Abstract
Trospium chloride is a quaternary ammonium compound, which is a competitive antagonist at muscarinic cholinergic receptors. Preclinical studies using porcine and human detrusor muscle strips demonstrated that trospium chloride was many-fold more potent than oxybutynin and tolterodine in inhibiting contractile responses to carbachol and electrical stimulation. The drug is poorly bioavailable orally (< 10%) and food reduces absorption by 70%– 80%. It is predominantly eliminated renally as unchanged compound. Trospium chloride, dosed 20 mg twice daily, is significantly superior to placebo in improving cystometric parameters, reducing urinary frequency, reducing incontinence episodes, and increasing urine volume per micturition. In active-controlled trials, trospium chloride was at least equivalent to immediate-release formulations of oxybutynin and tolterodine in efficacy and tolerability. The most problematic adverse effects of trospium chloride are the anticholinergic effects of dry mouth and constipation. Comparative efficacy/tolerability data with long-acting formulations of oxybutynin and tolterodine as well as other anticholinergics such as solifenacin and darifenacin are not available. On the basis of available data, trospium chloride does not appear to be a substantial advance upon existing anticholinergics in the management of urge urinary incontinence.
Keywords: urge incontinence, trospium, anticholinergic, overactive bladder
Introduction
Urge urinary incontinence (UUI), the most frequent type of urinary incontinence (UI), has as typical symptoms incontinence associated with urinary frequency (> 8 micturitions/day) and urgency (sudden, strong desire to urinate). Nighttime frequency (nocturia) and nocturnal incontinence (enuresis) disrupt sleep. Although the etiology is known in some cases (eg, neurologic diseases such as stroke, Parkinson’s disease, multiple sclerosis, or spinal cord injury or bladder outlet obstruction due to benign prostatic hyperplasia or prostate cancer), in most cases the cause is unknown. UUI and stress UI frequently coexist as mixed UI (Rovner et al 2002).
The most effective agents in suppressing inappropriate bladder contractions, enhancing bladder storage, and relieving UUI signs and symptoms are the anticholinergic/antispasmodic drugs (Rovner et al 2002; Herbison et al 2003), primarily oxybutynin and tolterodine. Other agents, such as tricyclic antidepressants, propantheline, dicyclomine, scopolamine, and flavoxate, are less effective, no safer, or have been inadequately studied and are generally not recommended (Rovner et al 2002). Propiverine is an anticholinergic also used in managing UUI but it is not available in many countries.
Unfortunately, even oxybutynin and tolterodine are only moderately effective in UUI (Herbison et al 2003), and their systemic (non-urinary tract) anticholinergic effects are bothersome, compromise adherence, and may limit efficacy by limiting dose escalation. The search for a “uroselective” anticholinergic agent has been intense within the pharmaceutical industry.
The purpose of this paper is to profile trospium chloride. Trospium chloride has been available for many years in Europe, manufactured by Madaus AG (Cologne, Germany) under the tradename Spasmo-lyt® and is also available as Uroplex® and SpasmoUrgenin®. This agent has recently been approved by the US Food and Drug Administration (FDA) for the treatment of overactive bladder (symptoms of urinary frequency, urgency, and UUI) under the tradename Sanctura® (Odyssey Pharmaceuticals, East Hanover NJ and Indevus Pharmaceuticals, Lexington, MA, USA).
A MEDLINE/PUBMED search was conducted to identify pertinent articles in the English language. Additional references were obtained from the bibliographies of these articles. In addition, proceedings of the Incontinence Society, European Association of Urology, American Urological Association, and American College of Obstetrics and Gynecology meetings were reviewed for relevant abstracts. Data over the time period of 1966 through June 2004 were reviewed.
Chemistry
Trospium chloride (Figure 1), also known as azonia-3-α-benziloyloxy-8-spiro-1′-pyrrolidinium chloride, has the molecular formula C25H30NO3Cl, and a molecular weight of 427.97. Its chemical name is spiro[8-azoniabicyclo[3,2,1]octane-8,1′-pyrrolidinium]-3-[(hydroxydiphenylacetyl)-oxy]chloride(1α,3β,5α)-(9CI). Solubility in water exceeds 50 mg per mL at room temperature while in light mineral oil it is 9.2 × 10–3 mg/mL (Langguth et al 1997). Its log partition coefficient between n-octanol and buffer at pH 7.4 is –1.22 (Langguth et al 1997). Synthesis and pharmacological activity of this compound were first described in 1966 (German language literature).
Figure 1.
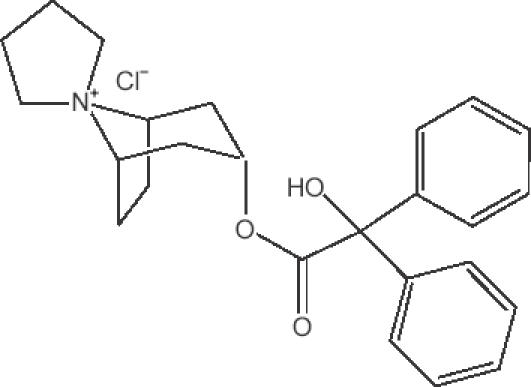
Chemical structure of trospium chloride.
Pharmacodynamics
Ex vivo studies
Ex vivo studies have evaluated the effect of trospium chloride on the activity of porcine and human detrusor muscle strips. Parameters studied included EC50 (bath concentration inducing 50% reversion of carbachol-induced tension), Rmax (% relaxation at final drug concentration in bath), IC50 (bath concentration causing 50% inhibition of maximum contractile response to electrical field stimulation), and MI (% inhibition of contraction amplitude at final drug concentration in bath). For porcine tissue, trospium chloride was significantly more potent than oxybutynin (EC50 values of 0.006 and 25 μmol/L and Rmax values of 100 [at 0.1 μmol/L] and 76.2 ± 8%, respectively). Corresponding values for EC50 and Rmax in human tissue were 0.003 and 10 μmol/L and 86 ± 13 (at 0.1 μmol/L) and 79 ± 20% (both tissues, p < 0.05) (Uckert et al 1998). In human tissue, EC50 and Rmax values for trospium chloride, oxybutynin, and tolterodine were 0.003, 10, and ≤ 1.0 μmol/L, respectively and 86 ± 13, 50 ± 7, and 70 ± 8%, respectively. Corresponding IC50 and MI values were 0.05, 10, and > 10 μmol/L and 80 ± 17, 53 ± 7, and 40 ± 16% (trospium chloride vs comparators, p < 0.05). In the carbachol and electrical field stimulation protocols, trospium chloride produced significant changes from control at all tested bath concentrations (1, 0.1, 0.01, 0.001, 0.0001, and 0.00001 μmol/L) (Uckert et al 2000). The effects of trospium chloride were dose-dependent in both ex vivo studies (Uckert et al 1998, 2000).
In vivo (animal) studies
The effect of trospium chloride on gastrointestinal (GI) motility in dogs was studied after single 0.1 and 0.5 mg/kg intravenous (IV) doses. At the 0.5 mg/kg dose level, complete inhibition of gastric and intestinal motor activity occurred for up to 0.5 h post-dose, the gastric and jejunal motility indices (MoI) were substantially reduced, and colonic contractions and the colonic MoI fell for 2.5 h post dose (Hatchet et al 1986).
In vivo (human) studies
In a placebo-controlled, crossover, double-blind trial conducted in 12 healthy human volunteers, the effect of trospium chloride on 24-h jejunal motility was evaluated. Trospium chloride 15 mg orally thrice daily significantly prolonged the duration of irregular contractile activity after meals (from mean 324 to 368 min, p < 0.02) and decreased its contraction frequency (from 2.24 to 1.08/min, p < 0.001) and amplitude (from 26.5 to 20.3 mm Hg, p < 0.001). In the fasting state, the cycle length of the migrating motor complex was significantly prolonged (from 77 to 116 min, p < 0.01), due primarily to an extended phase I (motor quiescence) (from 42 to 78 min, p < 0.025). Phase III was significantly shortened (from 7.3 to 3.8 min, p < 0.005), and showed a slower aboral migration velocity (from 8.4 to 4.7 cm/min, p < 0.005). Clustered contractions were significantly less frequent during postprandial (from 42 to 14 per 24 h, p < 0.01) and fasting periods (from 11 to 4 per 24 h, p < 0.01). Runs of clustered contractions were abolished by the drug. In summary, trospium chloride significantly reduced jejunal motor activity in healthy volunteers (Schmidt et al 1994).
The effects of single 0.2, 0.5, 1, and 1.5 mg IV doses of trospium chloride on gallbladder contractility were evaluated in 6 female volunteers using a double-blind, crossover design. Trospium chloride produced a dose-dependent inhibition of gallbladder contraction induced by a fat stimulus and measured using sodium iopodate (p < 0.0001). The two highest doses virtually abolished contractility (Matzkies et al 1992). Nine volunteers participated in a randomized, crossover, placebo-controlled trial evaluating the effect of single IV doses of placebo, 1.2 mg trospium chloride, and 5 mg biperiden on esophageal motility. Trospium chloride significantly reduced the amplitude of primary peristaltic pressure waves (from mean 67 mm Hg on placebo to 17 mm Hg, p < 0.01) but not their duration. The frequency of failed primary peristalsis was significantly increased by the drug (from 0 on placebo to 10%, p < 0.05) (unit = % of 120 water swallows). The percentage of secondary contractions elicited by air distension was significantly reduced by the drug (from 95% on placebo to 60%, p < 0.01). Trospium chloride also significantly increased the latency to onset of secondary contractions (from 7 s on placebo to 11 s, p < 0.05) and reduced their amplitude (from 65 mm Hg on placebo to 25 mm Hg, p < 0.01). The effects of trospium chloride were statistically indistinguishable from those of biperiden in all comparisons. Lastly, trospium chloride had no effect on esophageal evoked potentials. In summary, trospium chloride impairs esophageal motility (Pehl et al 1998).
Lastly, the effect of trospium chloride on GI motility was evaluated in 33 healthy volunteers participating in a double-blind, crossover, placebo-controlled trial (gallbladder N = 11, gastric emptying N = 12, gastoesophageal reflux and orocecal transit time N = 10). The 4 treatments were placebo and trospium chloride 10, 15, and 20 mg orally at 0600, 1400, and 2200 the day prior to study and at 0600 on the day of the study. Gallbladder ejection fractions were significantly reduced by 10 and 20 mg doses compared with placebo (p < 0.025 and p < 0.01, respectively), while the effects of the 10 and 20 mg doses were not significantly different. Gastric emptying was significantly delayed by the 15 mg dose compared with placebo (p < 0.02, no change in volume suggests an antisecretory response to the drug). The fractional time of esophageal pH below 4 as a percentage of the 24-h study period was significantly increased by the 15-mg dose compared with placebo (p < 0.05), as was the orocecal transit time (p < 0.001) (Pfeiffer et al 1993).
Pharmacokinetics
Healthy volunteers
The pharmacokinetic parameters of trospium chloride have been extensively reviewed recently (Guay 2003). Table 1 illustrates mean data after drug administration by the oral and IV routes. Trospium chloride is absorbed slowly after oral dosing, with mean times to peak plasma concentration of 5–6 h in healthy young volunteers and 3.5 h in healthy elderly volunteers. Using urinary excretion data, the mean ± SD oral bioavailability is 2.91 ± 0.90% (using trospium chloride data only) and 3.25 ± 1.02% (using total compound data). Mean (range) absolute bioavailability of a 20 mg dose based on serum concentration data, is 9.6% (4.0%–16.1%) (Anonymous 2004). Food reduces oral drug bioavailability by 70%–80%. Absorption after single intravesical (into the bladder) doses of 15 and 30 mg is essentially negligible.
Table 1.
Mean (± SD) pharmacokinetic parameters of trospium chloride after administration by the intravenous and oral routes (after Guay 2003)
| Populations | N | Dose (mg) | Route | Frequency | Cmax (mg/L) | tmax (h) | AUC (mg/L˙h) | t½ | CL (mL/min) |
|---|---|---|---|---|---|---|---|---|---|
| Healthy younger men | 6 | 0.5 | IV | 1 dose | ND | ND | 7.0 ± 2.2 | 1.6 ± 0.6 | 1405 ± 830a |
| 807 ± 391b | |||||||||
| Healthy elderly men | 18 | 20 | PO | 1 dose | 1.4 ± 88%c | 3.5 ± 77%c | 14.0 ± 79%c | 10.2 ± 47%c | ND |
| 20 | PO | q12 h × 9 | doses 1.9 ± 47%c | 3.2 ± 71%c | 15.0 ± 38%c | 12.7 ± 32%c | ND |
Total body clearance.
Renal clearance.
Percentage coefficient of variation.
Abbreviations: AUC, area under the plasma concentration-versus-time curve; CL, clearance; Cmax, peak plasma concentration; d, days; IV, intravenous; ND, not done; PO, oral; q12h, every 12 hours; tmax, time to Cmax; t ½, terminal disposition half-life.
Studies have been done using animal models to evaluate the process of absorption of trospium chloride and methods to augment it. Absorption of the drug across the intestinal epithelium is complex, involving P-glycoprotein-mediated secretion and saturable binding to intestinal mucus (Langguth et al 1997). Limited permeability across the epithelial cell layer accounts for the low bioavailability. Use of water/oil microemulsions or cyclodextrin did not enhance, and actually reduced, oral bioavailability (Langguth et al 1997). However, oral bioavailability was enhanced by ion pairing using N-alkylsulfates (6- or 7-carbon chain is optimal) or N-alkylsulfonates (7- or 9-carbon chain is optimal) (Langguth et al 1997). In addition, ion pairing using nonylsulfonate and heptylsulfonate may allow use of a transdermal formulation (transepidermal flux is increased by 7.1 ± 5.7-fold and 13.5 ± 23.0-fold, respectively, over trospium chloride alone) (Langguth et al 1987).
Trospium chloride is 50%–85% plasma protein bound. The mean ± SD apparent volume of distribution is 395 ± 140 L (Anonymous 2004). There are no published data regarding the penetration of the drug into the central nervous system. Renal excretion accounts for approximately 70% of drug clearance. Approximately 80%, 10%, and < 5% of urinary excretion is accounted for by parent compound, spiroalcohol metabolite, and hydrolysis/oxidation products, respectively. Cumulative 48-h urinary excretion after a single 0.5 mg IV dose was 278 ± 59 μg of parent compound and 10 ± 4 μg of the spiroalcohol metabolite (spiroalcohol accounts for a mean of 7.1% of total urinary excretion, range 3.2%–10.9%). Corresponding values after a single oral 10 mg dose were 158 ± 43 μg and 16 ± 12 μg (spiroalcohol accounts for a mean 15.8% of total urinary excretion, range 3.4%–30.4%). Renal clearance is 4-fold higher than creatinine clearance, indicating that filtration and secretion are involved (Anonymous 2004). Terminal disposition half-life (t1/2) is approximately 10–12 h (Anonymous 2004).
Trospium chloride exhibits dose-independence over the single dose range of 20–60 mg (measured by area under the serum concentration-versus-time curve [AUC] data) and dose-dependence when measured by peak concentration [Cmax] data (3-fold increase when doubling from 20 to 40 mg and 4-fold increase when tripling from 20 to 60 mg) (Anonymous 2004). Of interest, there appears to be circadian variability in trospium chloride pharmacokinetics, with a decrease in Cmax of up to 59% and AUC of up to 33% for evening relative to morning dosing (Anonymous 2004). The mean accumulation factor for a 20 mg twice daily oral regimen is 1.1 (90% CI 0.85–1.35).
Special populations
Age does not appear to significantly affect trospium chloride pharmacokinetics although actual data were not presented in the source document (Anonymous 2004). Conflicting results have been noted in studies evaluating the effect of gender on the pharmacokinetics of trospium chloride. After administration of a single oral 40 mg dose in 16 elderly subjects, AUC was 45% lower in females compared with males. In contrast, after 20 mg twice daily administration for 4 days in 12 elderly patients, Cmax and AUC were 68% and 26% higher, respectively, in females versus males (Anonymous 2004).
Trospium chloride Cmax is increased by means of 12% and 63% in subjects with mild (Child-Pugh class A) and moderate hepatic impairment (Child-Pugh class B), respectively, compared with healthy subjects. However, AUC is similar in the three groups. No data are available regarding the effect of severe hepatic impairment (Child-Pugh class C) (Anonymous 2004). Renal impairment significantly alters trospium chloride pharmacokinetics. Patients with severe renal impairment (creatinine clearance < 30 mL/min) demonstrate a 4.5-fold increase in AUC, 2-fold increase in Cmax, and a 2- to 3-fold increase in t1/2 compared with healthy volunteers. In this patient population, a 50% reduction in daily dose is recommended (Anonymous 2004).
Efficacy
Table 2 illustrates available English language efficacy/tolerability studies of trospium chloride in UUI, in which placebo and active control (oxybutynin, tolterodine) groups were used (Stohrer et al 1991; Madersbacher et al 1995; Junemann et al 1999; Cardozo et al 2000; Hofner et al 2000; Junemann et al 2000; Frohlich et al 2002; Anonymous 2004; Zinner et al 2004). These were randomized, double-blind, and parallel-group in design. The two studies used for US licensing purposes were the two studies described in the product information sheet (Anonymous 2004), one of which has been recently published (Zinner et al 2004).
Table 2.
Clinical efficacy/tolerability studies of trospium chloride in the management of urge urinary incontinence
| Reference | Duration (wk) | Regimens | N | Results (compared with baseline) |
|---|---|---|---|---|
| Stohrer et al 1991 | 3 | TC 20 mg bid | 27 | All patients had detrusor hyperreflexia due to spinal cord injury. TC significantly increased maximum cystometric capacity (median change 120 mL vs placebo 0 mL) (p < 0.001) and bladder compliance (10 vs 1 mL/cm H2O, p < 0.001), and reduced maximum detrusor pressure (–35.0 vs –2.5 cm H2O, p < 0.001). No significant intergroup differences were noted with maximum urine flow rate or residual urine volume (p = 0.8 for each). In the 6 patients with partial sensation who could complete a study diary, micturition intervals increased by approximately 30% with TC. Spontaneously reported AE occurred in 1 TC patient (constipation) and 5 Plac patients (nausea, dry mouth, tiredness plus decreased exercise capacity, constipation and nervousness plus constipation). |
| Plac bid | 28 | |||
| Cardozo et al 2000 | 3–3.5 | TC 20 mg bid | 104 | The study was stopped early as interim analysis demonstrated superiority of TC over Plac. By intent-to-treat analysis, the treatment effect of TC (TC effect-Plac effect) was +22.0 mL for maximum cystometric capacity (p = 0.0054), +45.0 mL for volume at first unsTable contraction (p = 0.0015), and +7.0 mL for volume at maximum contraction (p = 0.0113). The 2 treatments were not significantly different with respect to effect on bladder compliance, residual urine volume, or maximum detrusor pressure at first unsTable contraction. Investigators assessed 26 and 30 TC patients as markedly and slightly improved, respectively, while corresponding figures for Plac patients were 17 and 26 (patient assessments were similar). In a subgroup analysis of patients with bladder capacity ≥ 350 mL, the treatment effect of TC was again significant for maximum cystometric capacity (+58 mL, p < 0.001) and volume at first unsTable contraction (+90 mL, p < 0.001). AE occurred in 68% TC and 62% Plac patients. Xerostomia was the most frequent AE (43 TC and 18 Plac patients) followed by GI disorders. Dizziness occurred in 11 TC and 15 Plac, and headache in 25 TC and 36 Plac patients. Drug acceptability by the patient was noted as very good/good in 37/50% of TC and 29/40% of Plac patients (no statistical results for AE were available). |
| Plac bid | 104 | |||
| Frohlich et al 2002 | 3 | TC 20 mg bid | 314 | By intent-to-treat analysis, the treatment effect of TC over Plac was significant for maximum cystometric capacity (+52 mL, p < 0.0001), volume at first unsTable contraction (+48 mL, p = 0.0001) and volume at maximum contraction (+40 mL, p = 0.0042). Nonsignificant treatment effects were noted for maximum detrusor pressure at first unsTable contraction and residual urine volume. Per protocol analysis gave similar results. Clinical cure or marked improvement (per patient self-assessment) occurred in 47.9% and 19.7% of TC and Plac patients, respectively. The distributions of clinical responses (per patient self-assessment) were significantly different in the 2 groups (p < 0.0001, more cure/markedly improved with TC and more slightly improved/no improvement/worsened with Plac). The investigator assessment was similar to that of the patients. ≥ 1 AE occurred in 35.7% of TC and 38.9% of Plac patients. The most frequent AE were GI disorders (TC 21.7%, Plac 18.7%) and xerostomia was the most frequent individual AE (TC 14.0%, Plac 8.4%; p = 0.052). CNS disorders (mostly headache) occurred in 11.1% of TC and 17.7% of Plac patients (p = 0.033). The global judgments of tolerability by investigators and patients were similar (between treatments and between each other). |
| Plac bid | 203 | |||
| Junemann and Fusgen 1999 | 3 | TC 40 mg qd | 56 | The treatment effects of TC 40 mg qd and 40 mg bid over Plac were similar for maximum cystometric capacity (+45.3 and +61.4 mL, respectively; p = 0.0006 vs Plac for both), volume at first unsTable contraction (+53.5 and +75.2 mL, p = 0.0333 vs Plac for both), and volume at maximum contraction (+45.2 and +77.0 mL, p = 0.0015 vs Plac for both). Treatment effects for volume at first desire to urinate and residual urine volume were not significant. No data were available regarding the statistical comparison of the 2 active treatments. AE frequency was dose-dependent (Plac 28.3%, TC 40 mg qd 51%, TC 40 mg bid 64%) and were mainly the expected anticholinergic effects. |
| TC 40 mg bid | 56 | |||
| Plac | 58 | |||
| Hofner et al 2000 | 52 | TC 20 mg bid | 177 | Per protocol analysis revealed statistically-indistinguishable treatment effects for the 2 drugs while both drugs produced significant effects compared with baseline (maximum cystometric capacity: +115 mL for TC and +119 mL for Oxy; volume at first unsTable contraction: +66 mL for TC and +49 mL for Oxy; volume at first desire to urinate: +86 mL for TC and +75 mL for Oxy). Frequency fell 31% on TC and 34% on Oxy. The number of incontinent episodes fell by approximately 1 per day with both drugs. AE occurred in 64.8% of TC and 76.7% of Oxy recipients (p < 0.01). Treatment-emergent AE (ie, those judged to be possibly, probably, or definitely drug-related) occurred in 47.9% of TC and 58.9% of Oxy recipients (p = 0.02). GI AE occurred in 39% of TC and 51% of Oxy recipients (p = 0.02), while xerostomia occurred in 33% of TC and 50% of Oxy recipients (p < 0.01). Tolerability of treatment was judged very good by 63% of TC and 42% of Oxy recipients (p = 0.004). The investigator assessment of tolerability was similar to that of the patients, favoring TC (p = 0.008). |
| Oxy 5 mg bid | 58 | |||
| Madersbacher et al 1995 | 2 | TC 20 mg bid | 88a | All patients had detrusor hyperreflexia due to spinal cord injury. The treatment effects of the 2 drugs were statistically indistinguishable from each other but both agents produced significant effects (p < 0.001) compared with baseline (maximum cystometric capacity; +97 mL for TC and +163 mL for Oxy; maximum voiding detrusor pressure: –35.4 cm H2O for TC and –38 cm H2O for Oxy; bladder compliance: +17 mL/cm H2O for TC and +22.6 mL/cm H2O for Oxy; residual urine volume: +76.5 mL for TC and +114.1 mL for Oxy). The frequency of hyperreflexive waves fell over time in both groups in a similar pattern (p = 0.16). Premature study withdrawal occurred in 16% of Oxy and 6% of TC patients and occurred earlier in the Oxy compared with TC groups (after means of 7 and 14 days on treatment, respectively). However the reasons for withdrawal were not listed, making interpretation of these data difficult. The only AE mentioned was xerostomia, which occurred in 54% of TC (4% being severe) and 56% of Oxy (23% being severe) patients. |
| Oxy 5 mg tid | ||||
| Junemann and Al-Shukri 2000 | 3 | TC 20 mg bid | 57 | The change from baseline in number of frequency episodes over 24 h was –3.4, –2.6, and –1.9, and Tolt 2 mg bid 63 for TC, Tolt, and Plac recipients (p = NS for Tolt vs Plac and p = 0.01 for TC vs Plac but no data Al-Shukri Plac bid 60 for TC vs Tolt). AE occurred in 34, 32, and 15% of TC, Tolt, and Plac recipients, respectively, 2000 with corresponding rates of mild xerostomia of 85, 84, and 42% in those who had that AE. |
| Tolt 2 mg bid | 63 | |||
| Plac bid | 60 | |||
| Zinner et al 2004 | 12 | TC 20 mg bid | 253 | By intent-to-treat analysis, at 12 weeks TC was superior to Plac in reducing urinary frequency/24 h (by –2.4 and –1.3 events, p ≤ 0.001), urge incontinence episodes/24 h (by –59 and –44%, p ≤ 0.0001), urge micturitions/24 h (–2.3 and –1.1 events, p ≤ 0.0001), diurnal micturitions/24 h (–1.9 and –1.0 events, p ≤ 0.0001), nocturnal micturitions/24 h (–0.5 and –0.3 events, p ≤ 0.05) and increasing urine volume/micturition (by +32.1 and +7.7 mL, p ≤ 0.0001). The superiority of TC over Plac was apparent as early as the week 4 visit. TC also reduced the impact of incontinence on QOL more than did Plac (measured by Incontinence Impact Questionnaire; for all patients by –54 and –36 points, p ≤ 0.05; for females by –59 and –36 points, p ≤ 0.05; for males by –33 and –35 points, NS). The subscores significantly improved by TC were travel, social relationships, and emotional health (all p ≤ 0.05). The results of other TC were travel, social relationships, and emotional health (all p ≤ 0.05). The results of other end point analyses demonstrated no significant differences between males and females. The five commonest AE were dry mouth (TC 22%, Plac 7%), constipation (10% and 4%), headache (7% and 5%), abdominal pain (3% and 1%), and diarrhea (3% and 5%) (no statistical results available). Mean increase in heart rate in trospium chloride recipients was 3 beats/minute compared with placebo. |
| Plac bid | 256 | |||
| Anonymous (Study 2) 2004 | 12 | TC 20 mg bid | 323 | By intent-to-treat analysis, TC was superior to Plac in reducing urinary frequency/24 h (by –2.7 and –1.8 events, p < 0.001) and urge incontinence episodes/week (by –16.1 and –12.1 2004 events, p < 0.001) and increasing urine volume/micturition (by +35.6 and +9.4 mL, p < 0.001). The superiority of TC over Plac was apparent as early as the week 4 visit. Pooled AE data (Zinner et al 2004 + this study) are available in Table 3. Mean increase in heart rate in trospium chloride recipients was 4 beats/minute compared with placebo. |
| Plac bid | 325 |
The number in each group was not given. 95 patients enrolled but data were evaluated in only 88 patients. Randomization was 1:1, so it was likely that approximately equal numbers were in the two groups.
Abbreviations: TC, trospium chloride; Plac, placebo; AE, adverse event; bid, twice daily; GI, gastrointestinal; CNS, central nervous system; qd, once daily; Oxy, oxybutynin immediate-release; Tolt, tolterodine immediate-release; tid, three times daily; NS, not significant; QOL, quality-of-life.
The majority of published clinical trials have emphasized cystometric end points, not clinical ones, with the exception of the two trials described in the product information sheet (one remains unpublished). Trospium chloride is rather unique in this regard, and the paucity of clinical outcome data does make evaluation of this agent difficult, especially in comparison with established anticholinergics. Trospium chloride has been uniformly superior to placebo and equivalent to immediate-release formulations of oxybutynin and tolterodine. No data are available regarding comparative efficacy with the newer long-acting formulations of oxybutynin and tolterodine, as well as solifenacin and darifenacin. Although statistically superior to placebo, the absolute differences between trospium chloride and placebo are not substantial and may call into question the clinical importance of such differences.
Safety
Table 2 illustrates the safety data from the individual placebo- and active-controlled English language clinical trials of trospium chloride (Stohrer et al 1991; Madersbacher et al 1995; Junemann et al 1999; Cardozo et al 2000; Hofner et al 2000; Junemann et al 2000; Frohlich et al 2002; Anonymous 2004; Zinner et al 2004). Table 3 illustrates the product information sheet data, which cite pooled results from two studies (Anonymous 2004; Zinner et al 2004). As would be expected, most adverse events are an extension of the drug’s anticholinergic properties. An interesting finding from these latter data is the increased frequency of anticholinergic adverse events in those subjects 75 years of age and older (15% of trospium chloride recipients were in this age stratum) compared with younger subjects. This is felt to be pharmacodynamic in nature (ie, increased sensitivity) and not pharmacokinetic (Anonymous 2004).
Table 3.
Pooled adverse event data (percentage) from the two placebo-controlled trospium chloride (20 mg twice daily) trials described in the package insert (Anonymous 2004) a
| Event | Placebo | Trospium chloride |
|---|---|---|
| N | 590 | 591 |
| Gastrointestinal | ||
| Dry mouth | 5.8 | 20.1 |
| Constipation (new onset) | 4.6 | 9.6 |
| Upper abdominal pain | 1.2 | 1.5 |
| Worsened constipation | 0.8 | 1.4 |
| Dyspepsia | 0.3 | 1.2 |
| Flatulence | 0.8 | 1.2 |
| Nervous system | ||
| Headache | 2.0 | 4.2 |
| General | ||
| Fatigue | 1.4 | 1.9 |
| Renal/Urinary | ||
| Urinary retention | 0.3 | 1.2 |
| Eye | ||
| Dry eye NOS | 0.3 | 1.2 |
Those events judged to be at least possibly related to treatment with trospium chloride, reported in ≥ 1% of trospium chloride recipients, and more frequent in trospium chloride than placebo recipients.
Abbreviations: NOS, not otherwise specified.
A double-blind, randomized, placebo-controlled study in 29 healthy volunteers was performed to assess the maximum tolerated single oral dose of trospium chloride. The doses evaluated were 20, 40, 80, 120, 180, 240, and 360 mg. At each dose level, 9 subjects were randomized to active drug and 3 to placebo (exception: at 360 mg, the corresponding numbers were 8 and 2). There were essentially no inter-treatment differences (drug vs placebo) at doses of 120 mg and less. Anticholinergic effects of increasing intensity occurred at a dose of 180 mg and above (pupillary dilatation, decreased salivary flow, increased heart rate). With the 360 mg dose, vital signs were unchanged but subjects found the experience to be “quite unpleasant”. Pupillary effects were not seen until doses were greater than or equal to 180 mg. These three doses produced a long-lasting dilatation significantly different from that of placebo, but dose-dependence was not noted. A similar threshold was found for the hyposalivation effect and, in contrast to the pupillary effect, dose-dependence was noted. A similar threshold was found for the tachycardic effect and, like the pupillary effect, dose-dependence was not noted. Tachycardia appeared at 4 to 8 h post-dosing and disappeared by 12 h post-dosing. No significant effect on blood pressure was noted at any dose. No electrocardiographic effects occurred at any dose except a 10 to 40 ms reduction in the QT interval due to the tachycardia. Of recorded adverse events, only the frequency and intensity of xerostomia was dose-dependent. At lower doses, xerostomia was of mild intensity while after the 240 and 360 mg doses it was of moderate to severe intensity (Breuel et al 1993).
The effect of trospium chloride on QT interval was evaluated in a single-blind, randomized, placebo- and active (moxifloxacin)-controlled trial in 170 healthy volunteers. Subjects were randomized to five days of placebo, moxifloxacin 400 mg once daily, or various doses of trospium chloride (ranging from 20 to 100 mg twice daily). The QT interval was evaluated over a 24 h period at steady state. The QT interval was not affected by any dose of trospium chloride while moxifloxacin had the expected effect (mean Fredericia-corrected prolongation of 6.4 ms). Dose-dependent tachycardia was seen in trospium chloride recipients, with mean increases of 9.1 and 18.0 beats/minute in the 20 and 100 mg dose groups, respectively (Anonymous 2004).
In the gallbladder contractility study reviewed previously, xerostomia did not occur with the single 0.2 and 0.5 mg IV doses of trospium chloride but did occur in 3 of 6 subjects after the single 1.0 and 1.5 mg IV doses. At these latter doses, a transient dose-dependent tachycardia also occurred, reaching a peak at 0.25 h post-dosing (Matzkies et al 1992). Two case reports have also documented the potential for significant tachycardia after IV trospium chloride (Hasselkus 1998; Pfeiffer et al 1999). In one report, IV administration of 2 mg as premedication for endoscopy in 24 patients caused mean heart rate to rise from 81 to 125 beats/min within 1 min after dosing (Hasselkus 1998). In the other report, 31 patients again received the drug as premedication for endoscopy. In these patients, 1.2 mg of IV trospium chloride produced a rise in heart rate of approximately 14 beats/minute at 5, 10, and 15 minutes post-dosing (Pfeiffer et al 1999).
Two electroencephalographic (EEG) studies have been conducted to quantitate the central nervous system effects of trospium chloride, oxybutynin, tolterodine, and placebo in healthy volunteers (Pietzko et al 1994; Todorova et al 2001). The first study was a randomized, crossover design evaluating single doses of trospium chloride (1.2 mg IV, 45 mg oral) and oxybutynin (20 mg oral) in 12 subjects. Ten of these 12 subjects were also evaluated in a no drug state but this was not a placebo phase within the crossover design. No significant EEG effects were associated with trospium chloride by either route of administration. Oxybutynin caused a significant reduction in alpha and beta 1 activity (with eyes open, eyes closed, during reaction time testing). Heart rate significantly rose after IV trospium chloride administration, peaking at 20 min post-dosing with a 60% increase. Heart rate returned to baseline by 4 h post-dosing. No significant heart rate effect was noted with oral trospium chloride while oral oxybutynin caused a significant reduction, which peaked at 3 h post-dosing and did not return to baseline within the 4 h evaluation period. Adverse events included xerostomia (in 1, 2, and 1 trospium chloride oral, trospium chloride IV, and oxybutynin recipients, respectively), tachycardia (in 2 trospium chloride IV recipients), and headache (in 1 trospium chloride IV recipient; moderate to severe, occurring at 7 h post-dosing and lasting 3 h, requiring no treatment) (Pietzko et al 1994). The second study was a randomized, single blind design evaluating trospium chloride (15 mg thrice daily), oxybutynin (5 mg thrice daily), tolterodine (2 mg twice daily), and placebo, with each treatment lasting 1 day. Each of the sixty-four subjects was randomized to 1 of the 4 treatment groups. Trospium chloride and tolterodine did not induce any power change in 5 of 6 EEG frequency bands (delta, alpha 1, alpha 2, beta 1, beta 2) and caused isolated reductions in power of the theta band. In contrast, oxybutynin significantly reduced EEG power in 4 bands: theta, alpha 1, alpha 2, beta 1. Tolerability was rated as “very good” by 81.3, 62.5, 56.3, and 50% of placebo, tolterodine, trospium chloride, and oxybutynin recipients, respectively. Fifty-seven adverse events (36 being possibly drug-related) occurred in 30 subjects: 4 with placebo (maximum of 1/subject), 14 with tolterodine (3 had > 1 event), 15 with trospium chloride (4 had > 1 event), and 24 with oxybutynin (8 had > 1 event). In terms of central nervous system events, 3 events occurred in 3 placebo recipients, 5 events in 4 tolterodine recipients, 11 events in 8 trospium chloride recipients, and 17 events in 8 oxybutynin recipients. The events in trospium chloride recipients included headache in 5 subjects, tiredness in 2 subjects, and impaired concentration, restless sleep, cold sensation, and single myoclonus in 1 subject each (Todorova et al 2001). At the present time, there are no data to support the hypothesis that trospium chloride is less neurotoxic than non-quaternary anticholinergics due to reduced transit across the blood brain barrier (due to its quaternary amine structure).
Drug–drug interactions
In vitro studies have demonstrated that trospium chloride exerts negligible effects on cytochrome P450 (CYP) isozymes 3A4, 1A2, 2E1, 2C19, 2C9, and 2A6 in human liver microsomes. Although it is a reasonably potent inhibitor of CYP isozyme 2D6, the inhibition constant (ki) is 1000-fold higher than the Cmax achievable with the usual oral regimen. Hence, the likelihood of a clinically-important drug interaction with CYP isozyme 2D6 substrates and trospium chloride is very low (Anonymous 2004). However, no formal studies have been conducted evaluating potential drug–drug interactions with trospium chloride. Whether drugs actively secreted in the renal tubules affect trospium chloride pharmacokinetics (and vice versa) is unknown.
Dosing and administration
The usual dosage regimen is 20 mg orally twice daily. The drug should be taken at least 1 h before meals or on an empty stomach. The daily dose should be reduced by 50% in the presence of severe renal impairment (creatinine clearance < 30 mL/min), ie, 20 mg once daily at bedtime. In patients 75 years of age and over, dose reduction to 20 mg once daily should be considered based upon tolerability (Anonymous 2004).
Conclusion
A major deficiency of the database with trospium chloride is the relative lack of efficacy/tolerability data in the older population, especially those over 75 years of age. Subjects aged 75 years and above constituted only 15% of the FDA New Drug Application trial database. In this age stratum, the prevalence of anticholinergic adverse events was higher compared with younger subjects. In the “real world”, anticholinergics are prescribed most frequently to older subjects and it is reasonable to expect that the comorbidities and polypharmacy characteristic of this population will enhance drug toxicity. In fact, the majority of anticholinergic drugs for UUI are considered to be “potentially inappropriate drugs” for the elderly in the Beers criteria (exceptions: oxybutynin sustained-release and, by extension, tolterodine sustained-release) (Fick et al 2003). Based on the adverse event frequency data from the individual studies and the product information sheet, trospium chloride would fit the Beers criteria as a “potentially inappropriate drug” in the elderly.
Trospium chloride is one of the most recently approved anticholinergics for management of UUI in the US. Despite its commercial availability elsewhere for many years, there are no published comparative data available with the long-acting topical and oral oxybutynin and oral tolterodine formulations. The published database on the drug’s pharmacokinetics and drug interaction potential are similarly inadequate. In addition, no published quality-of-life or pharmacoeconomic data are available. In light of available data, trospium chloride does not appear to be a significant advance on existing anticholinergics for UUI. The quest for the magic “uroselective” bullet continues.
References
- Anonymous . Odyssey Pharmaceuticals, East Hanover, NJ and Indevus Pharmaceuticals. Lexington, MA, USA; 2004. Sanctura® (trospium chloride tablets) [product information] [Google Scholar]
- Breuel H-P, Murtz G, Boudy S, et al. Safety and tolerance of trospium chloride in the high dose range. Arzneimittelforschung. 1993;43:461–4. [PubMed] [Google Scholar]
- Cardozo L, Chapple CR, Toozs-Hobson P, et al. Efficacy of trospium chloride in patients with detrusor instability: a placebo-controlled, randomized, double-blind, multicentre clinical trial. BJU Int. 2000;85:659–64. doi: 10.1046/j.1464-410x.2000.00575.x. [DOI] [PubMed] [Google Scholar]
- Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- Frohlich G, Bulitta M, Strosser W. Trospium chloride in patients with detrusor overactivity: meta-analysis of placebo-controlled, randomized, double-blind, multi-center clinical trails on the efficacy and safety of 20 mg trospium chloride twice daily. Int J Clin Pharmacol Ther. 2002;40:295–303. [PubMed] [Google Scholar]
- Guay DRP. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacokinet. 2003;42:1243–85. doi: 10.2165/00003088-200342140-00004. [DOI] [PubMed] [Google Scholar]
- Hasselkus W. Inappropriate rise in heart rate caused by intravenous administration of trospium chloride during upper gastrointestinal endoscopy [letter] Endoscopy. 1998;30:580. doi: 10.1055/s-2007-1001350. [DOI] [PubMed] [Google Scholar]
- Hatchet T, Bueno L, Fioramonti J, et al. The use of a compact portable microcomputer system (EPSON HX20) to measure on-line the contractile activity of the digestive tract from eight channels application to pharmacological tests. J Pharmacol Meth. 1986;16:171–80. doi: 10.1016/0160-5402(86)90022-7. [DOI] [PubMed] [Google Scholar]
- Herbison P, Hay-Smith J, Ellis G, et al. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841–4. doi: 10.1136/bmj.326.7394.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofner K, Halaska M, Primus G, et al. Tolerability and efficacy of trospium chloride in a long-term treatment (52 weeks) in patients with urge-syndrome: a double-blind, controlled, multicentre clinical trial. Neurourol Urodyn. 2000;19:487–8. (abstract 85A) [Google Scholar]
- Junemann KP, Al-Shukri S. Efficacy and tolerability of trospium chloride and tolterodine in 234 patients with urge-syndrome: a double-blind, placebo-controlled, multicentre clinical trial. Neurourol Urodyn. 2000;19:488–90. abstract 85B. [Google Scholar]
- Junemann K-P, Fusgen I. Placebo-controlled, randomized, double-blind, multicentre clinical trial on the efficacy and tolerability of 1 × 40 mg and 2 × 40 mg trospium chloride (Spasmo-Lyt®) daily for 3 weeks in patients with urge-syndrome. Neurourol Urodyn. 1999;18:375–6. abstract 107. [Google Scholar]
- Langguth P, Kubis A, Krumbiegel G, et al. Intestinal absorption of the quaternary trospium chloride: permeability-lowering factors and bioavailabilities for oral dosage forms. Eur J Pharm Biopharm. 1997;43:265–72. [Google Scholar]
- Langguth P, Mutschler E. Lipophilisation of hydrophilic compounds. consequences on transepidermal and intestinal transport of trospium chloride. Arzneimittelforschung. 1987;37:1362–6. [PubMed] [Google Scholar]
- Lopez Pereira P, Miguelez C, Caffarati J, et al. Trospium chloride for the treatment of detrusor instability in children. J Urol. 2003;170:1978–81. doi: 10.1097/01.ju.0000085667.05190.ad. [DOI] [PubMed] [Google Scholar]
- Madersbacher H, Stohrer M, Richter R, et al. Trospium chloride versus oxybutynin: a randomized, double-blind, multicentre trial in the treatment of detrusor hyperreflexia. Br J Urol. 1995;75:452–6. doi: 10.1111/j.1464-410x.1995.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Matzkies F, Murtz G, Giannetti B, et al. Ultrasound studies of the effect of trospium chloride on gall-bladder kinetics. Arzneimittelforschung. 1992;42:1456–8. [PubMed] [Google Scholar]
- Pehl C, Wendl B, Kaess H, et al. Effects of two anticholinergic drugs, trospium chloride and biperiden, on motility and evoked potentials of the oesophasgus. Aliment Pharmacol Ther. 1998;12:979–84. doi: 10.1046/j.1365-2036.1998.00398.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Kaess H, Bodeker R-H, et al. Heart rate increase after intravenous administration of trospium chloride and scopolamine during endoscopic retrograde cholangiopancreatography [letter] Endoscopy. 1999;31:507. [PubMed] [Google Scholar]
- Pfeiffer A, Schmidt T, Holler T, et al. Effect of trospium chloride on gastrointestinal motility in humans. Eur J Clin Pharmacol. 1993;44:219–23. doi: 10.1007/BF00271361. [DOI] [PubMed] [Google Scholar]
- Pietzko A, Dimpfel W, Schwantes U, et al. Influences of trospium chloride and oxybutynin on quantitative EEG in healthy volunteers. Eur J Clin Pharmacol. 1994;47:337–43. doi: 10.1007/BF00191165. [DOI] [PubMed] [Google Scholar]
- Rovner E, Wyman J, Lackner T. Urinary incontinence. In: DiPiro J, Talbert R, Hayes P, et al., editors. Pharmacotherapy: a pathophysiologic approach. 5. Norwalk: Appleton & Lange; 2002. pp. 1543–56. [Google Scholar]
- Schmidt T, Widmer R, Pfeiffer A, et al. Effect of the quaternary ammonium compound trospium chloride on 24 hour jejunal motility in healthy subjects. Gut. 1994;35:27–33. doi: 10.1136/gut.35.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohrer M, Bauer P, Giannetti BM, et al. Effect of trospium chloride on urodynamic parameters in patients with detrusor hyperreflexia due to spinal cord injuries. a multicentre placebo-controlled double-blind trial. Urol Int. 1991;47:138–43. doi: 10.1159/000282207. [DOI] [PubMed] [Google Scholar]
- Todorova A, Vonderheid-Guth B, Dimpfel W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol. 2001;41:636–44. doi: 10.1177/00912700122010528. [DOI] [PubMed] [Google Scholar]
- Uckert S, Stief CG, Odenthal KP, et al. Responses of isolated normal human detrusor muscle to various sympatholytic drugs commonly used in the treatment of the overactive bladder. Arzneimittelforschung. 2000;50:456–60. doi: 10.1055/s-0031-1300230. [DOI] [PubMed] [Google Scholar]
- Uckert S, Stief CG, Odenthal KP, et al. Comparison of the effects of various spasmolytic drugs on isolated human and porcine detrusor smooth muscle. Arzneimittelforschung. 1998;48:836–9. [PubMed] [Google Scholar]
- Zinner N, Gittelman M, Harris R, et al. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004;171:2311–15. doi: 10.1097/01.ju.0000127742.73136.0c. [DOI] [PubMed] [Google Scholar]


