Abstract
Induced sputum analysis has recently emerged as a potential new clinical tool in the diagnosis and management of obstructive airway diseases such as asthma, chronic obstructive pulmonary disease, and other disorders including bronchiectasis. Its safety has been demonstrated in numerous studies, and its efficacy is superior to previous techniques for determining airway inflammation. It is a noninvasive and highly reproducible approach in generating a measurable index of inflammatory cells in the airways of the lungs. Recent studies have shown that exacerbations, particularly in patients with moderate to severe asthma, can be reduced by routine analysis of induced sputum samples. We now have the ability to clinically apply sputum measurements to manage asthmatics. Inflammatory markers and cell types in induced sputum can also be investigated using newer technologies with more sensitive qualitative and quantitative features than basic cellular analysis. This review outlines the procedure for sputum induction, characterizes inflammatory cell types in the sputum, and addresses recent advances in the field of sputum analysis.
Keywords: asthma, chronic obstructive pulmonary disease, cystic fibrosis, spirometry, inflammation
What are obstructive airway diseases?
Obstructive airway diseases are defined as respiratory disorders with narrowing of the bronchi and bronchioles occurring as a major part of their pathophysiology. This leads to increased resistance in the airways with resultant dyspnea, wheeze, and cough. Among the most prevalent of these conditions are asthma, chronic obstructive pulmonary disease (COPD), and bronchiectasis. These are characterized and diagnosed by specific physiological abnormalities which are determined by spirometric evaluation. Airway obstruction is defined by a reduced forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio on spirometry.
Current definitions also identify that they are associated with airway inflammation, but the characteristics of the inflammation are not specific to one disease or another. In the past, asthma was considered to be eosinophilic, while COPD was thought to be primarily neutrophilic. However, one-third of asthmatics have primarily non-eosinophilic lower airway inflammation, whereas one-third of COPD patients have eosinophilic inflammation. The inflammatory phenotypes in these airway diseases may be associated with differential prognoses and treatment successes with currently available pharmacologic agents (Hargreave and Leigh 1999; Wenzel 2004). Thus, many different types of inflammation result from many different causes in obstructive airway diseases, and the structural changes which result determine the physiological abnormalities. In addition, asthma and COPD are heterogeneous in their presentation and require a careful evaluation of patients on an individual basis in order to arrive at correct diagnoses and management plans.
There are also two other airway diseases that lack the obstructive characteristics of asthma and COPD. These are eosinophilic bronchitis, which is associated with prominent eosinophilic inflammation that often resolves without accompanying airway damage or remodeling, and chronic (nonobstructive) bronchitis that is associated with neutrophilic inflammation and has a similar pathophysiology to its obstructive counterpart, COPD. These airway diseases resolve on their own without damaging inflammatory sequelae or airway structural changes. This implies that acute inflammatory infiltration on its own is not a predictor of permanent damage to the airways and that different mechanisms exist to induce structural changes. In particular, chronic inflammation, in association with structural changes due to myofibroblast recruitment (Gizycki et al 1997) and smooth muscle hyperplasia (Hirst et al 2004), may be a more important process that leads to damage and alterations in the airway physiology.
Asthma is a chronic inflammatory disorder of the airways associated with widespread but variable airflow limitation that is at least partly reversible, either spontaneously or with treatment by inhaled bronchodilators (Global Initiative for Asthma 2004). Many of the physiological symptoms of asthma are caused by an underlying inflammatory component that contributes to bronchoconstriction, which leads to enhanced airway responsiveness to a variety of stimuli (Global Initiative for Asthma 2004). The most significant clinical risk factor for the development of asthma is atopy. Atopic asthma is defined as a heritable tendency to generate allergen-specific immunoglobulin E (IgE), which sensitizes the individual to the specific aeroallergen upon exposure. In atopic asthma, airway eosinophils and their activation products have been shown to correlate with disease severity and airway hyperresponsiveness (Wardlaw et al 1997). However, a significant proportion of severe asthmatic patients have a neutrophilic inflammation in their airways, which may contribute to asphyxic episodes of asthma (Fabbri et al 1998).
Not all asthma patients are atopic. Previously, those lacking demonstrable atopy were thought to possess sensitization to as yet unrecognized agents. Evidence now suggests that factors unrelated to IgE sensitization are responsible for the development and propagation of nonatopic asthma, although high total serum IgE, in the absence of identifiable allergen-specific IgE, can be seen in patients with nonatopic asthma. For instance, nonatopic asthma tends to have a stronger association with exacerbations related to stress (Klinnert 2003), and stress-related airway inflammation may be dependent on mast cell activation (Forsythe et al 2004). Another cause of nonatopic asthma is airway obstruction following occupational exposure to toxic agents, most commonly isocyanate exposure (Tarlo and Liss 2002). Interestingly, the inflammatory characteristics in atopic and nonatopic asthma seem to be similar in some cases.
COPD is defined by the Global Initiative for Obstructive Lung Disease (GOLD) as a “disease state characterized by airflow limitation that is not fully reversible” (Pauwels et al 2001). In COPD, the physiological changes in the airways are due to loss of elasticity and recoil, which in most circumstances (> 90% in Western countries) is due to cigarette smoke exposure (Barnes 2000, 2004). In addition to inflammatory infiltration, the lungs of COPD patients have an imbalance in their lung protease/antiprotease environment which is exacerbated by oxidative stress. Proteolytic enzymes, such as matrix metalloproteases, elastase, and oxidants are produced in excess in the airways of COPD patients, and antiproteases (including α1-antitrypsin and tissue inhibitors of metalloprotease [TIMPs]) are reduced or inactive (Barnes 2000). Antiproteases are sensitive to inactivation by oxidants, which further decreases the ability of the lungs to reduce the proteolytic burden in COPD. Inflammation contributes to the protease/antiprotease imbalance, resulting in reduced elastic recoil with consequent airflow limitation and architectural damage to the airways.
A clear differentiation of asthma and COPD is sometimes difficult to determine owing to their overlapping physiological and inflammatory profiles. The heterogeneity of these two airway diseases is evident upon closer examination of the different patient populations. COPD is thought to differ from asthma because of its progressive nature of non-reversible airway obstruction (O'Donnell DE et al 2004), while asthma possesses a degree of reversibility in conjunction with airway hyperresponsiveness in most cases. Some asthma patients are prone to airway remodeling, with resultant chronic, irreversible airflow limitation (Lange et al 1998; Ulrik and Backer 1999). The prevalence of or susceptibility of patients to chronic airflow limitation in asthma is not known. This may be due to a combination of rising prevalence of asthma (ISAAC Study 1998) associated with the fact that longstanding asthmatics are often smokers, which leads to worsened control of their disease (Lange et al 1998). Approximately one-third of all asthma patients that presented to emergency departments in one multicenter trial were smokers (Silverman et al 2003).
Another obstructive airway disorder, bronchiectasis, may be due to a range of underlying causes. Its main feature is chronic mucus secretion and infections, which are exacerbated by distortions in the airway structure. Among the most common early colonizers in the airways of patients with bronchiectasis are Gram-positive organisms such as Staphylococcus aureus. As the disease progresses, the airways become colonized by predominantly Gram-negative organisms such as Pseudomonas aeruginosa and the more aggressive Burkholderia cepacia complex (Meghdas et al 2004). Other colonizers include atypical mycobacteria and fungal infections such as Aspergillus fumigatus. One cause of bronchiectasis is cystic fibrosis (CF), which is the most common serious genetic disease in Caucasian populations (Rosenstein and Zeitlin 1998). It is characterized by excessive mucus production and increased mucus viscosity, which predisposes the patient to chronic bacteria-induced respiratory tract infections and contributes to airway constriction.
The diagnosis and management of obstructive airway diseases remains a major challenge for physicians and specialists alike. New and innovative techniques are being developed in an effort to enhance our ability to recognize and treat these diseases. In the following sections we address the applicability of the recently improved sputum induction technique to facilitate treatment of these diseases.
How are obstructive airway diseases diagnosed?
Most patients with obstructive airway disease never see a specialist and are primarily diagnosed and managed by their primary care physician. Patients who present with a wheeze or cough in the absence of predating viral infection, particularly in response to allergen exposure, are usually diagnosed with asthma. These patients are given prescriptions for β2-agonists and/or inhaled corticosteroids. These drugs are highly effective at controlling asthmatic exacerbations provided they are correctly prescribed and appropriately administered (LindenSmith et al 2004). Inhaled steroids were first made available in the early 1970s and had a significant impact on the treatment of asthma. Newer formulations of these drugs have reduced systemic bioavailability, which has improved their safety for use even in children (Haahtela et al 1991; Laitinen et al 1992).
Patients having difficulty in breathing that may be accompanied by a chronic, productive cough (chronic bronchitis) are diagnosed with COPD by their primary care physicians, particularly if they are smokers. There is no effective treatment for COPD, apart from strong and repeated recommendations to quit smoking and to enhance dietary and lifestyle practices. Treatment options for COPD are extremely limited because of the restricted range of available pharmacological therapies, apart from inhaled corticosteroids, long- or short-acting β-agonists, leukotriene receptor antagonists, and theophylline, which are effective in only a subset of patients. At this time, treatment options are being increased to include phosphodiesterase-4 inhibitors, which are not yet available for prescription use. However, COPD patients given these medications appear to show no overall improvement in their lung function (Barnes 2000). Treatment of COPD patients with inhaled steroids may reduce systemic inflammation and lower cardiovascular disease risk, but its effects on reversing airway obstruction are not significant (Sin et al 2004).
Airway obstruction is measured by spirometry, defined as a reduced FEV1 to FVC ratio. Disease severity is assessed in relation to percent predicted FEV1, which is based on healthy population reference values largely determined by age, sex, height, and ethnicity. Although airway obstruction in asthma has typically been defined as having a post-bronchodilator FEV1 below the lower limit of normal values, this value is variable and does not always follow a consistent trend. Occasionally, individuals with mild asthma may have normal FEV1 on spirometry and demonstrate variability between days or upon bronchodilator administration before measurement of airway hyperresponsiveness. The most common bronchoconstricting agents used in clinical measurements are methacholine and exercise testing. Bronchoconstrictors may act directly on the airway smooth muscle (methacholine, histamine) or indirectly by triggering the release of endogenous mediators in the lungs, such as exercise, cold air, hypertonic saline inhalation, or adenosine monophosphate. Recent studies have shown that a significant proportion of physician-diagnosed asthmatics never receive a physiological evaluation, such as simple spirometry (Asthma in America 1998; Asthma in Canada 2004) and may have been unnecessarily prescribed inhaled medications (Linden Smith et al 2004). In addition, as many as 6 out of 10 asthma patients do not achieve acceptable control over their asthma in Canada (Asthma in Canada 2004).
COPD classically encompasses a spectrum of disease, primarily emphysema (at autopsy) to chronic bronchitis symptoms (daily sputum for 3 months in at least 2 consecutive years). Similar criteria for airway obstruction are used for COPD, with airway obstruction again determined by FEV1. As more sensitive computer tomography imaging is now available, we can detect earlier, or more distal disease to determine clinically unrecognizable emphysematous changes in smokers. These patients may or may not have demonstrable airflow obstruction upon spirometric evaluation and administration of a full pulmonary function test (PFT). Moreover, physiological measurements of lung function are prone to error or false-positive results if the procedure is not properly carried out by healthcare professionals (Crapo et al 2003). For these reasons, it is occasionally difficult to differentiate the diagnosis based on physiological measurements alone. Since obstructive airway diseases have an inflammatory component, the measurement of lower airway inflammation should be part of their management.
How do we measure lower airway inflammation?
Until recently, we have had to rely on bronchoscopic evaluation or exhaled breath condensate to collect specimens from the lower airways to evaluate inflammation or infection in the lung. Fiberoptic bronchoscopic evaluation techniques are well established and have been shown to generate reproducible findings on inflammatory and immune profiles in numerous studies (Anon 1985). However, they are invasive and require the application of local or even general anesthesia for sample collection. In some patients, such as those with severe asthma, it is not possible to collect samples by bronchoscopy, as this may lead to exacerbations. Noninvasive measures of lower airway inflammation are the preferred approach, and although induced sputum acquisition has been available since the 1950s, only in the past two decades has this procedure been standardized to reduce variability and adverse effects in the patients.
The use of sputum in research has improved our understanding of airway diseases in many ways because it is noninvasive (in the case of spontaneous sputum) or relatively noninvasive (with induced sputum), and cell counts in sputum have the qualities of excellent and highly reproducible measurements that are accurate and sensitive and identify the presence, type, and severity of airway inflammation. These measurements can be obtained repeatedly and in exacerbations, as well as in all severities of disease. Induced sputum, in particular, has been shown to be a highly effective method for determining the inflammatory processes in the airways (Gibson et al 1989; Pizzichini et al 1996; Pizzichini et al 1997; Jayaram et al 2000). Increasingly, sputum induction has been used in clinical and research settings to study airway inflammation in both asthma and COPD (Fahy et al 1993; Keatings and Barnes 1997; Wielders and Dekhuijzen 1997; Rutgers et al 2001). Sputum induction can also be used for the assessment of infectious processes (for example, in tuberculosis and Pneumocystis carinii infection, particularly in AIDS patients) and avoids the use of invasive bronchoscopies in these cases (McWilliams et al 2002; Turner et al 2003). The protocol for the induction of sputum consists of administration of nebulized hypertonic saline at increasing doses, which is inhaled by the patient (Figure 1) (Paggiaro et al 2002). The introduction of nebulized saline allowed the development of a standardized protocol for effective collection, whereas previously the analysis of induced sputum was considered to be highly variable and unsuitable for determining the underlying inflammatory events. In general, the quality of the specimen in induced sputum is much better than spontaneously produced sputum, with improved cell viability and greater reproducibility of cell counts.
Figure 1.
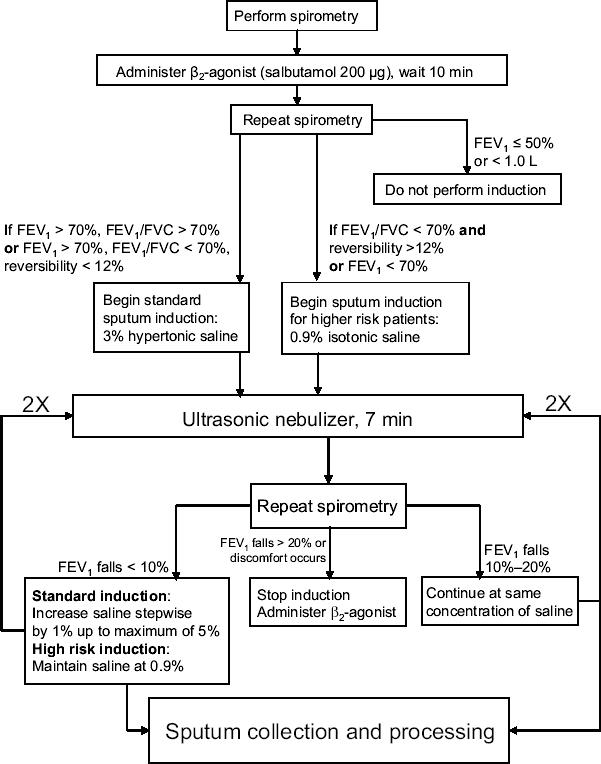
Example of a procedure for sputum induction. This scheme demonstrates a sputum induction protocol that is currently in use at the University of Alberta Hospital pulmonary function clinic. Patients are subjected to spirometry and given β2-agonist, followed by a second spirometric analysis. Based on the resulting FEV1 and FEV1/FVC ratio, the patient is given either 3% hypertonic saline for standard sputum induction or 0.9% isotonic saline for higher risk patients (to prevent the risk of exacerbations resulting from higher concentrations of saline). Patients who exhibit severe bronchoconstriction or poor lung volume are withdrawn from sputum induction. If the patient's asthma is severe from a clinical perspective, or requires oral prednisone for disease control, the patient should commence with high risk induction. Nebulized saline is administered on a repeated basis with accompanying spirometry to check for falling FEV1 or patient discomfort. Provided that spirometry results and patient comfort are within acceptable limits, a total of three steps of nebulized saline inhalation is conducted. Sputum is collected at each step following inhalation of saline and processed for analysis.
Following administration of hypertonic saline, the patient is encouraged to try to raise sputum through voluntary coughing, which benefits the process of expectoration. In cases where coughing is not spontaneously elicited, the patient is asked to cough deeply. Sputum is collected in a sterile vessel and separated for processing. The sputum consists of two major components: one is thicker mucous material that is distinct from saliva, which consists of mucus, cellular materials, and whole cells, and the second is serous fluid, which does not contain cells and is contaminated with saliva. Sputum processing involves the selection of sputum away from serous fluid, analysis of cell viability, and measurement of total and differential cell counts. The sputum can also be examined in detail by microscopy and other techniques for the presence of inflammatory mediators and cells. Typically, the yield of sputum is higher in patients with asthma and COPD than it is in normal individuals, although in a few patients (∼ 9%), it is difficult to generate sufficient sputum for analysis (Fahy et al 2001).
How safe is sputum induction?
Induced sputum has been standardized to ensure it is a safe tool and has been shown to be a reliable and valid approach for measuring inflammatory indices in asthma (Pizzichini et al 1996; Wong and Fahy 1997; de la Fuente et al 1998; Vlachos-Mayer et al 2000). Induced sputum is safe in children and those who have moderate to severe disease (Fahy et al 2001; Covar et al 2004). It has also been shown to be a reliable technique in COPD and other airway disorders (Rytila et al 2000; Kelly et al 2002; Crapo et al 2003; Turner et al 2003; Paran et al 2004).
The inhalation of hypertonic saline, or isotonic saline in some patients, is a bronchoconstrictive stimulus. The multicenter study carried out by Fahy and colleagues (2001) showed that FEV1 decreased ≥20% from the post-bronchodilator baseline in 14% of all subjects (n = 79) upon exposure to nebulized saline. Bronchoconstriction induced by saline inhalation is minimized or prevented by the administration of bronchodilators prior to the sputum induction protocol. Bronchoconstriction that occurs during saline administration can also be easily reversed by the administration of bronchodilators, and few reported complications result from this (2 out of 79 patients; bronchospasm to saline inhalation and sensitivity to methacholine were cited as reasons for complications in these patients) (Fahy et al 2001). It was advised that sputum induction not be carried out in patients with low lung volumes or poor FEV1 values. The occurrence of bronchoconstriction can be prevented and patient tolerance improved by using a relatively low output ultrasonic nebulizer without reducing the success of induction (Popov et al 1995; Kelly et al 2002). In addition, inhalation of a β2-agonist, such as albuterol (salbutamol), prior to administration of nebulized saline promotes bronchodilation and prevents bronchoconstriction (Popov et al 1995; Wong and Fahy 1997; de la Fuente et al 1998; Rytila et al 2000; Vlachos-Mayer et al 2000; Kelly et al 2002; Pizzichini 2002). Although there are no reports of fatalities associated with sputum induction, it is important to emphasize that this procedure should be done by a PFT nurse or technician trained in safe sputum induction with an attendant physician. Processing of sputum samples should be done by a technician trained in hematology to count and identify leukocytes and airway cells in sputum, as this is technically more demanding than assessing other bodily samples such as blood and urine.
In summary, the process of collection of induced sputum has developed significantly over the past decade to make it more tolerable to patients, even in those with severe bronchoconstriction. β2-Agonist administration following the procedure is routinely done to avoid further bronchoconstriction. Sputum induction can be carried out at any major hospital with a PFT laboratory and specially trained staff. The training for sputum induction requires just 1 week at a suitable PFT laboratory with a background in sputum induction.
What can sputum analysis show us?
Sputum can be analyzed for cellular markers to indicate the degree and type of inflammation in the airways of the patient at the time of collection. These measurements are critical in diagnosis and prediction of patient responses to specific treatments. Cell counts frequently correlate with respiratory physiology data and provide a useful confirmation of the disease state in the patient. In uncontrolled asthma (particularly in exacerbations), the most commonly observed inflammatory changes are elevated eosinophil numbers and increased products of eosinophil activation, including eosinophil cationic protein (ECP), major basic protein, and eosinophil-derived neurotoxin (Pizzichini et al 1996). Increasing levels of severity of asthma have been shown to correlate with sputum eosinophil levels as well as sputum ECP (Figure 2) (Louis et al 2000; Duncan et al 2003). Apoptotic eosinophils can also be detected and quantified in induced sputum as a putative measure of resolution of inflammation (Woolley et al 1996; Foresi et al 2000). Moreover, decreasing numbers of apoptotic eosinophils in sputum, in association with increased numbers of viable eosinophils, have been shown to correlate with the severity of asthma (Adachi et al 1995; Jang et al 2000; Duncan et al 2003).
Figure 2.
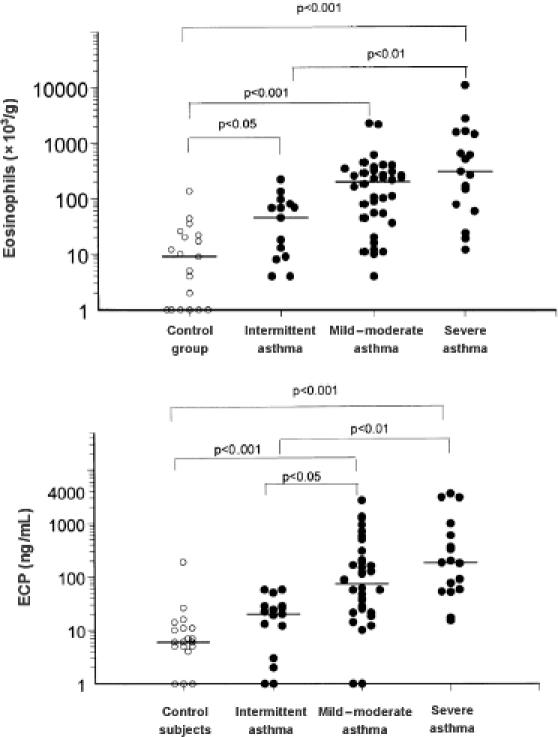
Eosinophil counts and concentrations of eosinophil cationic protein (ECP) in induced sputum of asthmatic and healthy nonatopic control subjects. Reprinted from Louis and colleagues (2000), with permission obtained from the authors and the American Thoracic Society.
Sputum analysis can also be used to determine the inflammatory response to inhaled glucocorticosteroids. Indeed, a single large dose (2400 μg) of inhaled budesonide was shown to result in a reduction in sputum eosinophil numbers 6 hours after administration (Gibson et al 2001).
A recent study by Green and colleagues (2002) demonstrated that the measurement of sputum eosinophilia can be used to manage symptoms in patients with moderate to severe asthma, which supports the use of induced sputum analysis in patient treatment. In fact, routine measurement of sputum eosinophil numbers in addition to management according to the British Thoracic Society (BTS) guidelines was a superior approach in the prevention of asthma exacerbations in one group (37 patients) compared with another group of patients who were solely managed according to the BTS guidelines (37 patients). Patients who had their sputum eosinophil numbers monitored had their steroid medication adjusted in line with changing eosinophil counts and were found to suffer fewer severe exacerbations that required hospitalization (Green et al 2002). These findings have been supported in a similar study in Canada (FE Hargreave, personal communication). These reports suggest that sputum induction and analysis may be an important procedure to assist in the management of asthma.
In COPD, the most common sputum change is neutrophilia and increased products of neutrophil activation, including proteases, myeloperoxidase, and elastase (Chung 2001; Williams and Jose 2001; Kim and Nadel 2004; O'Donnell RA et al 2004). In cigarette smokers with COPD, the degree of neutrophilia is loosely related to the degree of chronic airway obstruction (Stanescu et al 1996). This suggests that sputum neutrophils or their products may be used as early markers of the manifestation of COPD.
In as many as a third of all patients with COPD, eosinophils are also elevated in the sputum (Pizzichini et al 1998; Hargreave and Leigh 1999; Brightling et al 2000). Interestingly, COPD patients with eosinophilic sputum are more responsive to treatment with steroids (prednisone or prednisolone) (Pizzichini et al 1998). Patients with eosinophilic COPD demonstrated improved quality-of-life scores and FEV1 values following inhaled corticosteroid treatment. This indicates that sputum analysis may be important in defining a subset of COPD patients having an eosinophilic component that may benefit from treatment with inhaled corticosteroids. Glucocorticosteroids are effective at reducing eosinophilic inflammation to negligible levels; however, neutrophils are not typically thought to be responsive to steroid treatment. In fact, the survival and activity of neutrophils is enhanced by glucocorticoids, at least in vitro (Cox 1995; Strickland et al 2001). This suggests that it may be detrimental to administer glucocorticoids to patients who have a predominantly neutrophilic inflammation. However, one study suggested a beneficial effect by a 2-month treatment of COPD with high doses of inhaled beclomethasone (1500 μg/day) on reducing sputum neutrophil numbers (Confalonieri et al 1998). This observation is in contradiction to the perceived applicability of steroid treatment, and sputum analysis may be useful in determining the sensitivity of neutrophilic inflammation in the airways to high doses of steroids in further studies. Taken together, drugs used to treat asthma, COPD, and CF may benefit individual patients in a differential manner depending on the primary inflammatory cell type observed in the airways, particularly in the case of eosinophilic versus neutrophilic inflammation.
More detailed analyses of inflammatory cell function can also be carried out in research studies on sputum samples. A recent study has shown that eosinophils in asthmatic sputum show an activation profile that resembles purified peripheral blood eosinophils stimulated in vitro with chemical agonists (Lacy et al 2003). They were shown to redistribute an intracellular signaling molecule, called Rac2 GTPase, to their cell membranes, indicating activation of respiratory burst which leads to the release of damaging reactive oxygen species. This finding suggests that sputum eosinophils in asthmatics are fully activated and release cytotoxic mediators that can induce tissue injury in the airways. It also indicates that it is not the presence of eosinophils alone that correlates with their activation in the airways, as sputum eosinophils from normal subjects did not exhibit respiratory burst characteristics.
Sputum analysis gives us the opportunity to gauge the degree of inflammatory cell activation in airway diseases, which is an important indicator for the treatment and management of these disorders. It is proposed as a clinical measurement for asthma and COPD patients to determine the inflammatory processes and assist in diagnosis and management of disease. Sputum induction should be introduced with skilled technical support and an attending physician to prevent bronchoconstrictive episodes. In some cases, patients may not be able to generate sufficient sputum for analysis, and these may be directed towards alternative noninvasive techniques such as collection of exhaled breath condensates, which can be analyzed for the presence of inflammatory markers, although this approach has yet to be confirmed as a useful diagnostic approach (Effros et al 2005).
Potential new modalities of sputum analysis with clinical relevance
Several new approaches have appeared in the analysis of sputum samples that may provide important new avenues for the diagnosis and management of patients with asthma and COPD. Detailed sputum analysis is possible through the application of metabolomic techniques. Metabolomics is defined as the measurement of the complete metabolic response of an organism to an environmental stimulus or genetic modification, just as genomics describes the genetic expression of a cell or organism. One technique that can be applied in metabolomic analysis is nuclear magnetic resonance (NMR) spectroscopy. Using NMR, it is possible not only to detect, but also to quantify small amounts of metabolites in samples obtained from patients. In our case, we have measured the presence of oxidatively modified residues in sputum samples, such as modified tyrosine residues, which result from activation of airway eosinophils and neutrophils (Figure 3). The production of modified tyrosine residues occurs only during respiratory burst and degranulation of inflammatory cells and can be used to differentiate between eosinophils and neutrophils to indicate which type is likely to be dominant in the airways.
Figure 3.
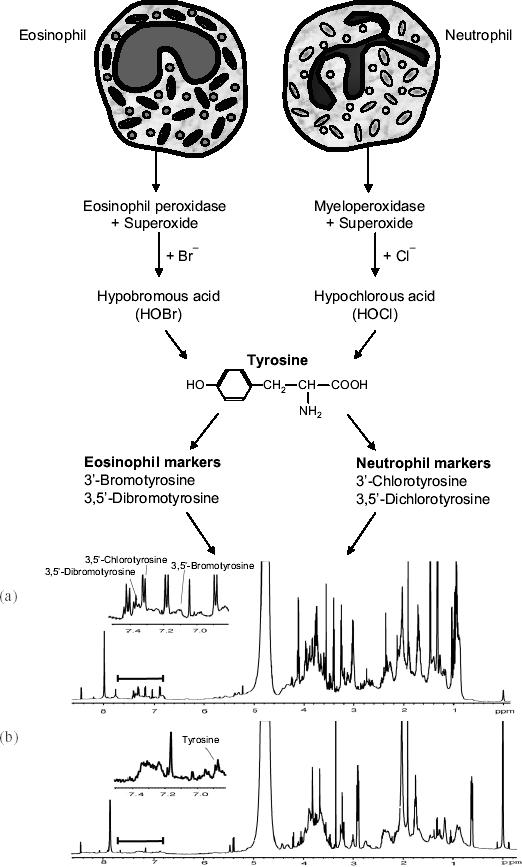
Detection of modified tyrosine residues from activated eosinophils and neutrophils in induced sputum as determined by nuclear magnetic resonance analysis. (a) Spectrum from an induced sputum sample obtained from a cystic fibrosis patient, compared with (b) a spectrum from control sputum. Peaks corresponding to tyrosine and some modified tyrosine residues are indicated. Spectral traces are from Saude et al 2004).
Specific modified tyrosine residues can be directly detected by NMR in sputum samples, which are complex biological mixtures that usually require extensive extraction protocols to determine the levels of these substances. Following a simple processing step, the sputum sample can be loaded into a high resolution NMR spectrometer for spectral analysis. In a recent study, it was possible to resolve 3-chlorotyrosine, 3-bromotyrosine, and 3,5-dibromotyrosine in sputum samples from CF patients following a spectral scan taking less than 10 minutes (Saude et al 2004). This confirms earlier studies that eosinophilic inflammation and activation occurs in CF patient airways (Halmerbauer et al 2000), although the contribution of eosinophils to the inflammatory processes in this disease is not clear. The levels of chlorinated tyrosine residues (3-chlorotyrosine) closely correlated with neutrophil percentages in sputum samples from CF patients (7 patients, r2 = 0.869) (Figure 4).
Figure 4.
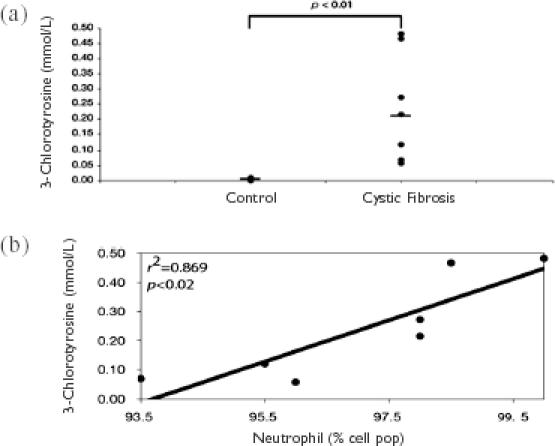
Increased 3-chlorotyrosine production in induced sputum of cystic fibrosis (CF) patients determined by nuclear magnetic resonance (NMR) analysis. Induced sputum samples from seven CF patients were analyzed by NMR to determine their levels of modified tyrosine residues. (a) The production of 3-chlorotyrosine, specific for neutrophil activation, was significantly elevated in CF sputum samples compared with control sputum samples. (b) Sputum levels of 3-chlorotyrosine correlated significantly with the percentage of sputum neutrophils from CF patients. Figures from Saude and et al (2004).
Taken together, there are many techniques that may be used for metabolomic analysis, and NMR is one of the most powerful approaches. The advantage of NMR analysis of sputum samples is that it will quantify activation products of inflammatory cells and extend measurements of total and differential cell counts, which is the usual approach in basic cellular analysis of sputum. Furthermore, as mentioned above, the presence of inflammatory cells alone in airway tissues is not always an indication that an active inflammatory process is occurring. Tissue pathology analysis traditionally assumes that an inflammatory cell infiltrate is an indication of inflammation (by measuring increased cell numbers in the tissue section or in bronchoalveolar lavage/sputum) without providing evidence to show that the inflammatory cells are concurrently activated. NMR spectroscopy analysis of sputum samples allows us to provide strong evidence of inflammatory cell activation. Moreover, it may be possible to discriminate between eosinophil and neutrophil activation in the airways, which will provide a rationale for modifications in drug treatment on an individualized basis.
Summary
Sputum induction is a novel, noninvasive, and highly reproducible technique for the analysis of cellular and inflammatory indices in obstructive airway diseases. This can be done quite easily if appropriate trained sputum laboratory staff are available to perform the inductions in a safe way. Sputum analysis is useful for continuous monitoring of airway inflammatory events in order to modify drug treatment or assist in management of patients with airway diseases in a highly individualized manner. In addition, modern techniques such as NMR spectroscopy may provide additional opportunities to manage these diseases, although more work is required before the clinical usefulness of NMR analysis on sputum samples is realized. The main drawbacks with sputum analysis are the relatively low yield of sputum in a small proportion of patients and the potential for bronchoconstrictive episodes induced by saline inhalation. Although there are no ways of overcoming poor sputum yield, bronchoconstriction can easily be reduced by the administration of bronchodilators.
References
- Adachi T, Motojima S, Hirata A, et al. Eosinophil viability-enhancing activity in sputum from patients with bronchial asthma. Contributions of interleukin-5 and granulocyte/macrophage colony-stimulating factor. Am J Respir Crit Care Med. 1995;151:618–23. doi: 10.1164/ajrccm.151.3.7881646. [DOI] [PubMed] [Google Scholar]
- Anon Summary and recommendations of a workshop on the investigative use of fiberoptic bronchoscopy and bronchoalveolar lavage in asthmatics. Am Rev Respir Dis. 1985;132:180–2. doi: 10.1164/arrd.1985.132.1.180. [DOI] [PubMed] [Google Scholar]
- Asthma in America. 1998 Accessed 9 Mar 2005. URL: http://www.asthmainamerica.com.
- Asthma in Canada. 2004 Accessed 9 Mar 2005. URL: http://www.asthmaincanada.com.
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Small airways in COPD. N Engl J Med. 2004;350:2635–7. doi: 10.1056/NEJMp048102. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–5. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- Confalonieri M, Mainardi E, Della PR, et al. Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease. Thorax. 1998;53:583–5. doi: 10.1136/thx.53.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covar RA, Spahn JD, Martin RJ, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. 2004;114:575–82. doi: 10.1016/j.jaci.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154:4719–25. [PubMed] [Google Scholar]
- Crapo RO, Jensen RL, Hargreave FE. Airway inflammation in COPD: physiological outcome measures and induced sputum. Eur Respir J Suppl. 2003;41:19s–28s. doi: 10.1183/09031936.03.00077902. [DOI] [PubMed] [Google Scholar]
- de la Fuente PT, Romagnoli M, Godard P, et al. Safety of inducing sputum in patients with asthma of varying severity. Am J Respir Crit Care Med. 1998;157:1127–30. doi: 10.1164/ajrccm.157.4.9610008. [DOI] [PubMed] [Google Scholar]
- Duncan CJ, Lawrie A, Blaylock MG, et al. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur Respir J. 2003;22:484–90. doi: 10.1183/09031936.03.00109803a. [DOI] [PubMed] [Google Scholar]
- Effros RM, Su J, Casaburi R, et al. Utility of exhaled breath condensates in chronic obstructive pulmonary disease: a critical review. Curr Opin Pulm Med. 2005;11:135–9. doi: 10.1097/00063198-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Fabbri L, Boschetto P, Caramori G. Neutrophils and asthma. In: Busse WW, Holgate ST, et al., editors. Inflammatory mechanisms in asthma. New York: Marcel Dekker; 1998. pp. 287–322. [Google Scholar]
- Fahy JV, Boushey HA, Lazarus SC, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163:1470–5. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Liu J, Wong H, et al. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147:1126–31. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- Foresi A, Teodoro C, Leone C, et al. Eosinophil apoptosis in induced sputum from patients with seasonal allergic rhinitis and with asymptomatic and symptomatic asthma. Ann Allergy Asthma Immunol. 2000;84:411–16. doi: 10.1016/S1081-1206(10)62274-0. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Ebeling C, Gordon JR, et al. Opposing effects of short- and long-term stress on airway inflammation. Am J Respir Crit Care Med. 2004;169:220–6. doi: 10.1164/rccm.200307-979OC. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Dolovich J, Denburg J, et al. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989;1:1346–8. doi: 10.1016/s0140-6736(89)92801-8. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Saltos N, Fakes K. Acute anti-inflammatory effects of inhaled budesonide in asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2001;163:32–6. doi: 10.1164/ajrccm.163.1.9807061. [DOI] [PubMed] [Google Scholar]
- Gizycki MJ, Adelroth E, Rogers AV, et al. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–73. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma. 2004 Accessed 9 Mar 2005. URL: http://www.ginasthma.com.
- Green RH, Brightling CE, Mc Kenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- Haahtela T, Jarvinen M, Kava T, et al. Comparison of a β2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325:388–92. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- Halmerbauer G, Arri S, Schierl M, et al. The relationship of eosinophil granule proteins to ions in the sputum of patients with cystic fibrosis. Clin Exp Allergy. 2000;30:1771–6. doi: 10.1046/j.1365-2222.2000.00988.x. [DOI] [PubMed] [Google Scholar]
- Hargreave FE, Leigh R. Induced sputum, eosinophilic bronchitis, and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S53–7. doi: 10.1164/ajrccm.160.supplement_1.14. [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Martin JG, Bonacci JV, et al. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S2–17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- ISAAC Study. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC) Eur Respir J. 1998;12:315–35. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- Jang AS, Choi IS, Lee S, et al. Bcl-2 expression in sputum eosinophils in patients with acute asthma. Thorax. 2000;55:370–4. doi: 10.1136/thorax.55.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram L, Parameswaran K, Sears MR, et al. Induced sputum cell counts: their usefulness in clinical practice. Eur Respir J. 2000;16:150–8. doi: 10.1034/j.1399-3003.2000.16a27.x. [DOI] [PubMed] [Google Scholar]
- Keatings VM, Barnes PJ. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med. 1997;155:449–53. doi: 10.1164/ajrccm.155.2.9032177. [DOI] [PubMed] [Google Scholar]
- Kelly MG, Brown V, Martin SL, et al. Comparison of sputum induction using high-output and low-output ultrasonic nebulizers in normal subjects and patients with COPD. Chest. 2002;122:955–9. doi: 10.1378/chest.122.3.955. [DOI] [PubMed] [Google Scholar]
- Kim S, Nadel JA. Role of neutrophils in mucus hypersecretion in COPD and implications for therapy. Treat Respir Med. 2004;3:147–59. doi: 10.2165/00151829-200403030-00003. [DOI] [PubMed] [Google Scholar]
- Klinnert MD. Evaluating the effects of stress on asthma: a paradoxical challenge. Eur Respir J. 2003;22:574–5. doi: 10.1183/09031936.03.00067903. [DOI] [PubMed] [Google Scholar]
- Lacy P, Abdel-Latif D, Steward M, et al. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol. 2003;170:2670–9. doi: 10.4049/jimmunol.170.5.2670. [DOI] [PubMed] [Google Scholar]
- Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a β2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992;90:32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- Linden Smith J, Morrison D, Deveau C, et al. Overdiagnosis of asthma in the community. Can Respir J. 2004;11:111–16. doi: 10.1155/2004/276493. [DOI] [PubMed] [Google Scholar]
- Louis R, Lau LC, Bron AO, et al. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- McWilliams T, Wells AU, Harrison AC, et al. Induced sputum and bronchoscopy in the diagnosis of pulmonary tuberculosis. Thorax. 2002;57:1010–14. doi: 10.1136/thorax.57.12.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghdas I, Loiez C, Baida N, et al. Epidemiology of infections associated to “Burkholderia cepacia complex” in the course of cystic fibrosis. Arch Pediatr. 2004;11:360–6. doi: 10.1016/j.arcped.2003.12.024. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, Aaron S, Bourbeau J, et al. State of the Art Compendium: Canadian Thoracic Society recommendations for the management of chronic obstructive pulmonary disease. Can Respir J. 2004;11(Suppl B):7B–59B. doi: 10.1155/2004/946769. [DOI] [PubMed] [Google Scholar]
- O'Donnell RA, Peebles C, Ward JA, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–42. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paggiaro PL, Chanez P, Holz O, et al. Sputum induction. Eur Respir J Suppl. 2002;37:3s–8s. doi: 10.1183/09031936.02.00000302. [DOI] [PubMed] [Google Scholar]
- Paran D, Fireman E, Elkayam O. Pulmonary disease in systemic lupus erythematosus and the antiphospholpid syndrome. Autoimmun Rev. 2004;3:70–5. doi: 10.1016/S1568-9972(03)00090-9. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154:308–17. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- Pizzichini E, Pizzichini MM, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–17. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- Pizzichini MM. Is sputum eosinophilia a good or poor predictor of benefit from inhaled corticosteroid therapy in asthma? Eur Respir J. 2002;20:1359–61. doi: 10.1183/09031936.02.00068102. [DOI] [PubMed] [Google Scholar]
- Pizzichini MM, Pizzichini E, Clelland L, et al. Sputum in severe exacerbations of asthma: kinetics of inflammatory indices after prednisone treatment. Am J Respir Crit Care Med. 1997;155:1501–8. doi: 10.1164/ajrccm.155.5.9154849. [DOI] [PubMed] [Google Scholar]
- Popov TA, Pizzichini MM, Pizzichini E, et al. Some technical factors influencing the induction of sputum for cell analysis. Eur Respir J. 1995;8:559–65. [PubMed] [Google Scholar]
- Rosenstein BJ, Zeitlin PL. Cystic fibrosis. Lancet. 1998;351:277–82. doi: 10.1016/S0140-6736(97)09174-5. [DOI] [PubMed] [Google Scholar]
- Rutgers SR, Timens W, Kauffman HF, et al. Markers of active airway inflammation and remodelling in chronic obstructive pulmonary disease. Clin Exp Allergy. 2001;31:193–205. doi: 10.1046/j.1365-2222.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- Rytila PH, Lindqvist AE, Laitinen LA. Safety of sputum induction in chronic obstructive pulmonary disease. Eur Respir J. 2000;15:1116–19. doi: 10.1034/j.1399-3003.2000.01522.x. [DOI] [PubMed] [Google Scholar]
- Saude EJ, Lacy P, Musat-Marcu S, et al. NMR analysis of neutrophil activation in sputum samples from patients with cystic fibrosis. Magn Reson Med. 2004;52:807–14. doi: 10.1002/mrm.20242. [DOI] [PubMed] [Google Scholar]
- Silverman RA, Boudreaux ED, Woodruff PG, et al. Cigarette smoking among asthmatic adults presenting to 64 emergency departments. Chest. 2003;123:1472–9. doi: 10.1378/chest.123.5.1472. [DOI] [PubMed] [Google Scholar]
- Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–5. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- Stanescu D, Sanna A, Veriter C, et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51:267–71. [Google Scholar]
- Strickland I, Kisich K, Hauk PJ, et al. High constitutive glucocorticoid receptor β in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med. 2001;193:585–94. doi: 10.1084/jem.193.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlo SM, Liss GM. Diisocyanate-induced asthma: diagnosis, prognosis, and effects of medical surveillance measures. Appl Occup Environ Hyg. 2002;17:902–8. doi: 10.1080/10473220290107101. [DOI] [PubMed] [Google Scholar]
- Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204–8. doi: 10.1183/09031936.03.00035303. [DOI] [PubMed] [Google Scholar]
- Ulrik CS, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J. 1999;14:892–6. doi: 10.1034/j.1399-3003.1999.14d27.x. [DOI] [PubMed] [Google Scholar]
- Vlachos-Mayer H, Leigh R, Sharon RF, et al. Success and safety of sputum induction in the clinical setting. Eur Respir J. 2000;16:997–1000. doi: 10.1183/09031936.00.16599700. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Moqbel R, Kay AB. Eosinophils and the allergic inflammatory response. In: Kay AB, editor. Allergy and allergic diseases. Oxford: Blackwell Scientific; 1997. pp. 171–97. [Google Scholar]
- Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170:579–80. doi: 10.1164/rccm.2407005. [DOI] [PubMed] [Google Scholar]
- Wielders PL, Dekhuijzen PN. Disease monitoring in chronic obstructive pulmonary disease: is there a role for biomarkers? Eur Respir J. 1997;10:2443–5. doi: 10.1183/09031936.97.10112443. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Jose PJ. Neutrophils in chronic obstructive pulmonary disease. Novartis Found Symp. 2001;234:136–41. doi: 10.1002/0470868678.ch9. [DOI] [PubMed] [Google Scholar]
- Wong HH, Fahy JV. Safety of one method of sputum induction in asthmatic subjects. Am J Respir Crit Care Med. 1997;156:299–303. doi: 10.1164/ajrccm.156.1.9610114. [DOI] [PubMed] [Google Scholar]
- Woolley KL, Gibson PG, Carty K, et al. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:237–43. doi: 10.1164/ajrccm.154.1.8680686. [DOI] [PubMed] [Google Scholar]


