Abstract
We develop a biophysical method for investigating chemical compounds that target the nucleic acid chaperone activity of HIV-1 nucleocapsid protein (NCp7). We used an optical tweezers instrument to stretch single λ-DNA molecules through the helix-to-coil transition in the presence of NCp7 and various chemical compounds. The change in the helix-coil transition width induced by wild-type NCp7 and its zinc finger variants correlates with in vitro nucleic acid chaperone activity measurements and in vivo assays. The compound-NC interaction measured here reduces NCp7’s capability to alter the transition width. Purified compounds from the NCI Diversity set, 119889, 119911, and 119913 reduce the chaperone activity of 5 nM NC in aqueous solution at 10 nM, 25 nM, and 100 nM concentration, respectively. Similarly, gallein reduced the activity of 4 nM NC at 100 nM concentration. Further analysis allows us to dissect the impact of each compound on both sequence-specific and non-sequence-specific DNA binding of NC, two of the main components of NC’s nucleic acid chaperone activity. These results suggest that DNA stretching experiments can be used to screen chemical compounds targeting NC proteins, and to further explore the mechanisms by which these compounds interact with NC and alter its nucleic acid chaperone activity.
Keywords: HIV-1 NC antagonists, nucleic acid chaperone activity, force-induced melting, helix-coil transition, transition width
Abbreviations: NC (nucleocapsid protein), ssDNA (single-stranded DNA), dsDNA (double-stranded DNA), DTP (Developmental Therapeutics Program), FIM (force-induced melting)
Introduction
HIV-1 nucleocapsid protein (NCp7) is a small (55 aa, 7 kDa), highly positively charged protein, which is essential for HIV-1 replication, and which is also a promising drug target. Currently, there are no drugs in clinical use that target HIV-1 NC. The NC protein plays the role of the nucleic acid chaperone protein in many stages of viral infection, reviewed in (1–5). The main structural features of HIV-1 NCp7 are the two non-equivalent CCHC-type zinc fingers, which are essential for NC’s ability to facilitate retroviral replication. In the immature virus, NCp7 is a domain of the larger Gag polyprotein, which is required for specific RNA packaging and for efficient viral assembly and budding. During virus maturation, viral protease processing of Gag results in a final product, NCp7, which is required for the minus strand transfer step during reverse transcription. In this step, melting and annealing of the very stable RNA and DNA hairpin structures, TAR and cTAR, are possible only in the presence of native NC, with the zinc fingers in the proper configuration (6–9). This step requires the optimum chaperone activity of NC, which significantly increases the rate of annealing of the two nucleic acid structures (10, 11). Note that NC proteins are binding proteins that aid in the transient melting of nucleic acid secondary structure, which is required for specific secondary structure rearrangements. These secondary structure rearrangements result in lower energy nucleic acid structures, and the proteins are therefore referred to as nucleic acid chaperones. Thus, these nucleic acid chaperones differ significantly from protein chaperones, which actively promote protein assembly.
A 2D NMR structure of a synthetic NCp9 (72 aa, also previously referred to as NCp7) revealed that the two highly folded zinc fingers of the CCHC-type are in close proximity, allowing Trp37 to bind to nucleic acids (12). A more recent NMR structure showed Trp37 of NCp7 directly bound to the GU bulge on the third stem loop (SL3) of ψ-packaging signal, an essential step in specific viral RNA recognition and packaging (13). Subsequent NMR structures of NCp7 bound to the ψ-site showed strong and comparable interactions between NCp7 and two of the four stem-loops, SL2 and SL3 (14). They also showed a stronger interaction between the two zinc fingers when NCp7 binds to SL3 than when it binds to SL2, which is probably mediated by the tight packing of Phe16 and Trp37 (14). It is possible that Phe16 is more important than Trp37 for the sequence-specific binding of NCp7, since the first zinc finger is more important in helix destabilization and the second one in the stability of binding to nucleic acids (7, 15–17). In addition to the nonelectrostatic interactions described above, strong electrostatic interactions due to NC’s numerous basic residues also contribute to its nucleic acid chaperone activity. For example, the basic residues are essential for annealing and duplex stabilization (18, 19). However, strong duplex stabilization due to the presence of highly positively charged binding ligands opposes duplex melting, which is an essential part of the nucleic acid chaperone activity of HIV-1 NC. Thus, it is the balance of NC’s capability to facilitate annealing through binding to dsDNA with its capability to destabilize GT-rich base-paired regions due to its sequence-specific binding to single-stranded TG sequences, which likely accounts for the enhanced chaperone activity of HIV-1 NCp7 (7, 9, 19–21).
These findings make NC a very desirable target in HIV-1 chemotherapy (22). Many groups are focusing on targeting the nucleocapsid protein (NCp7) in order to help stop the replication of HIV-1 in infected cells and in the blood supply (22), as well as to create a reliable anti-HIV-1 vaccine (23). Numerous chemical compounds have been investigated as possible HIV-1 NC antagonists. They were classified in two categories: irreversible zinc-ejector agents and agents that act by altering the zinc finger structure. Initially the zinc-ejector agents showed great promise (24). They can target the residues that coordinate the zinc ions, to a greater (Cys-49, Cys-36) or lesser (Cys-39) extent (25). Several studies proved that some compounds can have high selectivity for the CCHC zinc finger-type found only in retroviruses and a very few cellular proteins, such as poly(ADP-ribose) polymerase (26). However, the zinc ejector agents are still avoided because of their potential cytotoxicity at high concentrations. This study focuses on a small number of non-zinc-ejector compounds, identified in a previous study (27). These compounds were found by screening the influence of a large number of compounds on the efficiency of NC binding to d(TG)4 oligos in a high throughput luminescence based assay and confirmed by BIAcore analysis. This oligo sequence was chosen based on the sequence specific binding of NC suggested and measured by Fisher et al. (28), Vuilleumier et al. (29), and Urbaneja et al. (19, 30).
The screening of compounds can start by measuring the effect of the presence of each compound on the ability of NC to bind nucleic acids (12). The investigation of such compounds must be informed by a quantitative understanding of the individual effect of NC on the properties of the nucleic acids. In our experiments, the presence of nucleic acid chaperone proteins is observed through the unique signature of the protein on DNA stretching and relaxation curves. The effect of the compound on a protein can therefore be seen immediately and associated with a direct impact on the capability of the protein to act as a nucleic acid chaperone. These experiments are thus intended as an initial test to identify the mechanism by which these compounds are capable of altering NC’s activities.
Using an optical tweezers instrument, we can investigate the effect of these compounds on the nucleic acid chaperone activity of NC by stretching single DNA molecules in the presence of a fixed concentration of NC and various concentrations of compounds. In previous studies, we found that in the presence of NC, the force-induced melting transition width increases with protein concentration and saturates (9, 31). Although we have only a single DNA molecule in solution with the protein in excess, the fractional protein binding is proportional to protein concentration, which competes with the fixed concentration of sodium ions in solution for DNA binding. Therefore, the binding of NC to the lattice of DNA binding sites presented by the single DNA molecule follows the McGhee-von Hippel binding isotherm, which at low concentrations is directly proportional to the protein concentration in solution. Therefore, the increase in transition width depends linearly on protein concentration at low fractional binding. We have used our measurement of the concentration dependence of the transition width to quantify the association constant for NC to DNA, and determined a value of Ka = (1.22 ± 0.25) x108 M−1 for NC binding to the DNA lattice in 50 mM Na+ solution, in agreement with bulk measurements of this quantity (21). The large increase in the transition width induced by HIV-1 NCp7 is probably related to its sequence-specific preferential binding to ssDNA, as well as to the change of DNA elasticity upon binding of NC to ssDNA or dsDNA. The small hysteresis observed suggests that NCp7 can quickly release the ssDNA to allow rapid reannealing of the two complementary strands. The increase in the transition width and the extent of hysteresis are the features that distinguish the nucleic acid chaperone proteins studied in our laboratory from other nucleic acid binding proteins (ref. (9, 31, 32) and unpublished results).
When the protein is preincubated with a chemical compound that affects its zinc-finger structure (ejecting the zinc or altering the zinc-finger structure), the ability of the protein to influence the helix-coil transition is significantly lowered. This effect can be observed as a decrease of the transition width with increasing compound concentration. The effect could be associated with a possible decrease in NC’s ability to recognize the GT regions (i.e. reduction of sequence-specific binding), or with the screening of one or more positive charges of NC that are responsible for the electrostatic interaction of NC with DNA (i.e. reduction of non-sequence-specific binding), or a combination of these effects, both of them being likely important for NC’s chaperone capabilities.
In our experiments, we cannot determine structurally how the chemical compounds bind to the NC protein, whether they act as substrate analogs, conformational ligands, or zinc ejectors, but we can infer the effect on NC’s chaperone activity by measuring how the compounds alter the effect of the protein on the stretch-relax curves. This allows us to determine which aspects of the chaperone activity of NC are altered.
In this work, we report the use of this experiment to screen several compounds that proved to be active or moderate antagonists of the interaction between HIV-1 NCp7 and nucleic acids, in vitro and in vivo. These compounds were previously identified as reversible, non-covalent, non-redox binders, and were also shown to be non-zinc-ejectors. The experimental results refer to several compounds previously reported to have NCp7 antagonist activity (27), and to one example of a new series of compounds identified in recent studies by the Developmental Therapeutic Program (DTP), HIV Drug Resistance Program, National Cancer Institute (NCI), Frederick, MD, USA. It is worth noting that the new method reported here can be used to screen many types of compounds including fluorescent dyes, for which standard fluorescence titration assays can be strongly affected by a high fluorescence background.
Materials and Methods
The optical tweezers instrument and data acquisition were described previously (9, 31). HIV-1 NCp7 was prepared as described (33). All steps were performed at room temperature, except for the storage of compounds 6–14. The tethering buffer was always the same, i.e. 50 mM Na+ (10 mM HEPES, 5 mM NaOH, 45 mM NaCl, pH 7.5), prepared with de-ionized water. The chemical compounds were initially purchased from City Chemical (compound 1) or Sigma-Aldrich (compounds 2–5), or were provided by the Developmental Therapeutics Program, NCI (compounds 6–14) (http://dtp.nci.nih.gov) as part of an ongoing collaboration. Dissolving compounds 1–5 was done as follows: each day a certain amount of compound leading to a maximal 25, 50 or 100 μM compound concentration was placed in 250 mL of tethering buffer and filtered through a 0.2-μm filter. The solutions were kept in the dark, and used only for one day. The actual concentration of the bulk solution was calculated from the visible absorbance spectrum in a 1-mL quartz cuvette, on a Hamamatsu spectrophotometer. The values used for the extinction coefficients for compounds 1–5 at specific wavelengths are listed in the footnote (a) of Table 1. For compounds 2–4, they were provided by the vendor, i.e. Sigma, from technical references (34, 35), but for compound 5, tetrachlorogallein, which was provided by Sigma from the Salor catalog as a rare chemical, i.e. without technical data, we used the value of 1.2×104 M−1cm−1 at λ =560 nm from reference (36). We experimentally determined the extinction coefficient for compound 1 (gallein). In each of three independent determinations, between 3 and 4 mg of gallein was dissolved in 50 mL tethering buffer in a volumetric flask. This was allowed to dissolve fully overnight in the dark. The absorbance was determined at λ =531 nm, and the linear range of the spectrophotometer was determined from serial dilutions of the gallein stock solution. The extinction coefficient was determined to be (1.4±0.27)×104 M−1cm−1. Compounds 6–14 were requested from DTP at NCI. The amounts received were as low as 1–1.2 mg, such that we had to change the method of diluting each compound as follows: one day in advance we dissolved the powder directly in the original dark vial provided by adding a certain volume of de-ionized water (<2 mL) which led to the same bulk concentration of 2 mM for all compounds.
Table 1.
Experimental results describing the effects of investigated compounds on the chaperone activity of HIV-1 NCp7
| Part 1: Compounds assayed by Method A | |||||||
|---|---|---|---|---|---|---|---|
| Nr.crt. | Compound name Source & Product number CAS number | Compound concentrationa,b Incubation time (min) | KD (M−1) (this work)c | Anti-NC activityd (A, M or I) | KD (M−1) Ref. (27) | Antiviral activitye Ref. (27) | ff |
| 1 | Gallein
(City Chemical-G4117) 2103-64-2 |
100 nM
30 |
8.0E-08 | A | - | A | - |
| 2 | Erythrosine B
(Sigma-E7379) 16423-68-0 |
1250 nM
30 |
1.0E-06 | I | - | I | - |
| 3 | Tetrachlorogallein
(Sigma-S500739) 29817-83-2 |
2500 nM
30 |
2.5E-06 | I | - | A | - |
| 4 | Eosin Y
(Sigma-E6003) 17372-87-1 |
>3200 nM
30 |
>3.2E-06 | I | - | I | - |
| 5 | Tetrachlorofluorescein
(Sigma ) 6262-21-1 |
>8500 nM
30 |
>8.5E-06 | I | - | I | - |
| Part 2: Compounds assayed by Method B | |||||||
|---|---|---|---|---|---|---|---|
| Nr. crt. | NSC number | Compound concentrationa,b Incubation time (min) | KD (M−1) (this work)c | Anti-NC activityd (A, M or I) | KD (M−1) Ref. (27) | Antiviral activitye Ref. (27) | ff |
| 6 | 119889
(Tetrabromogallein) |
10 nM
30 |
1.0E-08 | A | 2.53E-07 | A | 25.3 |
| 7 | 119911 | 25 nM
30 |
2.5E-08 | A | 3.50E-07 | A | 14.0 |
| 8 | 378139 | 50 nM
30 |
5.0E-08 | A | 1.45E-04 | A | 2900 |
| 9 | 119913 | 100 nM
30 |
1.0E-07 | A | 3.79E-07 | A | 3.8 |
| 10 | 13778g | 200 nM
30 |
2.0E-07 | A | 2.0E-06g | Ag | 10 |
| 11 | 122391 | 500 nM
30 |
5.0E-07 | M | 1.07E-05 | A | 21.4 |
| 12 | 157411h | >500 nM
60 |
>5.0E-07 | I | 1.52E-05 | A | <30.4 |
| 13 | 119910 | 1500 nM
30 |
1.5E-06 | I | 4.07E-07 | M | 0.27 |
| 14 | 605868 | 2000 nM
30 |
2.0E-06 | I | 8.38E-06 | M | 4.2 |
All measurements were performed in 10 mM HEPES, 50 mM Na+ (45 mM NaCl and 5mM NaOH ), pH 7.5. The concentration of NCp7 was 4 nM when testing gallein and Eosin Y and 5 nM for all other compounds. Actual compound concentrations were obtained using absorbance measurements for compounds 1–5 only. Extinction coefficients used (in M−1cm−1) were as follows: (1.4±0.27)×104 for gallein at λmax=531 nm, 1.2×104 for tetrachlorogallein at λmax=560 nm, 9.16×104 for Eosin Y at λmax=518 nm, 8.8×104 for Erythrosine B at λmax=525 nm, and 4.44×104 for tetrachlorofluorescein at λmax=518 nm. Compounds 6–14 were received as aliquots of 1–1.2 mg powder from DTP at NCI and their concentrations in water were assumed to be maximal.
Values are estimated as averages of at least three experiments in which the helix-coil transition region matched that of naked DNA.
Values are estimated as the actual compound concentrations at which NC is completely saturated with compound and does not interact with the DNA molecule. The estimated dissociation constants are necessarily crude and may not correspond to equilibrium dissociations constants measured by other methods.
A, M or I are abbreviations for the activity level, according to the range of minimum compound concentration required to inhibit NC activity: A-active (concentration required<250 nM), M-moderate (250 nM<concentration required<500 nM)) and I-inactive (concentration required>500 nM).
In reference (27), these abbreviations refer to the anti-HIV-1 activity, defined as the percentage of cells from the human T-cell line CEM-SS that survived after incubation with HIV-1RF virus and various dilutions of compounds for six days, with A-active (80–100 % protection), M-moderate (50–80 % protection), and I-inactive (0–49 % protection), and not to the NC DNA or RNA binding ability. Compounds 1–5 were synthesized as xanthenyl-ring structures at DTP (NCI).
Values are estimated as the ratios between the dissociation constants estimated in this work (column 6) and the values published by Stephen et al. (column 8).(27)
Compound 13778 proved to work better as an HIV-1 entry inhibitor(41) due to its non-specific electrostatic interaction with positive residues. Preliminary data for 13778 yields a dissociation constant of 2×10−6 M. In this case, the ratio in the last column will be 10.
This compound (157411) induced dramatic changes to the helix-coil transition, at all concentrations (Fig. 2c) and it was difficult for us to determine whether it is active or not. We consider it inactive. See the Results section for details.
Protein sequence was as follows. The zinc finger sequences are represented using Italic font and the cysteine and histidine residues coordinating the zinc ions are underlined.
HIV-1 NCp7 = IQKGNFRNQRKTV KCFNCGKEGHIAKNCR APRKK GCWKCGKEGHQMKDCTERQAN
To tether single DNA molecules, a 5 μm streptavidin-coated polystyrene bead (Bangs Labs, Fishers, IN) was trapped in the optical tweezers and transferred by suction to a glass micropipette (World Precision Instrument, Sarasota, FL). Another bead was captured and held in the optical trap. A very dilute solution containing bacteriophage λ – DNA (~ 48,500 base pairs, biotin labeled on each 3′ terminus, obtained from Roche Applied Science, Indianapolis, IN) typically in 10 mM Hepes, pH 7.5, and 50 mM Na+ concentrations, was run through the cell until one molecule was captured between the two beads.
After capturing a single DNA molecule in the tethering buffer, the molecule was stretched to verify that the known force-extension curve was obtained in the absence of bound protein or compound. To measure the effect of the protein or compound on the helix-coil transition, a tethering buffer solution with 50 mM Na+ concentration, containing a fixed concentration of compound or NCp7 or a preincubated compound-NCp7 mixture, was added to the experimental cell until the buffer surrounding the captured DNA molecule was completely exchanged. The concentration of protein used throughout all experiments (5 nM, i.e. half of the saturating concentration) was chosen to avoid DNA aggregation and to observe an average effect of NC on the DNA helix-coil transition. Control stretching curves in the presence of high concentration (10 μM) of compound or NCp7 were taken for every new compound or protein aliquot to ensure that the compound did not have secondary effects on DNA stretching and relaxing curves and that the chaperone activity of the protein was normal. Although small molecules that bind to DNA often show significant effects on DNA stretching (37, 38), at the concentrations used in these experiments there were no changes in DNA stretching in the presence of the compounds used in this study. After doing these control experiments, another DNA molecule was captured and stretched in the presence of one or more NC protein-compound mixtures that had been previously incubated. The incubation time varied from 2 minutes to 3 hours. Two methods were used, denoted as A and B. In method A, a different DNA molecule was used for each preincubated mixture (Table 1, compounds 1 to 5). In an attempt to shorten the investigation time, method B was also used: one DNA molecule was stretched with mixtures prepared from 5 nM NCp7 and decreasing compound concentrations (Table 1, compounds 6–14). The titration was stopped when the chaperone activity of the NC protein reappeared, observed as an increase of the slope of the helix-coil transition, or until the DNA molecule broke. The second method is more suitable for an eventual high throughput method.
The efficiency of each compound was expressed as the value of the compound concentration at which the slope of the overstretching transition decreased to the slope observed in the absence of protein. Since the concentration of NCp7 was always 5 nM (or 4 nM in the case of gallein or Eosin Y), i.e. approximately half the saturated value, the minimum active concentration of the compound was considered to be the dissociation constant of compound binding to NCp7.
Results
In these studies we use single molecule force spectroscopy to monitor the helix-coil transition of DNA in the presence of compounds that may block the chaperone activity of HIV-1 NCp7. We showed in previous studies that the chaperone activity is directly related to the ability of the protein to alter the DNA helix-coil transition (9, 31). In the presence of HIV-1 NCp7, the slope of the helix-coil transition increases dramatically, representing an increase in the transition width (9, 31). The strong alteration in the shape of the transition curve can be quantified by fitting the data to a model for the DNA helix-coil transition. In previous studies, the fitting parameter used to describe the shape of the transition was the cooperativity parameter, σ (sigma), which results from the use of the Zimm-Bragg model for DNA melting. However, this is a two-state model, valid only for homopolymers. In our experiments, sequence heterogeneity can also increase the transition width as NC binds to ss and ds DNA. Therefore, in these studies we used a more general, model-independent parameter, i.e. the force transition width ΔF, given by the intersections of the tangent to the helix-coil transition with the two flanking curves corresponding to dsDNA and ssDNA regions, respectively (Fig. 1). The large increase in ΔF induced by NCp7 may include contributions from sequence-specific binding, change in the helix-coil boundary energy, or the ability of the protein to nucleate melting in the middle of DNA molecule, as well as a change in DNA elasticity upon NC binding to ds and ss DNA. These effects are induced by the presence of both zinc fingers, in their native conformation. Mutations of the zinc fingers of NC lower its chaperone activity, leading to decrease or loss of viral infectivity, in good agreement with the change in the transition width (7, 9). These findings suggest that the force transition width can be used to quantify the efficiency of each chemical compound in reducing the chaperone activity of NC. As we have shown previously (9), reduction in the capability of an NC protein to alter the helix-coil transition is not necessarily equivalent to reduction in NC binding to DNA, so the information provided by these experiments refers to actual reduction in nucleic acid chaperone activity, rather than simple reduction in NC-DNA binding by the compound being tested.
Figure 1.
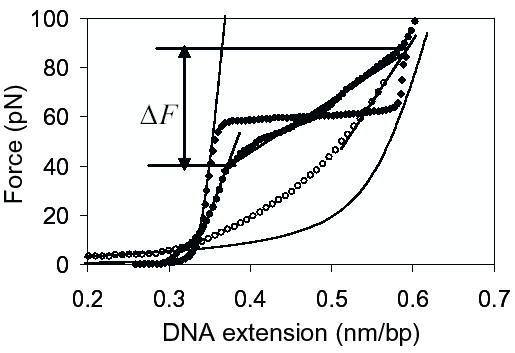
DNA stretching curves in the absence and presence of HIV-1 NCp7. Stretching curve (filled circles) for λ-DNA in the presence of 10 nM HIV-1 NCp7 (wild type). Stretching curve (open circles) for ssDNA in the presence of 10 nM HIV-1 NCp7 (31). The stretching curve for λ-DNA in the absence of protein (filled diamonds) is also shown, for reference. In addition, the method used for measuring the force-induced melting transition width is also illustrated here. ΔF is the difference in the forces at which the tangent to the experimental stretching curve of λ-DNA with NC intersects the tangents to the experimental stretching curves for dsDNA and ssDNA with NC bound on it. The left and right solid lines are the theoretical stretching curves for naked dsDNA and naked ssDNA given by the WLC and FJC models, respectively (40). Data are taken in 50 mM Na+ (45 mM NaCl and 5 mM NaOH), 10 mM HEPES, pH 7.5, and room temperature.
In each experiment, a single dsDNA molecule is stretched by pulling on one end of the DNA while the resulting force on the other end of the molecule is measured by the means of an optical trap (31). The results of typical experiments, in which λ-DNA is stretched in 50 mM Na+ buffer at pH 7.5, in the absence of protein, are shown in Fig. 1 (filled diamonds). In this case, the force (F) vs. extension curve begins to rise as the DNA helix is extended to near its normal B-form contour length of 0.34 nm/bp. At about 60 pN a sharp overstretching transition occurs, where very little additional force is required to stretch the DNA molecule to 1.7 times its contour length. We refer to this transition as the DNA helix-coil transition. At the end of this transition, the force rises again as the DNA, in which almost all regions have been converted into single stranded DNA, is stretched. The relaxation curve is almost identical to the stretching curve except for a small amount of hysteresis which is seen especially in low salt concentration and is probably due to regions of DNA that are reannealing more slowly than others. The transition width in 50 mM Na+ is about 3.6 pN, in agreement with theoretical predictions (39). The stretching curve for λ-DNA in the presence of 10 nM HIV-1 NCp7 (filled circles) displays the dramatic effect of NCp7 on the slope of the helix-coil transition and shows the method used to measure the transition width. The stretching curve for ssDNA in the presence of 10 nM HIV-1 NCp7 (open circles) measured previously in 25 mM Na+ (31), indicates major changes in the elasticity of ssDNA upon protein binding. The theoretical force-extension curves given by the worm-like chain (WLC) model for dsDNA (left solid line) and freely jointed chain (FJC) model for ssDNA (right solid line) in the absence of protein (40) are also shown, for comparison.
In the presence of a preincubated mixture of compound with NCp7, the slope of the overstretching transition decreases at increasing compound concentration until it reaches the value for naked DNA In contrast, none of the investigated compounds alone had any effect on the DNA stretching or relaxing curves in the investigated range, 0–10 μM (data not shown). For some compounds, the NCp7-induced change in slope did not decrease in the presence of moderate compound concentration, therefore we increased the compound concentration until we observed a small value for the slope of the transition, as for naked DNA, without exceeding 10 μM compound concentration. The naked DNA curve is shown in all parts of Fig. 2 for reference (black lines). The stretching curves are represented by solid lines, and the relaxation curves are represented by dashed lines. We also show the control DNA stretching curves in the presence of 5 nM HIV-1 NCp7, the concentrations used in this study (green lines). At 5 nM NC concentration, the transition width ΔF increases to about 30 pN (Fig. 2). To illustrate the differential efficiency of the compounds, we show data obtained with an active compound, gallein, and with two inactive compounds that also displayed unusual effects, i.e. Eosin Y and 157411 (Fig. 2). Except for one compound, Eosin Y, all compounds reduced the hysteresis significantly. These experiments also showed that NC without specific binding has a behavior that strongly resembles that of the small cations or polycations (polylysine and spermidine), which have no effect on the slope of the transition and show very little hysteresis (data not shown). The results are summarized in Table 1.
Figure 2.
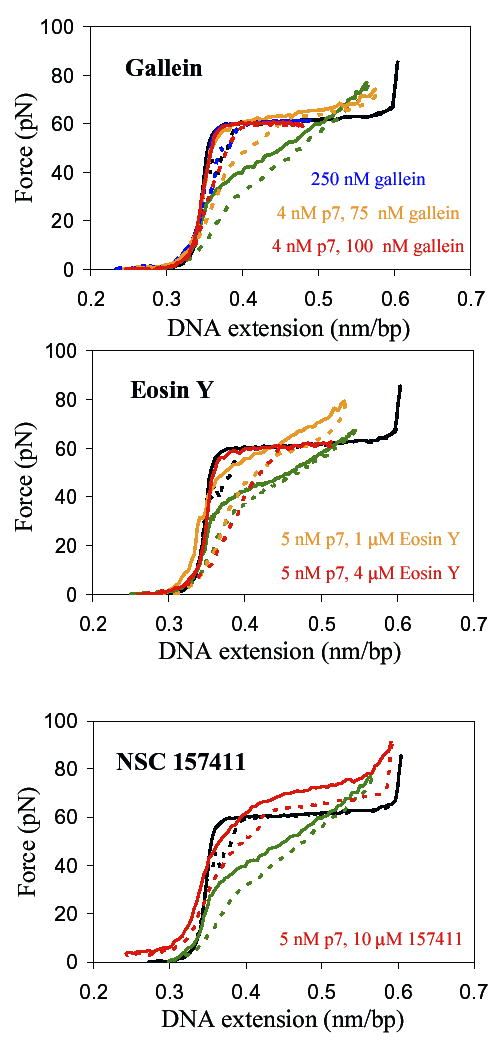
Typical results for single DNA molecule stretch-relax cycles in the absence or presence of HIV-1 NCp7 and some of the tested compounds. Solid lines are for stretching and dashed lines for relaxation. The black lines are for DNA only and the green lines are for DNA with 5 nM NCp7. The concentrations of NCp7 and/or compound are indicated in the legends. None of the compounds alone had any effect on DNA stretch-relax curves.
We classified these compounds based on the minimum amount of compound needed to remove the change in slope introduced by the protein in the helix-coil transition. The compounds were classified as active if the minimum compound concentration was below 250 nM, moderate for a concentration of 250 nM to 500 nM, and inactive when a compound concentration larger than 500 nM was required to remove evidence of protein activity. The concentration at which the activity of NCp7 was found to be negligible was used to obtain an approximate value for the effective dissociation constant of the compound to NCp7. For the rare chemical compounds received from DTP, the values estimated in this work are compared with the dissociation constants published by Stephen et al. (27). With one exception, the values obtained in our experiments were mostly within one order of magnitude of the previously reported values. In the case of some compounds, such as Eosin Y, the hysteresis never disappeared, suggesting that these compounds do not alter the affinity of NC for single-stranded nucleic acids. The effects of these compounds are likely important only at higher concentrations.
Screening the commercially-available gallein-like and fluorescein-like compounds
In initial studies, we purchased all the gallein- and fluorescein-like compounds (Fig. 1 in reference (27)) selected to explain the possible HIV-1 NC antagonism of the most efficient of the previously screened compounds, i.e. the gallein-like compounds. We used the first experimental method, A (see Materials and Methods section), i.e. for each mixture of a given compound and NC concentration, incubated for exactly 30 minutes, we used a different DNA molecule.
Gallein (compound 1, also known as alizarin violet) proved to be very efficient at inhibiting the chaperone activity and DNA binding of NC. The slope diminished to the slope in the absence of protein (Fig. 2a) at 100 nM gallein concentration (Table 1).
Given its previously shown anti-retroviral activity (27), tetrachlorogallein behaved differently than expected in our assay. The effect on the DNA stretching curve was dependent not only on the compound concentration, but also on the incubation time. A minimum time of 2 hours and a compound concentration of 1750 nM were needed to eliminate NC’s chaperone activity. This finding led us to develop and use the second experimental method, B (see Materials and Methods). In the case of tetrachlorogallein, this method proved to be very useful, because the mixture of compound with NC can be already incubated when we capture a new DNA molecule. Since it is preferable to compare the activities of these compounds at the same incubation time, we report in Table 1 only the average experimental result for 30 min incubation, which was 2500 nM.
The next compounds tested were Eosin Y, Erythrosine B, and Tetrachlorofluorescein (compounds 2, 4 and 5). We used method A to screen each of them with a fixed incubation time of 30 minutes. For these compounds, the concentrations at which the slope of the overstretching transition decreased to the no-protein state were higher than for gallein (Table 1). For Eosin Y the hysteresis never decreased to the hysteresis obtained for naked DNA (Fig. 2b), suggesting that the NC protein still has a strong interaction with the ssDNA and cannot unbind while the DNA is relaxed. However, the ability of NC to increase the transition width decreased very much in the presence of Eosin Y, indicating that the helix-destabilization and sequence specific binding of NC were significantly altered, but only at a high compound concentration of 4 μM. The next compound, Erythrosine B, was effective in preventing NC from binding to DNA, but only at more than 1 μM concentration, and the amount of hysteresis was as with no protein. Tetrachlorofluorescein had no effect on the chaperone activity of NC protein in the investigated range. While it is possible that tetrachlorofluorescein is effective at concentrations higher than 10 μM (in 50 mM NaCl), such high concentrations would likely be toxic for the host cells, so they were not tested. In general, we were able to confirm that the fluorescein–like compounds are much less active than the gallein-like compounds, as reported in the previous study (27).
Screening rare compounds provided by DTP
Compound 119889 (tetrabromogallein, despite the structure posted on the DTP website, which is for tetrabromofluorescein, as explained in reference (27)) and compound 119911 were active at very low concentrations and short incubation times, as short as 2 minutes, suggesting that the chemical affinity for NC is very high, the binding is stoichiometric, and the purity and the solubility in water are very high. The concentrations required for preventing NC’s chaperone activity were as low as 10 nM and 25 nM. Compounds 378139 and 119913, with concentration requirements of 50 nM and 100 nM, respectively, can also be classified as active. They are similar to the previous compounds, but are more complex derivatives of gallein or fluorescein. For compound 13778, longer incubation times (>30 min) and minimum concentration of 200 nM was needed to see its full effect on NC. This was somewhat surprising, as a previous bulk study had identified this compound as very active (41). However, in the bulk study, compound 13778 proved to be a different type of anti-HIV antagonist, blocking viral entry into CD4 cells (41), due to its non-specific electrostatic interaction with positive residues. This compound and its analogues can compete with viral gp120 protein of a few HIV-1 strains for binding to human CD4+ cells. This result shows that our single molecule method allows us to detect only compounds that specifically target NC’s chaperone activity. Thus, while 13778 may be a useful anti-HIV antagonist for other reasons, we see only a moderate effect on NC’s chaperone activity.
Compound 122391 can be classified as moderate because it is efficient at a high concentration (500 nM) but short incubation time (30 min). Apparently, compound 157411 removes the change in slope due to NCp7 at a relatively low ratio (500 nM) and at longer incubation time (1 hour), but its behavior is different from the other compounds studied here. The other compounds gradually decrease the slope, maintaining some hysteresis, but the overstretching region resembles the one in the presence or in the absence of NCp7. In contrast, 157411 increases the overstretching force at all concentrations tested (10 nM-10 μM) (Fig. 2c). The aggregation and the hysteresis never disappeared, suggesting that the clustered positive residues and at least one of the stacking residues are not affected. Perhaps this compound can interact only with the first zinc finger motif, reducing NC’s specific binding and helix-destabilization only.
The chemical structures at neutral pH of the compounds used in this study are shown in Fig. 3 (active inhibitors) and Fig. 4 (moderate inhibitors and inactive compounds). The drawings are adaptations of the 2D structures provided by the vendors or found in the DTP database http://dtp.nci.nih.gov.
Figure 3.
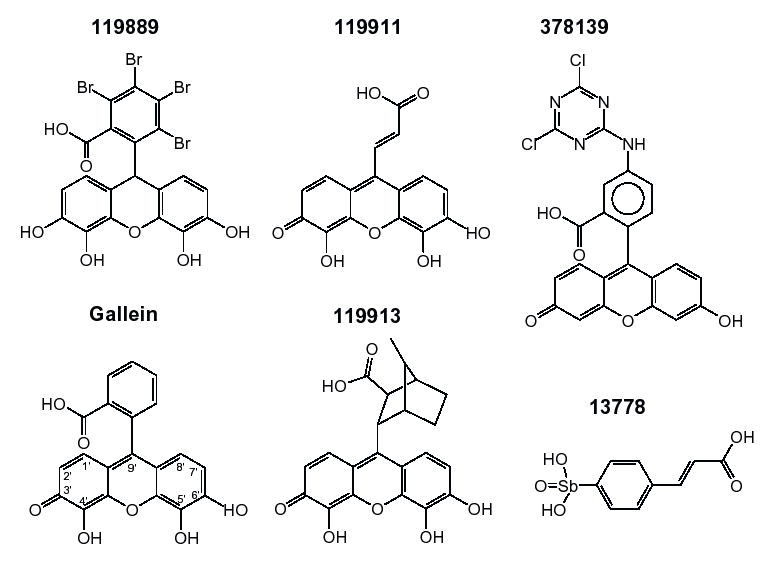
Chemical structures of compounds with high anti-HIV-1 NCp7 activity. For 119889, the structure posted on the NCI website is for tetrabromofluorescein and not for tetrabromogallein, as shown here, which is in fact the active compound. See Results and Table 1 for details.
Figure 4.
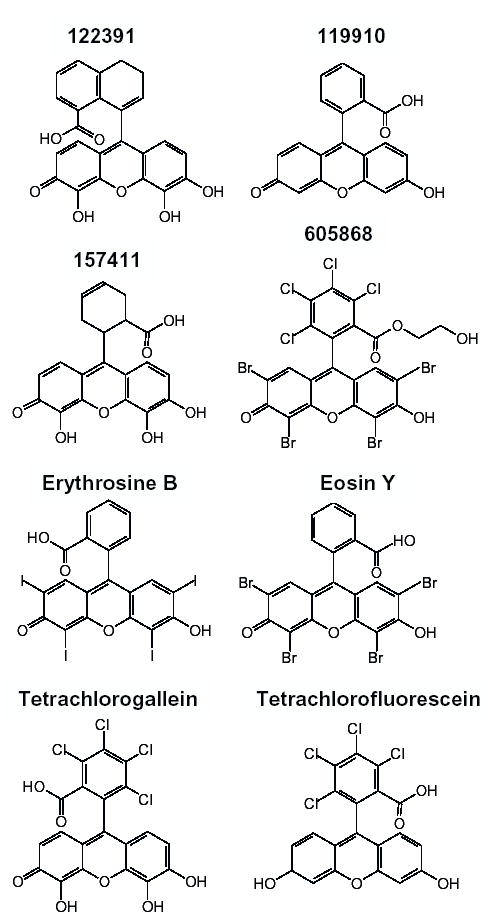
Moderate and inactive compounds. See Results and Table 1 for details. Also note that the two-dimensional representation for 605868 on the DTP web site is not in agreement with the three-dimensional representation or the chemical formula, which is what is shown here.
Secondary effects of HIV-1 NCp7-compound mixtures in binding to dsDNA
In the presence of NCp7 only, a small force is required to stretch dsDNA even at extensions well below the contour length of the molecule, i.e. F<15 pN (9, 31). In the presence of a compound:NC mixture, when NC is still active, this effect is still seen, suggesting that NC can bind to dsDNA as well. Another effect often observed is an increase in the midpoint of the helix-coil transition, suggesting that the NC sequence-specific binding to ssDNA is reduced as compared to dsDNA affinity. This effect cannot be assigned to binding of compound to naked DNA since control curves in the presence of high concentrations of compound showed no binding to both ss and ds DNA, reflected in the fact that the stretch-relax curves are identical to those measured for naked DNA. We also found that the bound NCp7 cannot be removed from DNA by just adding compound solution. This process may require a longer time (on the order of hours), despite the very low concentration of NCp7. NC dissociation from DNA is usually observed only after prolonged rinsing with buffer, when the concentration of NC becomes extremely small.
Discussion
In this study, single DNA molecule stretching experiments were used to determine if some chemical compounds found as possible HIV-1 inhibitory agents targeting NC are also efficient in lowering the chaperone activity of HIV-1 nucleocapsid protein NCp7 as observed in our single DNA molecule experiments. The compounds were chosen from a previous study (27) which focused on finding compounds with reversible non-covalent, non-redox, and non-chelating activity against HIV-1 NCp7. The gallein-like compounds were found to meet all criteria and the mechanism of antagonism was inferred from molecular modeling (27). Our previous results regarding the chaperone activity of NCp7 are consistent with the results of in vitro and in vivo assays (7, 9, 15). In addition, we can independently select only compounds that do not bind to dsDNA or ssDNA. Therefore, this single molecule method can complement the in vivo and in vitro studies in the process of identifying compounds that target NC.
Adding any compound after DNA titration with NCp7 had no effect on the chaperone activity of NC, confirming the fact that the compounds, which are small molecules, cannot compete for NC binding with the large λ-DNA molecule, which is a highly charged polyelectrolyte. It also indicates that NC remains bound to DNA even in the presence of the compound. This finding suggests that the compound has an antagonist effect only against free NC, and may therefore only be effective at reducing viral infection on a longer time scale. These compounds will bind free NC as it is made available during viral replication, thus having a gradual anti-HIV-1 effect.
The low compound concentrations required for 119889 and 119911 (10 nM and 25 nM, respectively) suggest that these compounds bind stoichiometrically and with high affinity to NC, interfering with all of the critical binding mechanisms, thus inhibiting NC’s chaperone activity. Other factors that could differentiate these compounds from the others are their possible high purity and solubility in water and in the experimental buffer.
Compound 13778 appears to be effective in strongly reducing the non-sequence specific binding of NC to ssDNA, since it reduces the hysteresis but not the dramatic increase in the slope of the overstretching-transition associated with the sequence-specific binding. This is probably the reason for which the concentration required to completely eliminate NCp7 binding to ssDNA is higher than for the most effective compounds (119889 and 119911). The moderate and nonspecific activity observed here is consistent with the recent results of the full study by Yang et al. (41), who showed that the activities of this compound are strongly dependent upon electrostatic interactions. Thus, while this compound is effective at inhibiting HIV-1 replication, it does not specifically target NC. The moderate activity observed in our experiments illustrates the fact that our method is really a probe for anti-NC chaperone activity, rather than for compounds that simply inhibit NC binding to DNA.
Gallein is poorly soluble in aqueous solutions and it is light-sensitive. It is used to identify rare metals due to its property to form ternary-complexes with divalent metals (36, 42). It is possible that in our experiments gallein interacts with the zinc fingers and lowers their contribution relative to that of the basic residues, since the stretching curves were slightly higher than with NCp7 only (Fig. 2a). However, in the previous work (27), all compounds were probed for Zn ejector properties using the Newport Green assay and Zn ejection activity was not detected.
Tetrachlorogallein, which also forms extractable complexes with divalent metals, a property which allows its use in the separation of rare metals (36), requires a higher incubation time (30 min-3 hours), similar to that found for the selective Zn ejectors studied by Huang et al. (26). It is unlikely that this compound inhibits the chaperone activity of NC by ejecting Zn. These long incubation times are probably due to the low binding energy characterizing the interaction between these compounds and NCp7. The apparent high compound concentrations and long incubation times required for tetrachlorogallein are probably due to the low solubility of the purchased products. It is possible that only high concentrations of compound can completely block the interactions between NC and ssDNA, either by the improved association to NC, given the relatively weak binding of these compounds to NC (KD = 10−6 to 10−7 M), or by creating clusters of compounds around NC molecules. For some compounds, a possible low content of active compound present in the purchased product, particularly as selected from the Rare Chemical Library, may contribute to the requirements of high compound concentrations (27).
As expected, the fluorescein-like compounds investigated here did not prevent NC binding to ssDNA at reasonable concentrations, suggesting that the binding sites of NCp7 for ssDNA are still free, even at very high compound concentrations. Regarding Erythrosine B, it is possible that, due to its rather gallein-like structure, in which the two critical OH groups are substituted with I moieties, it has weak electrostatic interactions with the basic residues of NC. Although Erythrosine B differs from Eosin Y only in the halogen ions (I substituted for Br), it seems to have about three times higher affinity for NC than Eosin Y. Tetrachlorofluorescein was totally inactive in the investigated range. We consider that compound 157411 is inactive, given its unusual effect on DNA stretching and relaxation curves.
The best NC inhibitors were 119889 and 119911. Gallein, 378139 and 119913 are also possible active NC inhibitors. The new compound 13778 can be considered a prospective inhibitor of NCp7 in light of our single DNA molecule experiments. Surprising results were obtained for compounds 157411 and 378139. The high compound concentrations required for 157411 inhibition of NCp7 activity and the unusual effect on the DNA stretching-relaxing curves induced by NCp7 when incubated with this compound, suggests that NC stabilizes DNA in the presence of this compound. Although compound 157411 inhibits NC’s helix-destabilization activity, which is an essential characteristic of effective nucleic acid chaperone activity (30, 43), we classify it as inactive because of the high compound concentration required to observe this effect. In addition to supporting the conclusions of the earlier study (27), we are also able to identify the specific characteristic of protein-DNA interaction that is altered by the compound, which in this case is its effect on NC’s specific binding to single-stranded nucleic acids and duplex destabilization. For example, in the case of 378139, the apparent dissociation constant measured in this work turned out to be much lower than the value determined previously (27). This compound was active in the in vivo anti-HIV assay, which confirms our predictions. All values of the dissociation constants determined in this study are in line with the general trend that the most active compounds are the ones with the lowest dissociation constant.
From these studies we can identify several common features of the most active compounds. A first look at their chemical structures (Fig. 3) implies that the most effective NC inhibitors have the hydroxyl moieties of gallein, and a fused three-ring aromatic structure. These features seem necessary but not sufficient to ensure a high affinity to NCp7, given the low affinity of some other compounds that share these features yet do not inhibit NCp7 (e.g. 122391, 157411, and tetrachlorogallein). The other side chains can consist of small groups or ions, but symmetry is very important. The 4′ and 5′-hydroxyl groups or halogen moieties may form hydrogen bonds with important residues of NCp7 near the zinc fingers (18). This would disrupt NC’s specific interactions with single-stranded nucleic acids, but may not strongly affect electrostatic binding to dsDNA. The amount of hysteresis persistent during titrations, comparable to that produced with NCp7 only, suggests that complexes formed of one NCp7 molecule and one or more molecules of chemical compound can still bind to ssDNA, sequence-specific or not, and the unbinding rate may be reduced. As we learned from data collected for other retroviral proteins, HIV-1 NCp7 has the highest force transition width and the smallest hysteresis, which imply that its high affinity for single-stranded nucleic acids (and duplex destabilization), sequence specificity, and high mobility between different binding modes, lead to its enhanced chaperone activity (to be published elsewhere). It is likely that in the presence of Eosin Y only the specific binding and electrostatic interaction of NC are affected but the aromatic residues can still be stacked within the nucleotides. For tetrachlorofluorescein, the hysteresis is comparable to that produced with NCp7 only, but this compound is inactive in the investigated range, i.e. it does not reduce the slope at all. Finally, for 157411 the high stretching force and the hysteresis observed indicate duplex-stabilization, suggesting that there is a reduction of ssDNA-binding relative to dsDNA-binding of NC in the presence of this compound. One possible general explanation of the effect of all these compounds on NC’s binding to DNA could be that the aromatic ring structures of the compounds (Fig. 4), which we showed are unable to intercalate DNA directly, might interfere with NC’s ability to stack its aromatic residues between bases, but that this capability varies, depending on the specific interactions that can be formed with these residues.
The main purpose of the present work is to show that the single molecule technique used herein can be used in the first stages of drug development as a reliable complement to the high-throughput assays. The amounts of protein and compounds required are low (1 μg and 1–5 mg, respectively), suggesting that this technique can be useful when candidate compounds are available in very low amounts, such as after initial synthesis. Most important, in addition to using this method for screening antagonists of all retroviral NC proteins, one can investigate the mechanism by which these antagonists alter NC’s nucleic acid chaperone activity. Thus, while this technique cannot substitute for initial high-throughput screening assays, the additional information about the mechanism by which the drugs alter NC’s chaperone activity can be used to further refine the types of drugs tested. In particular, in contrast to previous bulk methods, this single molecule method may be used to distinguish between simple competitive inhibitors and drugs that specifically target NC’s chaperone activity. Thus, single molecule force spectroscopy is a powerful new technique for determining the ability of any chemical compound to inhibit the nucleic acid chaperone activity of a protein at the single molecule level.
Acknowledgments
We thank Dr. Robert Schultz (DTP at NCI) for providing the rare compounds, Prof. Karin Musier-Forsyth (Univ. of Minnesota), Dr. Ioulia Rouzina (Univ. of Minnesota), and Dr. Irina E. Catrina (Univ. of Massachusetts, Medical School) for helpful discussions. Funding for this project was provided by NIH (GM 072462), NSF (MCB-0238190), and the Research Corporation, to MCW. This work was supported in part by funds from NCI under contract No. NO1-CO-12400 with SAIC-Frederick, Inc.
References
- 1.Berkowitz R, Fisher J, Goff SP. RNA packaging. Curr Top Microbiol & Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 2.Rein A, Henderson LE, Levin JG. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends in Biochem Sci. 1998;23:297–301. doi: 10.1016/s0968-0004(98)01256-0. [DOI] [PubMed] [Google Scholar]
- 3.Darlix JL, Lapadat-Tapolsky M, de Rocquigny H, Roques BP. First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J Mol Biol. 1995;254:523–537. doi: 10.1006/jmbi.1995.0635. [DOI] [PubMed] [Google Scholar]
- 4.Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. [PubMed] [Google Scholar]
- 5.Levin JG, Guo J, Rouzina I, Musier-Forsyth K. Progress in Nucleic Acid Research and Molecular Biology (Elsevier, Ed.) Vol. 80. Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Wu T, Anderson J, Kane BF, Johnson DG, Gorelick RJ, Henderson LE, Levin JG. Zinc Finger Structures in the Human Immunodeficiency Virus Type 1 Nucleocapsid Protein Facilitate Efficient Minus- and Plus-Strand Transfer. J Virol. 2000;74:8980–8988. doi: 10.1128/jvi.74.19.8980-8988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo J, Wu T, Kane BF, Johnson DG, Henderson LE, Gorelick RJ, Levin JG. Subtle Alterations of the Native Zinc Finger Structures Have Dramatic Effects on the Nucleic Acid Chaperone Activity of Human Immunodeficiency Virus Type 1. Nucleocapsid Protein J Virol. 2002;76:4370–4378. doi: 10.1128/JVI.76.9.4370-4378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julian N, Demene H, Morellet N, Maigret B, Roques BP. Replacement of His23 by Cys in a zinc finger of HIV-1 NCp7 led to a change in 1H NMR-derived 3D structure and to a loss of biological activity. FEBS Lett. 1993;331:43–48. doi: 10.1016/0014-5793(93)80294-5. [DOI] [PubMed] [Google Scholar]
- 9.Williams MC, Gorelick RJ, Musier-Forsyth K. Specific zinc finger architecture required for HIV-1 nucleocapsid protein's nucleic acid chaperone function. Proc Natl Acad Sci USA. 2002;99:8614–8619. doi: 10.1073/pnas.132128999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You JC, McHenry CS. Human immunodeficiency virus nucleocapsid protein accelerates strand transfer of the terminally redundant sequences involved in reverse transcription. J Biol Chem. 1994;269:31491–31495. [PubMed] [Google Scholar]
- 11.Hargittai MRS, Gorelick RJ, Rouzina I, Musier-Forsyth K. Mechanistic insights into the kinetics of HIV-1 Nucleocapsid Protein-facilitated tRNA annealing to the Primer Binding Site. J Mol Biol. 2004;337:951–968. doi: 10.1016/j.jmb.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Roques BP, Morellet N, de Rocquigny H, Demene H, Schueler W, Jullian N. Structure, biological functions and inhibition of the HIV-1 proteins Vpr and NCp7. Biochimie. 1997;79:673–80. doi: 10.1016/s0300-9084(97)83501-8. [DOI] [PubMed] [Google Scholar]
- 13.De Guzman RN, Wu ZR, Stalling CC, Pappalardo L, Borer PN, Summers MF. Structure of the HIV-1 Nucleocapsid Protein Bound to the SL3 psi-RNA Recognition. Element Science. 1998;279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- 14.Amarasinghe GK, Guzman RND, Turner RB, Chancellor KJ, Wu ZR, Summers MF. NMR Structure of the HIV-1 Nucleocapsid Protein Bound to Stem-Loop SL2 of the Psi-RNA Packaging Signal. Implications for Genome Recognition. J Mol Biol. 2000;301:491–511. doi: 10.1006/jmbi.2000.3979. [DOI] [PubMed] [Google Scholar]
- 15.Gorelick RJ, Chabot DJ, Rein A, Henderson LE, Arthur LO. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J Virol. 1993;67:4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dannull J, Surovoy A, Jung G, Moelling K. Specific binding of HIV-1 nucleocapsid protein to PSI RNA in vitro requires N-terminal zinc finger and flanking amino acid residues. EMBO J. 1994;13:1525–1533. doi: 10.1002/j.1460-2075.1994.tb06414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanchou V, Decimo D, Pechoux C, Lener D, Rogemond V, Berthoux L, Ottmann M, Darlix JL. Role of the N-terminal zinc finger of human immunodeficiency virus type 1 nucleocapsid protein in virus structure and replication. J Virol. 1998;72:4442–4447. doi: 10.1128/jvi.72.5.4442-4447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmalzbauer E, Strack B, Dannull J, Guehmann S, Moelling K. Mutations of Basic Amino Acids of NCp7 of Human Immunodefficiency Virus Type 1 Affect RNA Binding In Vitro. J Virol. 1996;70:771–777. doi: 10.1128/jvi.70.2.771-777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbaneja MA, Kane BP, Johnson DG, Gorelick RJ, Henderson LE, Casas-Finet JR. Binding Properties of the Human Immunodeficiency Virus Type 1 Nucleocapsid Protein p7 to a Model RNA: Elucidation of the Structural Determinants for Function. J Mol Biol. 1999;287:59–75. doi: 10.1006/jmbi.1998.2521. [DOI] [PubMed] [Google Scholar]
- 20.Buckman JS, Bosche WJ, Gorelick RJ. Human immunodeficiency virus-type 1 nucleocapsid Zn2+-fingers are required for efficient reverse transcription, initial integration processes, and protection of newly synthesized viral DNA. J Virol. 2003;77:1469–1480. doi: 10.1128/JVI.77.2.1469-1480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruceanu M, Urbaneja MA, Hixson CV, Johnson DG, Datta SA, Fivash MJ, Stephen AG, Fisher RJ, Gorelick RJ, Casas-Finet JR, Rein A, Rouzina I, Williams MC. Nucleic acid binding and chaperone properties of HIV-1 Gag and nucleocapsid proteins. Nucleic Acids Res. 2006;34:593–605. doi: 10.1093/nar/gkj458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice WG, Turpin JA. Virus-encoded zinc-finger as targets for antiviral chemotherapy. Rev Med Virol. 1996;6:187–196. doi: 10.1002/(SICI)1099-1654(199612)6:4<187::AID-RMV176>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of Human Immunodeficiency Virus Type 1 Infectivity with Preservation of Conformational and Functional Integrity of Virion Surface Proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonnell NB, De Guzman RN, Rice WG, Turpin JA, Summers MF. Zinc ejection as a new rationale for the use of cystamine and related disulfide-containing antiviral agents in the treatment of AIDS. J Med Chem. 1997;40:1969–1976. doi: 10.1021/jm970147+. [DOI] [PubMed] [Google Scholar]
- 25.Hathout Y, Fabris D, Han MS, Sowder RCn, Henderson LE, Fenselau C. Characterization of intermediates in the oxidation of zinc fingers in human immunodeficiency virus type 1 nucleocapsid protein P7. Drug Metab Dispos. 1996;24:1395–1400. [PubMed] [Google Scholar]
- 26.Huang M, Maynard A, Turpin JA, Graham L, Janini GM, Covell DG, Rice WG. Anti-HIV agents that selectively target retroviral nucleocapsid protein zinc fingers without affecting cellular zinc finger proteins. J Med Chem. 1998;41:1371–1381. doi: 10.1021/jm9708543. [DOI] [PubMed] [Google Scholar]
- 27.Stephen AG, Worthy KM, Towler E, Mikovits JA, Sei S, Roberts P, Yang Q-e, Akee R, Klausmeyer P, McCloud TG, Henderson LE, Rein A, Covell DG, Currens M, Shoemaker RH, Fisher RJ. Identification of HIV-1 nucleocapsid protein: nucleic acid antagonists with cellular anti-HIV activity. Biochem Biophys Res Comm. 2002;296:1228–1237. doi: 10.1016/s0006-291x(02)02063-6. [DOI] [PubMed] [Google Scholar]
- 28.Fisher RJ, Rein A, Fivash M, Urbaneja MA, Casas-Finet JR, Medaglia M, Henderson LE. Sequence-Specific Binding of Human Immunodeficiency Virus Type 1 Nucleocapsid Protein to Short Oligonucleotides. J Virol. 1998;72:1902–1909. doi: 10.1128/jvi.72.3.1902-1909.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vuilleumier C, Bombarda E, Morellet N, Gerard D, Roques BP, Mely Y. Nucleic Acid Sequence Discrimination by the HIV-1 Nucleocapsid Protein NCp7: A Fluorescence Study. Biochemistry. 1999;38:16816–16825. doi: 10.1021/bi991145p. [DOI] [PubMed] [Google Scholar]
- 30.Urbaneja MA, Wu M, Casas-Finet JR, Karpel RL. HIV-1 nucleocapsid protein as a nucleic acid chaperone: spectroscopic study of its helix-destabilizing properties, structural binding specificity, and annealing activity. J Mol Biol. 2002;318:749–764. doi: 10.1016/S0022-2836(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 31.Williams MC, Rouzina I, Wenner JR, Gorelick RJ, Musier-Forsyth K, Bloomfield VA. Mechanism for nucleic acid chaperone activity of HIV-1 nucleocapsid protein revealed by single molecule stretching. Proc Natl Acad Sci USA. 2001;98:6121–6126. doi: 10.1073/pnas.101033198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin SL, Cruceanu M, Branciforte D, Li PW-l, Kwok SC, Hodges RS, Williams MC. LINE-1 Retrotransposition Requires the Nucleic Acid Chaperone Activity of the ORF1 Protein. J Mol Biol. 2005;348:549–561. doi: 10.1016/j.jmb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Lee BM, De Guzman RN, Turner BG, Tjandra N, Summers MF. Dynamical behavior of the HIV-1 nucleocapsid protein. J Mol Biol. 1998;279:633–649. doi: 10.1006/jmbi.1998.1766. [DOI] [PubMed] [Google Scholar]
- 34.Conn HJ. Biological Stains. Sigma Chemical Company; Milwaukee, WI: 1990. [Google Scholar]
- 35.Floyd GJ. The Sigma-Aldrich Handbook of Stains. Dyes and Indicators, Aldrich Chemical Company, Inc.; Milwaukee, WI: 2003. [Google Scholar]
- 36.Mori I, Fujita Y, Fujita K, Koshiyama Y, Tanaka T. The spectrophotometric determination of trace vanadium with 3,4,5,6-tetrachlorogallein. Bulletin of the Chemical Society of Japan. 1986;59:3997–3999. [Google Scholar]
- 37.Mihailovic A, Vladescu I, McCauley M, Ly E, Williams MC, Spain EM, Nunez ME. Exploring the Interaction of Ruthenium(II) Polypyridyl Complexes with DNA Using Single-Molecule. Techniques Langmuir. 2006;22:4699–709. doi: 10.1021/la053242r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vladescu ID, McCauley MJ, Rouzina I, Williams MC. Mapping the phase diagram of single DNA molecule force-induced melting in the presence of ethidium. Phys Rev Lett. 2005;95:158102. doi: 10.1103/PhysRevLett.95.158102. [DOI] [PubMed] [Google Scholar]
- 39.Rouzina I, Bloomfield VA. Force-induced melting of the DNA double helix - 1. Thermodynamic analysis. Biophys J. 2001;80:882–893. doi: 10.1016/S0006-3495(01)76067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith SB, Cui YJ, Bustamante C. Overstretching B-DNA: The elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996;271:795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- 41.Yang Q-e, Stephen AG, Adelsberger WJ, Roberts P, Zhu W, Currens M, Feng YX, Crise BJ, Gorelick RJ, Rein AR, Fisher RJ, Shoemaker RH, Sei S. Discovery of Small-Molecule HIV-1 Entry Inhibitors that Target the gp120-binding domain of CD4. J Virol. 2005;79:6122–6133. doi: 10.1128/JVI.79.10.6122-6133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai R, Wang L, Tang Ha, Wu J, Ai J. Synthesis and pharmacological action of the solid complexes of rare earth (III) with alizarin violet. Lanzhou Daxue Xuebao. Ziran Kexueban. 1996;32:68–72. [Google Scholar]
- 43.Tsuchihashi Z, Brown PO. DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1994;68:5863–5870. doi: 10.1128/jvi.68.9.5863-5870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


