Abstract
Adverse drug reactions (ADRs) are a significant cause of morbidity and mortality and contribute to the incidence of adverse events, resulting in increased healthcare costs. Healthcare providers need to understand their role and responsibility in the detection, management, documentation, and reporting of ADRs, all essential activities for optimizing patient safety. The purpose of this article is to summarize findings from important ADR literature reviews and describe the components, and extent of participation, of the national ADR reporting program available in New Zealand. A series of recommendations to increase the detection of ADRs is also described.
Keywords: adverse drug reactions, post-marketing surveillance, pharmacists
Introduction
International attention to patient safety has been growing significantly since the publication of the US Institute of Medicine (IOM) report “To err is human: building a safer health system” (Kohn et al 1999). Similarly in New Zealand, the release of Professor Peter Davis's report on adverse events in New Zealand public hospitals in December 2001 (Davis et al 2001) led to an increased awareness of various issues related to patient safety, which have been further highlighted in more recent reports in the local literature (Davis et al 2002, 2003; Morton and MacMillan 2003; Briant et al 2004).
Adverse drug reactions (ADRs) are a significant cause of morbidity and mortality and contribute to the incidence of adverse events, resulting in increased healthcare costs (Lazarou et al 1998; Dormann et al 2000). Therefore, it is important to motivate healthcare providers to understand their role and responsibility in the detection, management, documentation, and reporting of ADRs, all essential activities for optimizing patient safety.
The purpose of this article is to summarize findings from important ADR literature reviews and to describe the components of the national ADR reporting program available in New Zealand and the extent of participation of health professionals in this program, particularly pharmacists. Finally, a series of recommendations to increase the detection of ADRs is analyzed as a means to improve patient safety through the promotion of a more active role on the part of the pharmacy professional.
Literature review
Adverse reactions are a recognized hazard of drug therapy. Although some ADRs are minor and resolve without sequelae, others can cause permanent disability or death. Many studies have assessed the incidence of ADRs in numerous settings, but these estimates vary considerably. This may be due to known underreporting of ADRs and differences in study methodology, populations studied, and ADR definitions.
Various definitions of an ADR have been used around the world. In New Zealand, as in many other countries, the World Health Organization's (WHO) definition for an ADR is used; that is, “any response to a drug which is noxious, unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function” (Uppsala Monitoring Center, WHO Collaborating Centre for International Drug Monitoring 2000). An adverse drug event (ADE) is any undesirable experience associated with the use of a medical product in a patient (Nebeker et al 2004). This broad definition includes ADRs and other events (including medication errors) related to the prescribing, preparation, dispensing, or administration of medications. In broad terms, an ADR is an adverse event with a causal link to a drug. Both ADRs and ADEs have been targeted to improve patient safety.
Epidemiological studies have suggested that ADRs account for about 5% of all hospital admissions (Einarson 1993; Lazarou et al 1998; Roughead et al 1998), although estimates of up to 28% of drug-related admissions have been suggested (Miller 1974; Jick 1994). Also, the risk of ADRs increases when a patient is hospitalized. Lazarou and colleagues (1998) conducted a meta-analysis of 39 prospective studies from US hospitals to determine the incidence of ADRs in hospitalized patients. Although the results have been criticized (Bates 1998; Kvasz et al 2000), the authors reported that ADRs may be the fourth to sixth leading cause of death in hospitalized patients, with fatal ADRs occurring in 0.32% of the cases.
The significant impact that ADEs and ADRs have on morbidity, mortality, and costs cannot be overemphasized, nor should it be ignored. ADRs have been reported to be associated with a greater length of hospital stay, which consequently increases healthcare costs. McDonnell and Jacobs (2002) assessed the potential preventability of ADRs directly related to a patient's hospital admission. They considered that 62.3% of these were potentially preventable. The findings of the IOM report (Kohn et al 1999) estimate the total US costs, including lost income, lost household production, disability, and healthcare costs, due to preventable ADEs at US$17 billion to US$29 billion.
As many ADRs often go unrecognized or unreported, an organized ADR monitoring program is one mechanism to more actively detect ADRs, and consequently positively affect the quality of patient care.
Clinical importance of ADR monitoring and reporting
During clinical trials, medicines are generally studied in a controlled environment, for a relatively small number of patients, and usually for a limited duration. While the approval process includes extensive safety testing, these trials sometimes exclude the elderly, the very young, and patients with comorbidities. Often patients on multiple drug therapy and patients with decreased renal and hepatic function are excluded. For these patient populations, any vulnerability to ADRs may be missed. Furthermore, it is extremely difficult to predict how practitioners will actually use medications in practice.
Once the drug is commercially available, the exclusion criteria applied in clinical trials no longer exist. Thus, exposure to the drug will last longer, as therapy may continue long term, increasing the possibility of previously undetected problems to arise and be identified. Additionally, adverse reactions may occur at such a low frequency that they are not being detected in the small numbers of patients included in clinical trials; consequently, widespread use of medicines in the general population can increase the chances for uncovering reactions not previously reported for a particular drug during the marketing approval process.
Post-marketing surveillance, also known as pharmacovigilance, is the process of identifying, reporting, and responding to risk-benefit issues arising with marketed medicines. Post-marketing surveillance programs use the information generated from these reports to update drug labeling and, on occasions, to reevaluate the approval or marketing decision. Even if the report does not warrant labeling changes, the information provided can signal potential problems with the use of certain drugs for which recommendations can be provided to decrease the risk, or be further investigated. Once the reports are studied and evaluated, the data generated can help to estimate risk patterns, such as identifying populations at risk of developing an ADR with certain medications, investigate the preventability of these ADRs to provide indicators for quality improvement, or signpost for interventions. The dissemination of this information is also a crucial aspect of pharmacovigilance, as it is needed for drug prescribing and regulation (Brewer and Colditz 1999).
In summary, post-marketing surveillance programs are essential in every country for monitoring the occurrence of ADRs, as the data derived from within the country may encourage national regulatory decision making. Thus, these programs may contribute to decreased morbidity, mortality, length-of-stay, healthcare costs, and liability associated with ADRs.
The New Zealand perspective
The New Zealand Quality of Healthcare Study (NZQHS) (Davis et al 2002, 2003) examined 6579 medical records using two-stage retrospective review applied to a representative sample of hospital admissions for the calendar year of 1998. The sample was drawn by systematic list selection, after the exclusion of specialist institutions, from 13 public hospitals providing acute care and with more than 100 beds. The main aim was to quantify the impact of adverse outcomes of healthcare management in the New Zealand public hospital system.
The NZQHS reported that 12.9% of public hospital admissions were associated with an adverse event, a rate that is similar to those recorded for Australia (16.6%) and the United Kingdom (10.8%) in comparable studies (Wilson et al 1995; Vincent et al 2001). Half of the events in this New Zealand study were shown to be preventable and occurred inside hospital, and, of these, 7.5% were associated with pharmacological treatment and 10.7% with therapy-related incidents (Davis et al 2002, 2003).
The Centre for Adverse Reactions Monitoring (CARM) in Dunedin is New Zealand's national monitoring centre for adverse reactions (New Zealand Pharmacovigilance Centre 2004). It collects and evaluates spontaneous reports of adverse reactions to medicines, vaccines, herbal products, and dietary supplements from health professionals in New Zealand. Currently, the CARM database holds more than 48 000 reports, providing New Zealand-specific information on ADRs to these products and serving to support clinical decision making when unusual symptoms are thought to be therapy related. CARM collaborates with and pools anonymized data with other national monitoring centers into the database of the WHO's International Drug Monitoring Programme.
CARM reports its findings to a government-appointed committee, the Medicines Adverse Reaction Committee (MARC). This committee makes recommendations to Medsafe (NZ Medicines and Medical Devices Safety Authority), which is a business unit of the Ministry of Health and is the authority responsible for the regulation of therapeutic products in New Zealand (New Zealand Medicines and Medical Devices Safety Authority 2004). Medsafe has the responsibility for implementing strategies that should result in the safer use of medicines. MARC meets four times a year to review published material, all fatal reports, and selected reports of significant, unusual, or serious reactions reported to CARM.
In addition to CARM, the Intensive Medicines Monitoring Programme (IMMP) monitors more closely selected new medicines using a method called prescription event monitoring, supplementing New Zealand's ADRs database. The IMMP aims at measuring the incidence of adverse reactions, their characterization, early identification of previously unrecognized reactions, and the construction of a risk profile for each new medicine. This network is able to keep New Zealand's international reputation in ADR monitoring and reporting abreast of the latest concerns around drug safety; for example, New Zealand led the world in taking regulatory action over agranulocytosis due to mianserin and liver toxicity due to nefazodone (Coulter and Edwards 1990; WHO 2003). All these programs constitute a significant contribution of New Zealand to the international ADR database and towards the improvement of safety of medicines worldwide.
The role of the pharmacist
Pharmacists have a central role in drug safety by contributing to the prevention, identification, documentation, and reporting of ADRs. All healthcare providers have roles to play in maintaining a balance between a medicine's benefits and risks. Once a drug is available to the public, making a determination about its safety is the shared responsibility of all who are part of the prescribing process, including patients. Pharmacists clearly understand that no drug product is completely safe and that pre-marketing trials do not fully identify the risks, particularly of recently marketed drugs. As part of the healthcare team, pharmacists advise on drug use or on the introduction to or withdrawal of a drug from the market and are often called upon in establishing the likelihood that an adverse event is in fact an ADR.
National drug monitoring programs throughout the world differ in their sources of participation in the reporting of ADRs by healthcare professionals. In contrast to Canada or the US, where the majority of the reports come from pharmacists, some countries, such as France, Ireland, Malaysia, New Zealand, the Nordic countries, and the UK, have the largest contribution of ADR reports coming from physicians (The Learning Centre 1999). The reasons for low reporting rates by pharmacists in these countries have not been adequately analyzed. It has been suggested that it may result from the simple fact that pharmacists are excluded from reporting ADRs to the national reporting program, which is the situation in the Nordic countries (eg, Finland and Sweden) (van Grootheest et al 2004). A study in the UK concluded that hospital pharmacists require continuing stimulation and education about reporting in order to raise further the profile of their role in reporting of suspected ADRs to their national pharmacovigilance program (Davis et al 1999).
Even among countries where pharmacists are allowed to report ADRs to their national program, lower reporting rates by pharmacists are observed. New Zealand is a good example of this case. As shown in Figure 1, between January and June 2004, only 5.7% of CARM reports were submitted by pharmacists (personal communication, CARM, 18 Mar 2005) compared with about 70% of ADR reports submitted to the MEDWATCH program in the US by pharmacists, most of which are from hospital-based pharmacy practitioners. Canada shows similar trends to the US in ADR reporting; for example, in the fiscal year of 1998–1999, at the British Columbia Regional ADR Centre, most ADR reports were generated by pharmacists (38.8% and 34.8% by hospital and community pharmacists, respectively), physicians' reports accounting for only 10.8% (The Learning Centre 1999).
Figure 1.
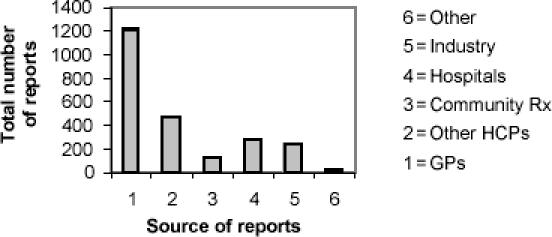
Sources of adverse drug reaction (ADR) reports in New Zealand, January–June 2004. Abbreviations: GPs, general practitioners; HCPs, healthcare professionals; Rx, pharmacists.
Predisposing factors for underreporting
Underreporting of ADRs is a common phenomenon in spontaneous post-marketing surveillance programs. Underreporting may delay signal detection and cause underestimation of the size of a problem. Correcting for underreporting is difficult, because its extent is unknown and variable. Having a better understanding of the predisposing factors, particularly as they relate to the pharmacy profession, can assist pharmacy practitioners in establishing ways to decrease underreporting.
Barriers to improved monitoring and reporting of ADRs have been analyzed in various studies (Sweis and Wong 2000; Green et al 2001; van Grootheest et al 2002; Kelly et al 2004) and can be summarized as:
fear of personal and organizational liability
lack of resources for surveillance and reporting
labor-intensive, complex, and time-consuming reporting processes
ambiguity in interpreting whether the medication was the cause of the adverse event
minimal feedback provided to reporters
no incentives, rewards, or motivation to report
lack of knowledge and confidence to distinguish between significant ADRs and minor ones
surveillance and reporting functions without a leader.
The Australian report by Kelly and colleagues (2004) looked more closely into the factors influencing ADR reporting in hospitals and classified them into predisposing and disabling factors (Table 1). Through a questionnaire to all health professionals involved in the ADR reporting process, it was found that knowledge appeared to be a greater influence on ADR reporting than attitudes and beliefs, particularly among doctors. Interestingly, pharmacists indicated a high level of knowledge and were identified and utilized by other healthcare professionals as key facilitators of the reporting process.
Table 1.
Factors influencing adverse drug reaction (ADR) underreporting
| Predisposing factors |
|---|
| Knowledge |
| Unsure how to report an ADR |
| Unsure who is responsible for reporting ADRs |
| Unsure which drug was responsible for the ADR |
| Unsure if the reaction was a side effect rather than an ADR |
| Attitudes and beliefs |
| Believed only safe drugs are allowed on the market |
| Concerned about confidentiality of information |
| Reporting could show ignorance |
| Concerned about legal liability by reporting |
| Difficult to admit harm to patient |
| Willing to publish or report only unusual cases in the literature |
| Disabling factors |
| Too busy to send ADR reports |
| Form unavailable when needed |
| Insufficient data to complete a report |
Source: Adapted from Kelly et al (2004).
Factors influencing the underreporting by pharmacists specifically have also been investigated by some authors. Sweis and Wong (2000) conducted a survey of UK hospital pharmacists which showed that they were more likely to report serious and rare ADRs and those associated with newly marketed drugs (predisposing factor: attitudes or beliefs). Van Grootheest and colleagues (2002) surveyed community pharmacists in The Netherlands, showing that the most frequently mentioned barriers to reporting were the ADR assumed to be already known or uncertainty about the causal relationship between the ADR and a drug (predisposing factor: knowledge) and the reporting procedure being too time-consuming (disabling factor: time).
Factors influencing the underreporting by New Zealand pharmacists have not yet been investigated or reported, but would be useful for establishing corrective actions.
Policy and procedures for ADR reporting in hospitals
Although CARM produces the highest rate of reporting ADRs in the world, both in terms of reports per 1000 doctors and reports per million population (New Zealand Pharmacovigilance Centre 2004), having national programs as the only source of information on ADR trends does not allow individual healthcare organizations, such as hospitals, to analyze the contribution of drug-induced illness to the morbidity, mortality, length of stay of patients, and the overall healthcare costs of the individual organization. As mentioned previously, it is well known that there is significant underreporting not only for ADRs, but for all ADEs. Most hospitals in New Zealand rely on spontaneous reporting of ADRs by their staff directly to CARM (personal communication, CARM, 18 Mar 2005), without any type of analysis of the report prior to its submission. As shown in Figure 1, ADR reports submitted by hospitals represent only about 10% of the total reports received by CARM. Similar types of reporting systems in other parts of the world have been shown to detect only a small percentage of ADRs that occur in hospitals (Cullen et al 1995).
Given the perceived failure of spontaneous reporting systems and the paucity of ADR reports, hospital accreditation commissions in countries such as Canada or the US recommended the institution of more active methods of ADR detection to supplement spontaneous reports in hospitals. In these countries, it is usually the department of pharmacy staff who coordinate the hospital's ADR reporting program and, in most cases, do so under the direction of the institution's pharmacy and therapeutics (P&T) committee. All ADR reports received are initially screened (and occasionally analyzed for causality or severity) by pharmacists before being presented to the P&T committee for review and further action. The American Society of Health-System Pharmacists (ASHP) has developed a very useful guideline for ADR monitoring and reporting by pharmacists in organized healthcare systems, which summarizes not only the role of the pharmacist, but also the crucial components of a comprehensive ADR reporting program in hospitals (ASHP 1995).
Taking the above into consideration, it appears to be important for hospitals to establish their individual policies and procedures for ADR reporting to supplement a country's spontaneous reporting system. In an attempt to have an initial understanding of this issue in New Zealand, we informally (via email communication with hospital pharmacists) investigated if District Health Boards (DHBs) had individual policies for ADR reporting, and whether these policies covered the components of a comprehensive in-house ADR reporting program as outlined by the ASHP guidelines. No source other than the written policies was utilized for the latter review.
Of the 22 DHBs to which we put the simple question “Do you have in-house policies or procedures for reporting ADRs within your DHB to supplement reporting to CARM?”, 14 responded. Eight DHBs had specific ADR reporting policies in place, three were working on writing one, and three did not have a formal policy in place, though their pharmacy departments did issue advice on reporting directly to CARM. Table 2 provides the results of how many DHBs included the specific components of an “ideal” in-house ADR program in their policy.
Table 2.
Features of adverse drug reaction (ADR) reporting policies in some New Zealand District Health Boards (DHBs)
| Components for an ideal in-house ADR program (ASHP 1995) | Nr of DHBs |
|---|---|
| Provides a definition of an ADR | 2 |
| Classifies ADRs according to type | 1 |
| Uses detection identifiers (ie, triggers that signal an investigation is warranted for ADR), such as emergency box usage, use of medicine to treat a symptom rather than a disease (eg, antihistamines) | 2 |
| Provides management guidance for ADRs | 3 |
| Evaluates all ADRs individually (ie, all reports of suspected ADRs are reviewed and differentiated from obvious medication errors) | 3 |
| Includes probability assessment (ie, by the use of scales or algorithms to determine the likelihood that the event is medicine related) | 0 |
| Provides severity ranking for all ADRs | 0 |
| Addresses the multidisciplinary responsibility for reporting | 6 |
| Uses in-house reporting forms (other than or in addition to those of CARM) | 7 |
| Tracks the pattern and incidence of ADRs within the DHB, which are reviewed by relevant committees (eg, P&T), for action and feedback | 0 |
| Recommends pharmacist's involvement in the ADR reporting procedure | 5 |
| Has provisions for follow-up reporting of all reported ADRs (eg, documentation in medical notes, patient informed, medic alert bracelet etc) | 6 |
| Has provisions to notify the patient's general practitioner about the ADR | 5 |
| Has provisions for further notification to CARM | 5 |
Abbreviations: CARM, Centre for Adverse Reactions Monitoring; P&T, pharmacy and therapeutics.
The results summarized in Table 2 show that for the eight hospitals that indicated having ADR policies and procedures available, generally these can be considered adequate in ensuring that if an ADR occurs, a multidisciplinary team (including the pharmacist) is involved in the reporting procedure, and that the message is documented and conveyed properly to minimize the risk of re-occurrence. However, the policies at these hospitals may be inadequate for providing useful guidance in assessing individual ADRs, as they lack provisions for detecting, classifying, determining causality, assigning probability, or managing ADRs, all essential for an ideal in-house ADR reporting program.
Although generalizations cannot be made from this informal investigation, the review of the policies from the eight DHBs that responded to having established policies for reporting ADRs suggests that important features of an in-house ADR reporting program within hospitals are being omitted, and that a more profound investigation into the subject may be warranted to help clarify the New Zealand perspective. More comprehensive guidelines on ADR monitoring and reporting by pharmacists than those provided in the Pharmacy Practice Handbook (Pharmaceutical Society of New Zealand 2004) may also assist pharmacists in New Zealand to become more actively involved and exert leadership in the development, maintenance, and ongoing evaluation of ADR programs within their practice settings.
Improving ADR reporting
The reporting of ADRs needs continuous stimulation. It is important to achieve the development of a positive attitude towards pharmacovigilance among healthcare professionals, including pharmacists, so that ADR reporting becomes an accepted and understood routine. Research into pharmacist ADR reporting has shown that those who undergo training are more likely to report (Sweis and Wong 2000; Green et al 2001) and that continued educational initiatives are needed for the multidisciplinary team to sustain a successful ADR monitoring and reporting program (Kelly et al 2004).
Considering that the ADR reporting rates by New Zealand pharmacists are significantly lower than those of physicians, as well as significantly lower than those contributed by pharmacists in other countries, the following recommendations have been suggested to stimulate pharmacists to overcome some of the potential barriers to ADR reporting, improve their ADR detection skills, and participate more actively in ADR prevention and management strategies; all of these may help them not only to achieve better ADR reporting rates, but to improve the quality and safety of medication use by their patients.
Making ADR reporting forms accessible
ADR reporting forms should be carried by clinical pharmacists during their routine ward rounds, as well as being readily available in all the wards for other members of the multidisciplinary team to use them (ASHP 1995; Uppsala Monitoring Center 2000). If no hospital-based reporting forms are available, use the ones provided by CARM. Alternatively, an in-house version can be developed. This alternative provides the opportunity to make the form more user-friendly, whereby only the first part of the reporting form is required to be completed by the reporting person; the rest can then be completed by the clinical pharmacist or other member of the team who can assess the reaction in more depth.
Assuring that the ADR program is multidisciplinary
A collaborative multidisciplinary approach to ADR monitoring and reporting is essential for a successful program (ASHP 1995; Uppsala Monitoring Center 2000). Nursing is a critical component of the ADR process because nurses spend the most time with the patients, and physician knowledge and experience is essential in the assessment and evaluation of an ADR. By assigning a clinical pharmacist to the wards, the detection and assessment of ADRs can be greatly enhanced, as many ADRs are discussed on rounds but rarely documented. Also, establishing contacts with the medical records and emergency departments may add significant value to the ADR detection process. Asking members of these departments to contact the pharmacy when they suspect an ADR is likely to contribute to a broader multidisciplinary ADR program. The hospital laboratory can provide assistance in the detection of ADRs by reporting patients with elevated levels of certain medications or in the detection of antibiotic-associated diarrhea with a report of positive Clostridium difficile toxin assays (Vitillo 2000).
Centralizing ADR reporting activities
Completed CARM reports, or the equivalent in-house ADR form, should initially be forwarded to a central area, such as the Medicines Information Center (MIC), for further assessment (Michel and Knodel 1986; ASHP 1995; Andrew et al 2001). A fax line, email, and online ADR reporting forms can also be available to facilitate communication in alerting the multidisciplinary team to an ADR (Vitillo 2000). Once in the central area, ADR reports must be followed up by a pharmacist. Thus, pharmacovigilance must be integrated in the activities of dispensing, MIC, and ward pharmacists.
Targeting antidote medication
This is a widely used method to improve detection of ADRs (Classen et al 1991; Dormann et al 2000). Pharmacists screen orders, usually assisted by a computerized alerting system, for antidote medications, discontinuation orders, dosage decreases, and laboratory test orders to detect ADRs. Reports of toxic plasma concentrations of medications with low therapeutic indexes are also used.
Talking to patients
Pharmacists play an integral role in gathering information from their patients, as well as in educating patients on various aspects of medication use, including safety (ASHP 1995). Many patients are not aware of important risk information about their medications, so they would not know what to expect if they experienced a potentially harmful reaction caused by one of their medications.
Providing feedback
ADR report information should be disseminated to the reporters and to all the healthcare professional staff members (ASHP 1995) in the form of newsletters (Vitillo 2000), such as an “ADR Bulletin”, which should be available on a regular basis for educational purposes and should reflect local efforts in the monitoring, prevention, detection, and management of ADRs.
Seeking administrative support
Submitting a summary of all the ADR reports to the P&T (ASHP 1995; Vitillo 2000) or other equivalent hospital medicines committee before forwarding them to CARM may prove to be valuable. These reports can be sent under the hospital medicines committee's name; thus the names of individual healthcare professionals and patients can be kept confidential. Collaborative efforts with national and international institutions working in pharmacovigilance can prove to be extremely valuable and can provide useful resources for ADR monitoring programs.
Providing incentives for ADR reporting
Examples include issuing certificates or recognition awards or pens with reminding logos on ADR reporting, which have been used as incentives to motivate departments other than pharmacy to report ADRs (Vitillo 2000).
Conclusions
The effectiveness of an ADR monitoring and reporting program depends on the awareness of all healthcare providers. It is important to address within the pharmacy profession that ADR surveillance is a priority and a professional responsibility. It is essential that programs aimed at increasing ADR surveillance include processes that are user friendly and lack negative associations or stigma. More studies on ADR monitoring and reporting in New Zealand are necessary.
ADR awareness programs have been developed in various institutions to increase the detection of ADRs, implement strategies for successful prevention and management of ADRs, and consequently contribute to improving the safety of medication use. International ADR programs indicate that a multidisciplinary approach and the involvement of the P&T or other equivalent hospital medicines committee are essential to assure more effective education and communication among all healthcare professionals on the clinical importance of ADR surveillance. Lastly, it is also important to assure that the awareness programs are ongoing, to avoid ADR surveillance losing “momentum”. Several strategies for promoting successful, ongoing ADR programs have been provided in this review.
References
- Andrew I, Baker S, Chalmers A. Implementing a system for tackling under-reporting of adverse drug reactions within a district general hospital. Int J Pharm Pract. 2001;001(9 Suppl):R71. [Google Scholar]
- [ASHP] American Society of Health-System Pharmacists. 1995 Guidelines on adverse drug reaction monitoring and reporting [online]. Accessed Nov 2004. URL: www.ashp.org/bestpractices/MedMis/MedMis_Gdl_ADR.pdf.
- Bates DW. Drugs and adverse drug reactions. How worried should we be? [editorial] JAMA. 1998;279:1216–17. doi: 10.1001/jama.279.15.1216. [DOI] [PubMed] [Google Scholar]
- Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA. 1999;28:824–9. doi: 10.1001/jama.281.9.824. [DOI] [PubMed] [Google Scholar]
- Briant R, Ali W, Lay-Yee R, et al. Representative case series from public hospital admissions 1998. I: drug and related therapeutic adverse events. NZ Med J. 2004;117(1188) [online]. Accessed Sep 2004. URL: http://www.nzma.org.nz/journal/117-1188/747/256d96007f6b4e/$FILE/ImprovingQualitySystemsApproach.pdf. [PubMed]
- Classen DC, Pestotnik SL, Evans S, et al. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266:2847–51. [PubMed] [Google Scholar]
- Coulter DM, Edwards IR. Mianserin and agranulocytosis in New Zealand. Lancet. 1990;336:785–7. doi: 10.1016/0140-6736(90)93248-n. [DOI] [PubMed] [Google Scholar]
- Cullen DJ, Bates DW, Small SD, et al. The incident reporting system does not detect adverse drug events: a problem for quality improvement. Jt Comm J Qual Improv. 1995;21:541–8. doi: 10.1016/s1070-3241(16)30180-8. [DOI] [PubMed] [Google Scholar]
- Davis P, Lay-Yee R, Briant R, et al. Occasional paper nr 3. Wellington: NZ Ministry of Health; 2001. Adverse events in New Zealand public hospitals: principal findings from a national survey. [Google Scholar]
- Davis P, Lay-Yee R, Briant R, et al. Adverse events in New Zealand public hospitals. I: occurrence and impact. NZ Med J. 2002;115(1167) [online]. Accessed Sep 2004. URL: http://www.nzma.org.nz/journal/115-1167/271. [PubMed]
- Davis P, Lay-Yee R, Briant R. Adverse events in New Zealand public hospitals. II: preventability and clinical context. NZ Med J. 2003;116(1183) [online]. Accessed 10 Sep 2004. URL: http://www.nzma.org.nz/journal/116-1183/624. [PubMed]
- Davis S, Coulson RA, Wood SM. Adverse drug reaction reporting by hospital pharmacists: the first year. Pharm J. 1999;262:366–7. [Google Scholar]
- Dormann H, Muth-Selbach U, Krebs S, et al. Incidence and costs of adverse drug reactions during hospitalisation. Computerised monitoring versus stimulated spontaneous reporting. Drug Saf. 2000;22:161–8. doi: 10.2165/00002018-200022020-00007. [DOI] [PubMed] [Google Scholar]
- Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–40. doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- Green CF, Mottram DR, Rowe PH, et al. Attitudes and knowledge of hospital pharmacist to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51:81–6. doi: 10.1046/j.1365-2125.2001.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H. Adverse drug reactions: the magnitude of the problem. J Allergy Clin Immunol. 1994;74:555–7. doi: 10.1016/0091-6749(84)90106-4. [DOI] [PubMed] [Google Scholar]
- Kelly M, Kaye KI, Davis SR, et al. Factors influencing adverse drug reaction reporting in New South Wales teaching hospitals. J Pharm Pract Res. 2004;34:32–5. [Google Scholar]
- Kohn LT, Corrigan JM, Donaldson MS. Institute of Medicine report: To err is human: building a safer health system. Washington: National Academy Pr [online]; 1999. Accessed Sep 2004. URL: http://bob.nap.edu/html/to_err_is_human. [PubMed] [Google Scholar]
- Kvasz M, Allen IE, Gordon MJ, et al. Adverse drug reactions in hospitalized patients: a critique of a meta-analysis. Medscape Gen Med. 2000;2(2) [online]. Accessed Mar 2005. URL: http://www.medscape.com/viewarticle/408052. [PubMed] [Google Scholar]
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36:1331–6. doi: 10.1345/aph.1A333. [DOI] [PubMed] [Google Scholar]
- Michel DJ, Knodel LC. Program coordinated by a drug information service to improve adverse drug reaction reporting in a hospital. Am J Hosp Pharm. 1986;43:2202–5. [PubMed] [Google Scholar]
- Miller RR. Hospital admissions due to adverse drug reactions: a report from the Boston Collaborative Drug Surveillance Program. Arch Intern Med. 1974;134:219–23. [PubMed] [Google Scholar]
- Morton J, MacMillan S. Adverse events in New Zealand healthcare. NZ Med J. 2003;116(1183) [online]. Accessed Sep 2004. URL: http://www.nzma.org.nz/journal/116-1183/623. [PubMed]
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- New Zealand Medicines and Medical Devices Safety Authority. 2004 Medsafe [online]. Accessed Sep 2004. URL: http://www.medsafe.govt.nz/other.htm.
- New Zealand Pharmacovigilance Centre. Centre for Adverse Reactions Monitoring (CARM) [online] 2004 Accessed Sep 2004. URL: http://carm.otago.ac.nz/index.asp?link=carm.
- Pharmaceutical Society of New Zealand. Pharmacy practice handbook [online] Accessed Nov 2004. URL: http://www.psnz.org.nz/practice/handbook/intro.htm.
- Roughead EE, Gilbert AL, Primrose JG. Drug-related hospital admissions: a review of Australian studies published 1988– 1996. Med J Aust. 1998;165:405–8. doi: 10.5694/j.1326-5377.1998.tb138996.x. [DOI] [PubMed] [Google Scholar]
- Sweis D, Wong ICK. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf. 2000;23:165–72. doi: 10.2165/00002018-200023020-00006. [DOI] [PubMed] [Google Scholar]
- The Learning Centre. Continuing pharmacy education; fall 1999. Canada: University of British Columbia; 1999. Pharmacists are number one. [Google Scholar]
- Uppsala Monitoring Center, WHO Collaborating Centre for International Drug Monitoring. Guidelines for setting up and running a pharmacovigilance centre. Uppsala: Uppsala Monitoring Center; 2000. Safety monitoring of medicinal products. [Google Scholar]
- van Grootheest AC, Mes K, de Jong-van den Berg LTW. Attitudes of community pharmacists in The Netherlands towards adverse drug reaction reporting. Int J Pharm Pract. 2002;10:267–72. [Google Scholar]
- van Grootheest K, Olsson S, Couper M, et al. Pharmacists' role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf. 2004;13:457–64. doi: 10.1002/pds.897. [DOI] [PubMed] [Google Scholar]
- Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. BMJ. 2001;322:517–19. doi: 10.1136/bmj.322.7285.517. (Erratum: BMJ 2001, 322:1395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitillo JA. Adverse drug reaction surveillance: practical methods for developing a successful monitoring program. Medscape Pharmacists. 2000;1(2) [online]. Accessed Mar 2005. URL: http://www.medscape.com/viewarticle/408575.
- [WHO] World Health Organization. Pharmaceuticals newsletter. Nefazodone. 2003 Nr 1 [online]. Accessed Oct 2004. URL: www.who.int/medicines/library/pnewslet/news2003.pdf.
- Wilson RM, Runciama WB, Gibberd RW, et al. The Quality in Australian Health Care Study. Med J Aust. 1995;163:458–71. doi: 10.5694/j.1326-5377.1995.tb124691.x. [DOI] [PubMed] [Google Scholar]


