Abstract
Toll-like receptor 4 (TLR4) is the transuding subunit of lipopolysaccharide receptor and an important intracellular signal pathway of the innate immune response. Published data about the relationship between TLR4 mutations and atopy/asthma are not consistent. This study was performed to detect two commonly reported Asp299Gly and Thr399Ile polymorphisms in TLR4 gene in Singaporean Chinese and their possible association with atopy-related phenotypes. A total of 117 unrelated Singapore residents were randomly selected for this study. Among them, atopy was evident in 66 subjects while 61 had allergic rhinitis. Twenty-one patients had concomitant asthma and 17 were atopic. In all subjects, neither the Asp299Gly nor Thr399Ile polymorphism was found by DNA sequencing. This discrepant result could be due to the ethnic variation of allelic distribution in TLR4 gene. Although it is still debatable whether there is any role of TLR4 polymorphisms on atopy-related phenotypes, one needs to develop an appropriate model to investigate the interactions between genetic variations and environmental factors that contribute to the complex traits in allergic diseases.
Keywords: TLR4 polymorphisms, Singaporean Chinese, atopy
Introduction
Toll-like receptor 4 (TLR4) is one of the principal receptors for bacterial endotoxin recognition and an important intracellular signal pathway of the innate immune response (Aderem et al 2000; Lien et al 2000). Recently, there is evidence that endotoxin exposure during early life is protective against the development of atopy and asthma (Strachn 2000; Liu 2002). TLR4 is therefore reasonably suspected as a candidate gene for studying interaction between genetic variation and environmental factors for the development of atopic diseases.
Human TLR4 gene is located at chromosome 9q32-33 and comprises three exons spanning about 10 kb (http://www.ncbi.nlm.nih.gov; AF177765). Twelve rare amino acid variants of TLR4 were reported in 2001 (Smirnova et al 2001). Raby and colleagues have identified an additional 29 polymorphisms of this gene (Raby et al 2002). However, most association studies have concentrated on two positions: an A to G polymorphism at nucleotide 896 from the start codon of the human TLR4 gene resulting in an aspartic acid to glycine substitution at position 299 of the amino acid sequence (Asp299Gly); and a C to T polymorphism at nucleotide position 1196 resulting in a threonine to isoleucine substitution at position 399 of the amino acid sequence (Thr399Ile). Many studies have reported an association between polymorphisms at TLR4 Asp299Gly and Thr399Ile with clinical diseases (Lorenz et al 2002; Michel et al 2003; Werner et al 2003; Yang et al 2004). In 2000, Arbour and colleagues first reported that TLR4 mutation were associated with endotoxin hyporesponsiveness in humans (Arbour et al 2000). Using an in vitro cultured cell approach, the same group reported that Asp299Gly mutation interrupted TLR4-mediated lipopolysaccaride (LPS) signaling (Schwartz 2001b). The study provides genetic evidence that human TLR4 Asp299Gly mutation could be associated with differences in LPS responsiveness and, consequently, could alter the ability of the host to respond to environmental stimulus (Schwartz 2001b).
Recently, polymorphisms of TLR4 were reported to be associated with the severity of atopy (Yang et al 2004) and with a modified response to endotoxin in asthmatics (Werner et al 2003). However, some studies were unable to confirm these two common polymorphisms on human TLR4 gene or its associated atopy-related phenotypes (Raby et al 2002; Noguchi et al 2004). This study is therefore designed to screen the existence of these two common TLR4 poly-morphisms (Asp299 Gly and Thr399Ile) and the association with atopy-related phenotypes in a Singaporean Chinese population.
Materials and methods
A total of 117 unrelated Singapore Chinese residents, aged from 9 to 72 years with a mean age of 31.7 ± 11.7 years, were investigated. Among them, atopy was evident in 66 subjects, and 51 were non-atopic. In atopic subjects, 61 experienced allergic rhinitis. Twenty-one patients had concomitant asthma and 17 of them were atopic. They were randomly called during a national rhinitis survey study to attend a rhinologic examination in the ENT outpatient clinic of the National University Hospital of Singapore. All subjects were carefully interviewed by an attending physician for their medical and family history, and a thorough inspection of the nose was performed. Peripheral blood samples were taken for allergy screening tests and for DNA extraction. All study subjects gave their informed consent prior to the study. This study was approved by the Institutional Review Board of the National University of Singapore and funded by the Academic Research Fund from the same university.
Diagnosis of atopy and its related diseases were made according to the following criteria (Bousquet et al 2001):
Atopy: a positive serum specific IgE (equal or more than 0.35 U/mL) to at least one of the inhalant allergens (house dust mites) tested.
Rhinitis: the occurrence of two or more symptoms (nasal obstruction, rhinorrhea, sneezing, and itchy nose) on most days during the past year. If patients also had atopy, a diagnosis of allergic rhinitis was given.
Asthma: a history of paroxysmal attacks of breathlessness commonly associated with a tightness of the chest and wheezing as previously confirmed by a physician.
Genomic DNA was extracted using a modified phenol-chloroform procedure. The polymorphism of TLR4 was identified by sequence analysis in 117 subjects. PCR was carried out in a volume of 25 μL containing 30 ng genomic DNA, 10 × buffer, 0.2 mmol/L dNTPs, and 1 unit Taq polymerase (Qiagen, Hilden, Germany). The primers were: 5′-TCT AGA GGG CCT GTG CAA TT-3′ (forward) and 5′-TGA AAA CTC ACT CAT TTG TTT CAA-3′ (reverse) at a concentration of 0.4 μmol/L (Lorenz et al 2002). PCR were optimized using denaturing for 2 min at 94 °C. The PCR cycle was set at 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 40 s totally for 35 cycles, followed by 72 °C for 5 min. The sizes of the generated PCR products were 496 bp. Before sequencing, the PCR products were purified with QIAquick PCR purification kit (Qiagen, Hilden, Germany). Sequencing reactions were performed using the BIG Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA, USA). The sequenced products were purified by ethanol precipitation, following which the DNA pellet was dissolved in 4 μL of loading dye (Deionized formamide, PE Applied Biosystems, Foster City, CA, USA) and analyzed on an Applied Biosystems Genetic Analyser 3100 (PE Applied Biosystems, Foster City, CA, USA) in the Department of Pediatrics, National University of Singapore.
Results and discussion
In all 117 subjects, we were unable to identify polymorphisms either at TLR4 Asp299Gly or Thr399Ile. It appeared that the allele frequencies of mutation at these two positions are rare (lower than 1%) in the Singaporean Chinese population. This study indicated that all sequenced samples had Asp at amino acid 299 and Thr at amino acid 399 (Figure 1). In addition, we were unable to detect any polymorphism within the DNA fragments that were amplified and sequenced in this study.
Figure 1.
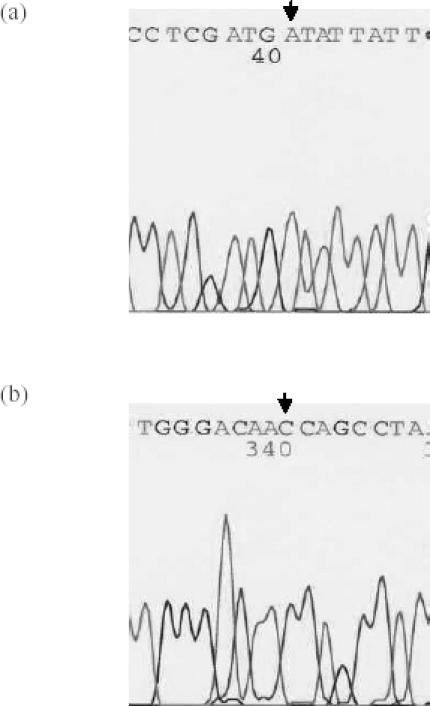
Sequence analysis of TLR4 at amino acid position of 299 and 399. (a) AA homozygote at amino acid position 299; (b) CC homozygote at amino acid position 399. All sequences are 5′-3′.
Allele frequencies of TLR4 Asp299Gly and Thr399Ile are found to be low in published studies. Asp299Gly is present at frequencies between 3.3% and 9.4%, while those of Thr399Ile are between 3.7% and 11% in several Caucasian studies (Table 1). A study performed in 32 asthmatic Japanese showed that Gly299 variant was undetectable in all patients (Noguchi et al 2004). In another Japanese study, the Asp299Gly allele of the TLR4 gene was undetectable in any of the specimens from 107 healthy volunteers and 168 patients with various diseases (Okayama et al 2002). From the available data, it seems that the polymorphisms of TLR4 at amino acid position of 299 and 399 are rare in Asian population.
Table 1.
Summary of association studies regarding the polymorphisms at Asp299Gly and Thr399Ile
| Author(s) | Country/ethnic group | Nr of study subjects | Asp299Gly G allele frequency (%) | Thr399Ile T allele frequency (%) | Association with diseases | |
|---|---|---|---|---|---|---|
| Yes/No | Diseases for comparison | |||||
| Agnese 2002 | US | Patients: 77 Controls: 39 | NA | NA | Yes | Gram-negative infections |
| Lorenz 2002 | France | Patients: 91 Controls: 73 | 6–8 | 6.6–11 | Yes | Gram-negative septic shock |
| Werner 2003 | Germany | 334 | 8.3 | 8.6 | Yes | Modify endotoxin effects on asthma |
| Michel 2003 | Australia | 118 | 6.6–9.4 | 6.6–9.4 | Yes | Systemic inflammatory hyporesponsiveness 100% linkage disequilibrium |
| Reindl 2003 | Australia | 190 | 6.05 | 5.8 | Yes | Multiple sclerosis |
| Yang 2004 | US (Caucasians) | Family: 336 | 5.9–8.1 | NA | Yes and No | Atopy severity, but not asthma |
| Raby 2002 | Canada (various populations) | 90 | NA | NA | No | Asthma or atopy-related phenotypes |
| Noguchi 2004 | Japan | 32 | Not detected | NA | No | Asthma |
| Folwaczny 2004 | Germany | Patients: 122 Control: 122 | 4.13.3 | 4.53.7 | No | Chronic periodontitis |
| Smirnova 2001 | US (Caucasians) | 348 | 7 | 7 | NA | NA |
| Okayama 2002 | Japan | Patients: 168 Controls: 107 | Not detected | NA | NA | NA |
Abbreviations: NA, not available.
Exposure to bacterial endotoxin has received considerable attention as a potential risk factor for the development of asthma and atopic diseases (Schwartz 2001a). As a receptor for LPS, TLR4 is one of the most commonly investigated candidate genes for studying interaction between genetic variation and environmental factors for the development of atopic or atopy-related diseases. Although associations between polymorphisms of Asp299Gly and Thr399Ile at TLR4 gene and atopic diseases have been reported by some studies, they may not be the key players in the development of atopic phenotypes. Otherwise, one could expect to confirm these polymorphisms in a different population since allergic disease is a common disease worldwide.
TLR4 is a candidate gene for atopic diseases because of its important biologic characteristics. It is possible that polymorphisms at other positions of TLR4 might play an important role in atopy-related phenotypes and that is in combination with the environmental exposure of bacterial endotoxin. Alternatively, it is also possible that genetic susceptibility to asthma and atopic-related phenotypes might be different in various populations. This notion was supported by the recent genome-wide studies for identifying the asthma susceptibility loci in ethnically diverse populations (Xu et al 2001; Hakonarson et al 2002). In view of the conflicting evidence, future studies are needed to identify the key genes for atopic phenotypes and to investigate the interactions between genetic and environmental factors influencing the complex trait of allergic diseases.
In summary, we were unable to detect the polymorphisms at Asp299Gly or Thr399Ile of TLR4 in Singaporean Chinese. Our results suggest that these two polymorphisms in TLR4 could not be the key player in Singaporean Chinese with atopy or atopic-related phenotypes.
References
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186:1522–5. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma (ARIA) (ARIA Workshop Report) J Allergy Clin Immunol. 2001;108:S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- Folwaczny M, Glas J, Torok HP, et al. Toll-like receptor 2 and 4 in periodontal disease. Clin Exp Immunol. 2004;135:330–5. doi: 10.1111/j.1365-2249.2004.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakonarson H, Halapi E. Genetic analyses in asthma: current concepts and future directions. Am J Pharmacogenomics. 2002;2:155–66. doi: 10.2165/00129785-200202030-00001. [DOI] [PubMed] [Google Scholar]
- Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AH. Endotoxin exposure in allergy and asthma: reconciling a paradox. J Allergy Clin Immunol. 2002;109:379–92. doi: 10.1067/mai.2002.122157. [DOI] [PubMed] [Google Scholar]
- Lorenz E, Mira JP, Frees KL, et al. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- Michel O, Le Van TD, Stern D, et al. Systemic responsiveness to LPS and polymorphisms in the toll-like receptor 4 gene in human beings. J Allergy Clin Immunol. 2003;112:923–9. doi: 10.1016/j.jaci.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Noguchi E, Nishimura F, Fukai H, et al. An association study of asthma and total serum immunoglobin E levels for toll-like receptor polymorphisms in a Japanese population. Clin Exp Allergy. 2004;34:177–83. doi: 10.1111/j.1365-2222.2004.01839.x. [DOI] [PubMed] [Google Scholar]
- Okayama N, Fulimura K, Suehiro Y, et al. Simple genotype analysis of the Asp299Gly polymorphism the toll-like receptor 4 gene that is associated with lipopolysaccharide hyporesponsiveness. J Clin Lab Anal. 2002;16:56–8. doi: 10.1002/jcla.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby BA, Klimecki WT, Laprise C, et al. Polymorphisms in toll-Like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Respir Crit Care Med. 2002;166:1449–56. doi: 10.1164/rccm.200207-634OC. [DOI] [PubMed] [Google Scholar]
- Reindl M, Lutterotti A, Ingram J, et al. Mutations in the gene for toll-like receptor 4 and multiple sclerosis. Tissue Antigens. 2003;61:85–8. doi: 10.1034/j.1399-0039.2003.610108.x. [DOI] [PubMed] [Google Scholar]
- Schwartz DA. Does inhalation of endotoxin cause asthma? Am J Respir Crit Care Med. 2001a;163:305–6. doi: 10.1164/ajrccm.163.2.ed2000a. [DOI] [PubMed] [Google Scholar]
- Schwartz DA. Inhaled endotoxin, a risk for airway disease in some people. Respir Physiol. 2001b;128:47–55. doi: 10.1016/s0034-5687(01)00264-x. [DOI] [PubMed] [Google Scholar]
- Smirnova I, Hamblin MT, Mcbride C, et al. Excess of race acid polymorphisms in the toll-like receptor 4 in humans. Genetics. 2001;158:1657–64. doi: 10.1093/genetics/158.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachn DP. Family size, infection and atopy: the first decade of the “hygiene hapothesis”. Thorax. 2000;55:S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M, Topp R, Wimmer K, et al. TLR4 gene variants modify endotoxin effects on asthma. J Allergy Clin Immunol. 2003;112:323–30. doi: 10.1067/mai.2003.1648. [DOI] [PubMed] [Google Scholar]
- Xu J, Meyers DA, Ober C, et al. Collaborative Study on the Genetics of Asthma: genomewide screen and identification of gene-gene interactions for asthma-susceptibility loci in three US populations: collaborative study on the genetics of asthma. Am J Hum Genet. 2001;68:1437–46. doi: 10.1086/320589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IA, Barton SJ, Porke S, et al. Toll-like receptor 4 polymorphism and severity of atopy in asthmatics. Genes Immun. 2004;5:41–5. doi: 10.1038/sj.gene.6364037. [DOI] [PubMed] [Google Scholar]


