15). Galanopoulou showed that the estrogenic derivatives of testosterone maintain KCC2 expression low, facilitating therefore the appearance of depolarizing GABAA receptor responses. Nevertheless, preliminary data from this laboratory show that early postnatal administration of testosterone or its metabolites (PN0-2) does not affect the SNR KCC2 expression in females (Galanopoulou, unpublished data), again suggesting that longer periods of administration of these hormones or actual hormonal milieu are needed to alter the KCC2 levels and accordingly the in vitro GABA responses. Indeed, Cooke et al. (Cooke et al., 1999) reported that the continuous presence of circulating testosterone is required for the maintenance of sex differences in the volume of medial amygdala in adult rats. Similarly, the sexually dimorphic nucleus of the preoptic area has also been shown to require the continuous presence of circulating testosterone to maintain its volume (Bloch and Gorski, 1988).
The dissociation between the in vivo and in vitro sex hormone-induced effects on SNR function
The data concerning EGABA should be contrasted with the data showing that the SNR-based muscimol-sensitive network involved in seizure control can be modified long-tern by a relative short period of exposure to testosterone and its metabolites.
One of the reasons for the discrepancy between the in vivo and in vitro effects may be the distinct maturational pattern resulting from the early postnatal hormonal treatment. We previously demonstrated that in intact rats, the switch from SNR muscimol proconvulsant to anticonvulsant effects in vivo (Velíšková and Moshé, 2001) follows the switch from the depolarizing to the hyperpolarizing GABA responses in the SNR neurons in vitro (Kyrozis et al., 2006). In males, the anticonvulsant muscimol effects occur after PN25 but the hyperpolarizing GABA responses are already present at PN17. The maturation in females is faster than in males: the in vivo muscimol anticonvulsant effects occur after PN15, while the in vitro GABA effects are hyperpolarizing around PN10. The results from the present study in PN15 rats suggest that the natural testosterone exposure in males castrated at PN3 or the perinatal hormonal administration in females may lead to a maturational pattern similar to that observed in PN 21 intact male rats. At this age, muscimol infusions still have proconvulsant effects (Velíšková and Moshé, 2001), while the EGABA responses are hyperpolarizing (Kyrozis et al., 2006).
Other factors beyond the local SNR GABAergic neuron effects may help explain the dissociation between the in vivo and in vitro data. Although it has been suggested that the nigral dopaminergic system may not be directly involved in seizure control (Albala et al., 1986; Gale, 1988), it may play a supporting role during seizures and thus hormone-induced changes in this system may affect the muscimol-induced in vivo effects. Sex differences in the SN dopaminergic system have been described including sex-dependent responses to muscimol (Robinson et al., 1981; Miller, 1983; Reisert et al., 1987; Dluzen and McDermott, 2000; Galanopoulou, 2006). Fin ally, the SNR input/output structures may be differentially affected by the hormonal treatments. The natural or exogenously administered circulating sex hormones influence the whole brain and the organizational effects of the sex hormones are not restricted to the SNR. Previously, using the 2-deoxyglucose mapping technique, we found that SNR muscimol infusions induce sex-dependent uptake pattern differing in several input/output SNR structures (Velisek et al., 2005). Thus, the in vivo effects of SNR muscimol on seizures may be dependent by other structures within the SNR seizure-controlling network and may not simply reflect only the local SNR properties. Ongoing studies in our laboratory are exploring this possibility.
Conclusions
Clinical evidence shows gender- and age-related expression in many seizure syndromes (Hauser, 1994; Christensen et al., 2005). The incidence of epilepsy is generally higher in males than in females (Hauser, 1997). As the SNR is part of a seizure controlling system, understanding the sex differences and the role of sex hormones in modulation of this system should offer significant insights in the pathophysiology and treatment of seizures.
Acknowledgments
Acknowledgements: The authors thank Drs. L. Velísek, A.S. Galanopoulou and J. Heida for valuable scientific discussions during the data collection and for critical reading of the manuscript. This work was supported by NINDS research grant NS-20253, a CURE grant, Centro Studi e Richerche “E. Fermi”, Roma, Italy, and a Heffer family research grant.
References
- Albala BJ, Moshé SL, Cubells JF, Sharpless NS, Makman MH. Unilateral peri-substantia nigra catecholaminergic lesion and amygdala kindling. Brain Res. 1986;370:388–392. doi: 10.1016/0006-8993(86)90500-7. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Cytoarchitectonic analysis of the SDN-POA of the intact and gonadectomized rat. J. Comp. Neurol. 1988;275:604–612. doi: 10.1002/cne.902750408. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Neonatal androgen and estrogen treatments masculinize the size of motoneurons in the rat spinal nucleus of the bulbocavernosus. Cell. Mol. Neurobiol. 1997;17:687–697. doi: 10.1023/A:1022590104697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–181. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc. Natl. Acad. Sci. U S A. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Gender differences in neurotoxicity of the nigrostriatal dopaminergic system: implications for Parkinson’s disease. J. Gend. Specif. Med. 2000;3:36–42. [PubMed] [Google Scholar]
- Galanopoulou AS. Sex- and cell-type-specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur. J. Neurosci. 2006;23:2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp. Neurol. 2003a;184:1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp. Neurol. 2003b;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Gale K. Progression and generalization of seizure discharge: anatomical and neurochemical substrates. Epilepsia. 1988;29:S15–34. doi: 10.1111/j.1528-1157.1988.tb05795.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Jordan CL, Segovia S, Guillamon A. The role of the androgen receptor in CNS masculinization. Brain Res. 2005;1035:13–23. doi: 10.1016/j.brainres.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Sexual differentiation of brain structure in rodents. In: Sergio M.e.a., editor. Sexual Differentiation: Basic and Clinical Aspects. Raven Press; New York: 1984. pp. 65–77. [Google Scholar]
- Goy RW, McEwen BS. MIT Press; Cambridge: 1980. Sexual Differentiation of the Brain. [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J. Neurobiol. 2003;54:502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;2(35 Suppl):S1–6. doi: 10.1111/j.1528-1157.1994.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Hauser WA. Incidence and prevalence. In: Engel J Jr., Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 47–57. [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kelly DD. Sexual Differentiation of the Nervous System. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 3 Edition Elsevier; New York: 1991. pp. 959–973. [Google Scholar]
- Kyrozis A, Chudomel O, Moshé SL, Galanopoulou AS. Sex-dependent maturation of GABA(A) receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci. Lett. 2006;398:1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in developing male rat forebrain. Endocrinology. 1999;140:3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Steroid hormones: Effect on brain development and function. Horm. Res. 1992;37(suppl 3):1–10. doi: 10.1159/000182393. [DOI] [PubMed] [Google Scholar]
- Miller JC. Sex differences in dopaminergic and cholinergic activity and function in the nigrostriatal system of the rat. Psycho neuro endocrinology. 1983;8:225–236. doi: 10.1016/0306-4530(83)90059-8. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J. Comp. Neurol. 2005;487:217–226. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Moshé SL, Brown LL, Kubová H, Velíšková J, Zukin RS, Sperber EF. Maturation and segregation of brain networks that modify seizures. Brain Res. 1994;665:141–146. doi: 10.1016/0006-8993(94)91164-9. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA. Sexual differences of the hypotheses and their determination by the gonads. Am. J. Anat. 1936;58:195–225. [Google Scholar]
- Ravizza T, Friedman LK, Moshé SL, Velíšková J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int. J. Dev. Neurosci. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Reisert I, Han V, Lieth E, Toran-Allerand D, Pilgrim C, Lauder J. Sex steroids promote neurite growth in mesencephalic tyrosine hydroxylase immunoreactive neurons in vitro. Int. J. Dev. Neurosci. 1987;5:91–98. doi: 10.1016/0736-5748(87)90054-2. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl-co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM, Becker JB. Gonadectomy attenuates turning behavior produced by electrical stimulation of the nigrostriatal dopamine system in female but not male rats. Neurosci. Lett. 1981;23:203–208. doi: 10.1016/0304-3940(81)90041-0. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc. Natl. Acad. Sci. U S A. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savu L, Benassayag C, Vallette G, Nunez EA. Ligand properties of diethylstilbestrol: studies with purified native and fatty acid-free rat alpha 1-fetoprotein and albumin. Steroids. 1979;34:737–748. doi: 10.1016/0039-128x(79)90088-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Dorsa DM. Estrogen and androgen differentially modulate the growth-associated protein GAP-43 (neuromodulin) messenger ribonucleic acid in postnatal rat brain. Endocrinology. 1994;134:1321–1328. doi: 10.1210/endo.134.3.8119173. [DOI] [PubMed] [Google Scholar]
- van der Schoot P. Effects of dihydrotestosterone and oestradiol on sexual differentiation in male rats. J. Endocrinol. 1980;84:397–407. doi: 10.1677/joe.0.0840397. [DOI] [PubMed] [Google Scholar]
- Velísek L, Velíšková J, Ravizza T, Giorgi FS, Moshé SL. Circling behavior and [14C]2-deoxyglucose mapping in rats: possible implications for autistic repetitive behaviors. Neurobiol. Dis. 2005;18:346–355. doi: 10.1016/j.nbd.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann. Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Velíšková J, Moshé SL. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006;6:83–87. doi: 10.1111/j.1535-7511.2006.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšková J, Löscher W, Moshé SL. Regional and age specific effects of zolpidem microinfusions in the substantia nigra on seizures. Epilepsy Res. 1998;30:107–114. doi: 10.1016/s0920-1211(97)00096-x. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Xu SG, Garant DS, Sperber EF, Moshé SL. Effects of substantia nigra ~-vinyl-GABA infusions on flurothyl seizures in adult rats. Brain Res. 1991;566:108–114. doi: 10.1016/0006-8993(91)91687-v. [DOI] [PubMed] [Google Scholar]