Abstract
Selectins mediate tethering and rolling of leukocytes to the vascular endothelium, the first adhesive step in the recruitment of immune cells to inflamed tissues. Thus, selectins play a key role in the pathogenesis of common inflammatory skin disorders such as atopic dermatitis or psoriasis. As a consequence of their key functions, selectins have received much attention as potential target structures for new therapies. Indeed, a number of agents including small-molecule as well as peptide compounds interfering with selectin functions have been developed to treat inflammatory disorders. However, many of the selectin-directed compounds have not held up to the high expectations, in some cases due to overlapping and mutually compensating functions of selectins or suboptimal pharmacokinetic properties of the compounds, while other agents appear to be more promising candidates and have already entered clinical trials. Selectively targeting the functions of one or several selectins involved in the cascade of leukocyte recruitment promises exciting new therapeutic options, but, at the same time, bears considerable imponderables, which will be discussed in this review article.
Keywords: lymphocyte recruitment, adhesion molecules, chemokines, inflammation, target molecules, selectins
Introduction
Recruitment of lymphocytes in a tissue-specific fashion is, on the one hand, a requirement for immune surveillance and defense against pathogens and tumors, but, on the other hand, plays a key role in the pathogenesis of inflammatory diseases, such as psoriasis, eczematous disorders, drug-induced eruptions, or lichen planus of the skin (Groves and Kupper 1996; Robert and Kupper 1999). Consequently, insight into mechanisms of leukocyte recruitment to the skin is essential not only for our understanding of the pathophysiology of inflammatory skin disorders, but also for the development of novel and selective therapies to treat such diseases, which heavily impact quality of life as well as the economic burden of healthcare systems worldwide. Given that the first steps of tissue-selective trafficking of leukocytes are mediated primarily by selectins, these molecules are being explored as promising therapeutic target structures.
Tethering and rolling of leukocytes: the selectin-mediated first contact to the vessel wall
The first steps of leukocyte localization to all tissues include tethering and rolling along the vessel wall (Figure 1) – rather loose and transient adhesive interactions that are essential for the subsequent firm adhesion, activation, and transmigration (von Andrian and Mackay 2000). Members of the selectin family of adhesion molecules are the primary mediators of these initial contacts (Groves et al 1991; Shimizu et al 1991; von Andrian et al 1991; Smith et al 1993; Springer 1994; Butcher and Picker 1996). Selectins are single-chain transmembrane adhesion molecules, which have been named for their lectin-like domain that attaches to carbohydrate ligands presented on glycoprotein scaffolds (Varki 1994; Feizi 2001; Ley 2001). Three selectins have been described: E- and P-selectin are expressed by endothelial cells, and L-selectin is expressed by leukocytes. E-selectin (CD62E) is primarily regulated on the transcriptional level and is strongly induced upon activation of endothelial cells by proinflammatory stimuli (Cotran and Gimbrone 1986; Bevilacqua and Stengelin 1989). In contrast, P-selectin (CD62P) is rapidly mobilized to the cell surface from its storage vesicles, the cytoplasmic Weibel-Pallade bodies, upon activation of endothelial cells (Bonfanti and Furie 1989; McEver and Beckstead 1989). In addition, there is some transcriptional regulation of P-selectin (Ulbrich et al 2003). The pivotal role of P- and E-selectin for lymphocyte rolling has been demonstrated in a large variety of experimental approaches interfering with adhesive interactions of selectins and their carbohydrate ligands (Carlos and Harlan 1994; Todderud et al 1997). Of note, several studies have demonstrated considerably overlapping and mutually compensating functions of the selectin family members, a finding that raises important consequences and constraints for the rational design of selectin-blocking drugs (Labow et al 1994; Jung and Ley 1999; Weninger et al 2000; Collins et al 2001). Consistent with these studies, a function-blocking antibody specifically directed against E-selectin did not significantly alleviate psoriasis, one of the most common chronic inflammatory skin disorders, in a clinical trial (Bhushan et al 2002). Likewise, specifically blocking L-selectin in a recent controlled clinical trial has failed to improve psoriatic lesions (Hardtke et al 2005). These observations in clinical settings have strongly supported and complemented the results from experimental studies showing that some selectin-mediated functions may be redundant and that interfering with a single selectin alone is not sufficient to interrupt the inflammatory chains in some inflammatory diseases. This notion is further corroborated by several small-molecule compounds that interfere with the functions of more than one selectin. The list of such compounds includes efomycine M, a non-carbohydrate macrolide inhibitor of both E- and P-selectin functions. Blocking both E- and P-selectin by efomycine M significantly diminished rolling of leukocytes on cutaneous microvessels and markedly alleviated chronic inflammatory skin conditions in a T cell-mediated murine model of psoriasis as well as in human psoriatic skin transplanted onto scid/scid mice (Schön et al 2002). Another promising example exhibiting broader activity against all three known selectins is bimosiamose, a compound that appeared to be efficacious in early clinical trials (Aydt and Wolff 2002–2003; Hicks et al 2005). Likewise, broader clinical efficacy may be expected from some other recently described compounds interfering with the functions of more than one selectin (Boehncke and Schön 2003; Ulbrich et al 2003).
Figure 1.
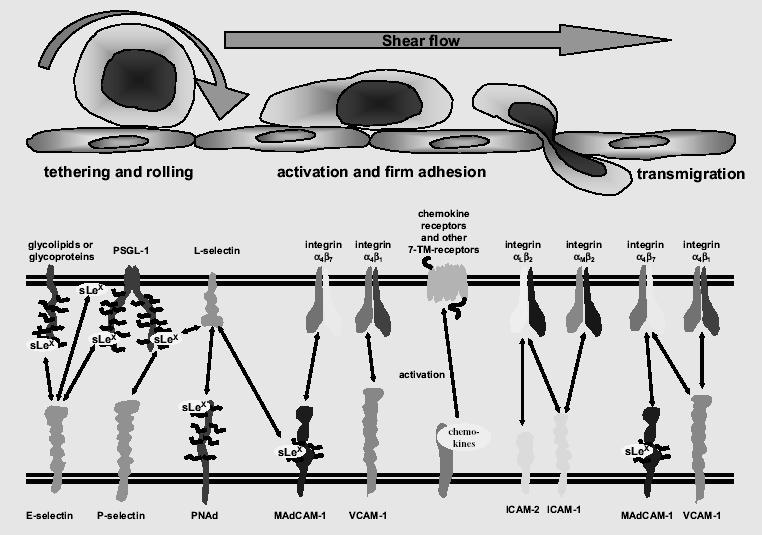
Molecular interactions involved in lymphocyte extravasation in the skin (modified from von Andrian and Mackay 2000; Schön et al 2003). The upper part of the figure depicts key steps of interactions with the endothelial lining resulting in extravasation of lymphocytes. The bottom part shows selected adhesion molecules which mediate these events.
The lymphocyte-expressed L-selectin (CD62L) binds to endothelial cell selectin ligands (Figure 1). Although some of its ligands, such as PNAd (Peripheral Node Addressin), may be induced in chronic inflammatory skin disorders, the contribution of L-selectin to cutaneous recruitment of lymphocytes appears to be limited due to its preferential expression by naive and a subpopulation of memory T cells rather than activated T cells. Thus, it has been proposed that L-selectin/PNAd interactions may be more important for extravasation of naive T cells in lymph nodes (von Andrian and Mempel 2003; Ley and Kansas 2004), a prerequisite for the priming of naive T lymphocytes. The topographic distribution of L-selectin at the tips of microvilli of rolling lymphocytes is thought to be important for easy contact formation with endothelial ligands (Fors et al 2001). Following ligand binding, L-selectin is shed from the cell surface at the trailing edge of rolling lymphocytes. The shedding of L-selectin is a proteolytic process mediated by metalloproteases (Condon et al 2001; Zhao et al 2001). It is thought that shedding is required for proper lymphocyte rolling (Hafezi-Moghadam and Ley 1999), since inhibition of L-selectin shedding resulted in increased LFA-1/ICAM-1-mediated firm adhesion and, consecutively, transmigration of lymphocytes (Hafezi-Moghadam et al 2001), supporting the hypothesis of a regulatory function of the shedding process.
In addition to the expression of L-selectin, lymphocytes express transmembrane glycoproteins bearing Sialyl-LewisX (sLeX) moieties, that function as E- and P-selectin ligands (Varki 1994). Among these ligands, the sLeX-bearing CLA (cutaneous lymphocyte-associated antigen), a specially glycosylated form of PSGL-1 (P-selectin glycoprotein ligand-1, CD162) (Fuhlbrigge et al 1997), is preferentially expressed by lymphocytes localizing to the skin. Accumulating experimental evidence has been published demonstrating that CLA is indeed functionally involved in tissue-specific recruitment to this organ (Picker et al 1990, 1991). Topographic specialization of microvascular endothelial cells within the skin is suggested by the observation that CLA-bearing T lymphocytes appear to extravasate preferentially through the endothelium of the superficial dermal plexus (Kunstfeld et al 1997), a cutaneous compartment potentially accessible for topically applied selectin inhibitors.
Selectin-mediated rolling of lymphocytes is complemented by VLA-4 (very late-activation antigen-4, CD49d/CD29), a heterodimeric adhesion receptor of the integrin family that binds to VCAM-1 (vascular cell adhesion molecule-1) and MAdCAM-1 (mucosal addressin cell adhesion molecule-1), two members of the immunoglobulin (Ig) superfamily of adhesion molecules (Figure 1). It has been reported that VLA-4 also mediates rolling of leukocyte subsets under certain conditions (Berlin et al 1995; Reinhardt et al 1997; Singbartl et al 2001). It is thought that its involvement in lymphocyte rolling is due, at least in part, to the topographic presentation of VLA-4 on microvilli of rolling cells, similar to the decoration of Lselectin, thus enabling easy contact formation with endothelial cell-expressed counter-receptors (Berlin et al 1995). The ligand binding affinity of VLA-4 can be rapidly up-regulated via the p56lck Src kinase signaling pathway following T lymphocyte stimulation (Feigelson et al 2001). This rapid change in integrin activity is thought to be crucial for the transition from rolling to firm adhesion of lymphocytes. While some aspects of the interplay of VLA-4- and selectin-mediated adhesive interactions involved in lymphocyte rolling still remain to be unraveled, it appears that there is some redundancy in their functions. In cutaneous inflammation in rats, all three receptors, E-selectin, P-selectin, and VLA-4, are apparently required for rolling of memory T lymphocytes (Issekutz AC and Issekutz TB 2002). It is possible that tissue-specific (micro)-environmental factors regulate their relative contributions to lymphocyte recruitment. In addition to VLA-4, the integrin LFA-1 has been reported to mediate neutrophil rolling under certain conditions (Henderson et al 2001), and both VLA-4 and LFA-1 can mediate selectin-independent recruitment of neutrophils and monocytes to sites of chronic inflammation (Birner et al 2000). Fractalkine may be another player contributing to endothelial capture and firm adhesion of leukocytes without an involvement of selectins (Fong et al 1998; Haskell et al 2000).
Finally, in addition to rolling of lymphocytes through direct contact to the endothelial lining, recent research has highlighted the importance of platelets for rolling under certain conditions (Klinger and Jelkmann 2002; Liu and Kubes 2003). While P-selectin has been implicated in adhesive interactions of platelets and leukocytes, the exact contribution of this process to cutaneous inflammation has not been completely defined yet (Geng et al 2004; Ludwig et al 2004).
Selectins as molecular targets for antiinflammatory therapies
Given that selectins mediate the critical first step in the cascade of leukocyte recruitment during inflammatory responses, it is not surprising that these molecules have received much attention as potential target structures for selective and specific therapies. Indeed, few areas in biomedical and pharmaceutical research reflect the excitement and enthusiasm, but also a number of drawbacks and disappointments associated with new developments as vividly as selectin-directed antiinflammatory strategies targeting lymphocyte extravasation. Indeed, as they appear to meet the “desire” for a mechanistic basis of diseases and, consecutively, rational therapeutic strategies, selectins are appealing target structures (Boehncke and Schön 2003; Schön et al 2003). This is reflected by a variety of selectin-directed agents including small-molecule compounds, carbohydrate mimetics, and antibodies generated by several companies to treat inflammatory disorders (synopsis shown in Table 1). However, a closer look at the complex and intertwined molecular crosstalk underlying lymphocyte recruitment may dampen some illusions or dishearten overenthusiastic approaches. In addition, recruitment to inflamed tissues may be tightly connected with other cellular functions of immunocytes such as chemokine receptor-mediated activation that follows selectin-mediated adhesion. As a consequence, inflammatory diseases encountered by clinicians are rarely predictable from single molecular pathways, and the design or selection of compounds targeting specific molecules involved in the inflammatory cascade is often difficult and may face considerable imminent problems (Kaila and Thomas 2002). This is reflected by the fact that some of the programs searching for inhibitors of the selectin family have been stopped for the indication of inflammatory disorders (Table 1), a notion that may reflect inherent difficulties in targeting this very initial step of leukocyte recruitment.
Table 1.
Selectin-directed compounds developed for the treatment of inflammatory disorders
| Target | Compound | Company | Status | Reference |
|---|---|---|---|---|
| Selectins | CY-1503 | Cytel Corp | Preclinical | Kerr et al 2000 |
| Selectins | BMS-190394 | BMS | Preclinical | Birnbaum et al 1997 |
| Selectins | Aventis | Preclinical | Marinier et al 2001 | |
| Selectins | OC-229648 | Ontogen Kanebo | Preclinical | |
| Selectins | efomycine M | none | Preclinical | Schön et al 2002 |
| E-selectin | ESA-2 | Novartis | Preclinical | Thomson et al 1993; Bantely and Ernst 2001; Hafezi-Moghadam et al 2001; Biedermann et al 2002 |
| Selectins | OJ- R9188 | Nippon Organon | Preclinical | Ikegami-Kuzuhara et al 2001 |
| Selectins | TBC-1269 | Texas Biotechnology Revotar | Phase I/II | Abraham et al 1999; Anaya-Prado et al 2002 |
| E- and P-selectin | PS3 | None | Preclinical | Yvin et al 2002 |
| E- and P-selectin | EP-5C7 | Protein Design Labs | Phase I | Berg et al 1995; Alon et al 1998 |
| E- and L-selectin | EL-246 | LygoCyte | Preclinical | Carraway et al 1998 |
| E-selectin | CDP-850 | Celltech | Phase II | Bhushan et al 2002 |
| P-selectin | rhPSGL-1 Ig | Genetics Institute | Phase II | Khor et al 2000 |
| L-selectin | LD201t1 | WyethNeXStar | Preclinical | Hicke et al 1996 |
Overall, there are several challenges that may make approaches to target molecules involved in leukocyte recruitment very complex and unpredictable, and this is mirrored by the fact that some of the previous approaches were not satisfying in preclinical or clinical trials. This includes trials using the sialyl LewisX-mimetic Cylexin (Kerr et al 2000), an anti E-selectin-directed monoclonal antibody (Bhushan et al 2002), or a L-selectin-directed antibody (Hardtke et al 2005). Several reasons, which are not mutually exclusive, can be delineated:
Specificity for only one selectin or a suboptimal combination of selectin specificities;
Low IC50 values;
Short half-lives or other unfavorable pharmacokinetic properties; or
Lability of selectin–selectin ligand interaction, which is essential for short-lived lymphocyte–endothelial interactions.
So, what are the challenges that we are facing? Perhaps the most important reason for the limited predictability of antiinflammatory approaches through targeting selectins is functional redundancy; ie, as outlined above, selectins may have considerable functional overlaps. In particular, this is true for endothelial E- and P-selectin (Labow et al 1994; Jung and Ley 1999; Collins et al 2001), allowing them to compensate for each other in case of loss of function of one of the players. While prominent examples have been reported in the selectin field (Labow et al 1994; Jung and Ley 1999; Collins et al 2001), this problem is by no means unique to this family, since similar (maybe even more pronounced) redundancy problems are faced when targeting the chemokine/chemokine receptor system (Zlotnik and Yoshie 2000; Onuffer and Horuk 2002).
It was long believed that CLA expressed by skin-homing T cells is mainly an E-selectin ligand (Berg et al 1991; Rossiter et al 1994). However, the carbohydrate moieties mediating tethering and rolling of lymphocytes along the endothelial lining also bind to P- and L-selectin (Labow et al 1994; Jung and Ley 1999; Collins et al 2001). Activated endothelial cells in inflamed tissues may express PNAd, a ligand for lymphocyte-expressed L-selectin, although L-selectin itself is not expressed by endothelial cells. This mechanism may compensate for lymphocyte rolling when E- and P-selectin are selectively blocked, eg, by small-molecule- or antibody-based therapeutics (Alon and Feigelson 2002; Dwir et al 2002). In addition, T cells may roll along the vessel wall through cluster formation with activated platelets (Diacovo et al 1996, 1998; Ludwig et al 2004), and even in the complete absence of selectins and their ligands, rolling of some lymphocytes may occur through interactions of endothelial VCAM-1 with the lymphocyte-expressed VLA-4 integrin (Jung and Ley 1999). Similar problems may be encountered when glycosylation of selectin ligands is targeted, eg, by proteasome inhibitors (Zollner et al 2002). Rational drug design of compounds modeled after functionally relevant groups, or high-throughput screenings assessing the functions of single molecules as readout parameters might, therefore, confront the aforesaid problems.
Recent examples of approaches that may have been too specific (thus neglecting functional redundancy) include the failure of antibodies directed specifically against E-selectin or L-selectin to alleviate psoriasis in controlled clinical trials (Bhushan et al 2002; Hardtke et al 2005), or the limited clinical response to recombinant human (rh) PSGL-1 Ig (Khor et al 2000). In that respect, concepts interfering with a group of molecules with overlapping functions involved in a given step of lymphocyte extravasation may bear some advantages, such as efomycine M or the TBC-1269 compound (bimosiamose) blocking several selectins (Abraham et al 1999; Schön et al 2002). However, the success of such broader strategies is not guaranteed, as exemplified by the insufficient response to the pan-selectin inhibitor OJ-R9188 (Ikegami-Kuzuhara et al 2001).
Another group of potential problems relates to unexpected effects; ie, targeting of certain molecules may result in unexpected or even paradoxical outcomes. As a possible example, a regulatory effect of chemokines on lymphocyte rolling has recently been identified, inasmuch as chemokines may exert (paradoxical) antiadhesive effects through G-protein-independent destabilization of L-selectin (Grabovsky et al 2002).
An alternative or additional approach to target leukocyte rolling along the skin microvasculature through modulation of selectin functions is interference with fucosyltransferase(FucT)-VII and -IV, key enzymes in the generation of selectin-ligand carbohydrate moieties. The rationale behind this approach comes from the observation that selectin ligand activity is absent or reduced dramatically in FucT-VII single or FucT-IV and -VII double knockout mice. Cutaneous inflammatory responses are markedly reduced in several models of inflammatory diseases using animals deficient for one or both enzymes (Maly et al 1996; Weninger et al 2000; Homeister et al 2001; Asadullah et al 2002). The relevance of this impaired function in human patients is highlighted by leukocyte adhesion deficiency II (LAD II), a rare congenital immune defect (Gu et al 2004). One potential approach to interfere with expression of FucT-VII is inhibiting its transcription using NF-κB inhibitors such as the proteasome inhibitor PS-519 or antioxidants such as N-acetyl cysteine (Zollner et al 2002). However, given that interfering with the pleiotropic effects of NF-κB may have many other effects on the immune system and other cells, the low specificity of this approach is a potential disadvantage. Another strategy might encompass the inhibition of FucT activity by small-molecule compounds. As posttranslational glycosylation of proteins by FucTs occurs in the Golgi apparatus, it might, however, be challenging to identify small molecules penetrating both the cell membrane and the Golgi membrane. At least two different companies (GlaxoSmithKline, Kyowa Hakko Kogyo) have reported recombinant expression of FucT-VII protein (Shinkai et al 1997; Smithers et al 1997), suggesting that this latter approach has been pursued. In addition, panosialins A and B have been isolated as inhibitors of FucTVII from the culture broth of Streptomyces sp., which inhibit FucT-VII activity and cell binding to immobilized selectin ligands in vitro (Shinoda et al 1998).
Overall, it is generally not easy to predict the clinical feasibility and efficacy of selectively targeting selectin functions in the treatment of inflammatory disorders. It appears that at least two levels of complex interactions influence the success of therapeutic strategies aimed at lymphocyte extravasation; ie, distinct players mediating consecutive pathogenic steps (“vertical”, eg, chemokine receptor activation followed by integrin-mediated firm arrest), as well as synchronous action of several molecules mediating a given event (“horizontal”, eg, selectins and chemokine receptors simultaneously involved in lymphocyte rolling).
Acknowledgments
This work was supported by a Rudolf Virchow Award from the Deutsche Forschungsgemeinschaft to MPS.
References
- Abraham WM, Ahmed A, Sabater JR, et al. Selectin blockade prevents antigen-induced late bronchial responses and airway hyperresponsiveness in allergic sheep. Am J Respir Crit Care Med. 1999;159:1205–14. doi: 10.1164/ajrccm.159.4.9806002. [DOI] [PubMed] [Google Scholar]
- Alon R, Chen S, Fuhlbrigge R, et al. The kinetics and shear threshold of transient and rolling interactions of L-selectin with its ligand on leukocytes. Proc Natl Acad Sci U S A. 1998;95:11631–6. doi: 10.1073/pnas.95.20.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Anaya-Prado R, Ramos-Kelly JR, Toledo-Pereyra LH, et al. Multiple selectin blockade with a small-molecule selectin inhibitor does not affect survival after a second inflammatory challenge with nonlethal LPS. J Invest Surg. 2002;15:171–80. doi: 10.1080/08941930290085921. [DOI] [PubMed] [Google Scholar]
- Asadullah K, Lowe JB, Schottelius A. Differential influences on skin inflammation models in mice deficient of alpha(1,3)fucosyltransferase VII. Arch Dermatol Res. 2002;294:43. [Google Scholar]
- Aydt E, Wolff G. Development of synthetic pan-selectin antagonists: a new treatment strategy for chronic inflammation in asthma. Pathobiology. 2002–2003;70:297–301. doi: 10.1159/000070746. [DOI] [PubMed] [Google Scholar]
- Bantely R, Ernst B. Synthesis of Sialyl Lewis X mimetics. Modifications of the 6-position of galactose. Bioorg Med Chem Lett. 2001;11:459–62. doi: 10.1016/s0960-894x(00)00692-2. [DOI] [PubMed] [Google Scholar]
- Berg EL, Fromm C, Melrose J, et al. Antibodies cross-reactive with E- and P-selectin block both E- and P-selectin functions. Blood. 1995;85:31–7. [PubMed] [Google Scholar]
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule-1. J Exp Med. 1991;174:1461–6. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, et al. Alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–22. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Stengelin S. Endothelial leukocyte adhesion molecule-1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–3. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bhushan M, Bleiker TO, Ballsdon AE, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. Br J Dermatol. 2002;146:824–31. doi: 10.1046/j.1365-2133.2002.04743.x. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Schwärzler C, Lametschwandtner G, et al. Targeting CLA/E-selectin interactions prevents CCR4-mediated recruitment of human Th2 memory cells to human skin in vivo. Eur J Immunol. 2002;32:3171–80. doi: 10.1002/1521-4141(200211)32:11<3171::AID-IMMU3171>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Patterson M, Kloner RA. The effect of CY-1503, a sialyl LewisX analog blocker of the selectin adhesion molecules, on infarct size and “no reflow” in the rabbit model of acute infarction/reperfusion. J Mol Cell Cardiol. 1997;29:2013–25. doi: 10.1006/jmcc.1997.0393. [DOI] [PubMed] [Google Scholar]
- Birner U, Issekutz TB, Walter U, et al. The role of alpha(4) and LFA-1 integrins in selectin-independent monocyte and neutrophil migration to joints of rats with adjuvant arthritis. Int Immunol. 2000;12:141–50. doi: 10.1093/intimm/12.2.141. [DOI] [PubMed] [Google Scholar]
- Boehncke WH, Schön MP. Interfering with leukocyte rolling – a promising therapeutic approach in inflammatory skin disorders? Trends Pharmacol Sci. 2003;24:49–52. doi: 10.1016/s0165-6147(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Bonfanti R, Furie BC. PADGEM (GMP140) is a component of Waibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–12. [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- Carraway MS, Welty-Wolf KE, Kantrow SP, et al. Antibody to Eand L-selectin does not prevent lung injury or mortality in septic baboons. Am J Respir Crit Care Med. 1998;157:938–49. doi: 10.1164/ajrccm.157.3.9707129. [DOI] [PubMed] [Google Scholar]
- Collins RG, Jung U, Ramirez M, et al. Dermal and pulmonary inflammatory disease in E-selectin and P-selectin double-null mice is reduced in triple-selectin-null mice. Blood. 2001;98:727–35. doi: 10.1182/blood.v98.3.727. [DOI] [PubMed] [Google Scholar]
- Condon TP, Flournoy S, Sawyer GJ, et al. ADAM17 but not ADAM10 mediates tumor necrosis factor-alpha and L-selectin shedding from leukocyte membranes. Antisense Nucleic Acid Drug Dev. 2001;11:107–16. doi: 10.1089/108729001750171353. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Gimbrone MAJ. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986;164:661–6. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo TG, Catalina MD, Siegelman MH, et al. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med. 1998;187:197–204. doi: 10.1084/jem.187.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo TG, Puri KD, Warnock RA, et al. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–5. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- Dwir O, Steeber DA, Schwarz US, et al. L-selectin dimerization enhances tether formation to properly spaced ligand. J Biol Chem. 2002;277:21130–9. doi: 10.1074/jbc.M201999200. [DOI] [PubMed] [Google Scholar]
- Feigelson SW, Grabovsky V, Winter E, et al. The Src kinase p56(lck) up-regulates VLA-4 integrin affinity. Implications for rapid spontaneous and chemokine-triggered T cell adhesion to VCAM-1 and fibronectin. J Biol Chem. 2001;276:13891–901. doi: 10.1074/jbc.M004939200. [DOI] [PubMed] [Google Scholar]
- Feizi T. Carbohydrate ligands for the leukocyte-endothelium adhesion molecules, selectins. Results Probl Cell Differ. 2001;33:201–23. doi: 10.1007/978-3-540-46410-5_11. [DOI] [PubMed] [Google Scholar]
- Fong AM, Robinson LA, Steeber DA, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–19. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fors BP, Goodarzi K, von Andrian UH. L-selectin shedding is independent of its subsurface structures and topographic distribution. J Immunol. 2001;167:3642–51. doi: 10.4049/jimmunol.167.7.3642. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kieffer JD, Armerding D, et al. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- Geng JG, Chen M, Chou KC. P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr Med Chem. 2004;11:2153–60. doi: 10.2174/0929867043364720. [DOI] [PubMed] [Google Scholar]
- Grabovsky V, Dwir O, Alon R. Endothelial chemokines destabilize L-selectin-mediated lymphocyte rolling without inducing selectin shedding. J Biol Chem. 2002;277:20640–50. doi: 10.1074/jbc.M201763200. [DOI] [PubMed] [Google Scholar]
- Groves RW, Allen MH, Barker JN, et al. Endothelial leukocyte adhesion molecule-1 (ELAM-1) expression in cutaneous inflammation. Br J Dermatol. 1991;124:117–23. doi: 10.1111/j.1365-2133.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Groves RW, Kupper TS. Leukocyte recruitment in cutaneous inflammation. In: Peltz G, editor. Leukocyte recruitment in inflammatory disease. New York: Springer; 1996. pp. 71–84. [Google Scholar]
- Gu YC, Bauer TR, Ackermann MR, et al. The genetic immuno-deficiency disease, leukocyte adhesion deficiency, in humans, dogs, cattle, and mice. Comp Med. 2004;54:363–72. [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–48. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Thomas KL, Prorock AJ, et al. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–72. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke M, Friedrich M, Philipp S, et al. Anti-L-selectin therapy is not effective in psoriasis: a randomized trial [abstract] Arch Dermatol Res. 2005;296:427. [Google Scholar]
- Haskell CA, Cleary MD, Charo IF. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion. J Biol Chem. 2000;275:34183–9. doi: 10.1074/jbc.M005731200. [DOI] [PubMed] [Google Scholar]
- Henderson RB, Lim LH, Tessier PA, et al. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med. 2001;194:219–26. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke BJ, Watson SR, Koenig A, et al. DNA aptamers block Lselectin function in vivo. Inhibition of human lymphocyte trafficking in SCID mice. J Clin Invest. 1996;98:2688–92. doi: 10.1172/JCI119092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AE, Abbitt KB, Dodd P, et al. The anti-inflammatory effects of a selectin ligand mimetic, TBC-1269, are not a result of competitive inhibition of leukocyte rolling in vivo. J Leukoc Biol. 2005;77:59–66. doi: 10.1189/jlb.1103573. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Thall AD, Petryniak B, et al. The alpha(1,3)fucosyltransferases FucTIV and Fuc-TVII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–26. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Ikegami-Kuzuhara A, Yoshinaka T, Ohmoto H, et al. Therapeutic potential of a novel synthetic selectin blocker, OJ-R9188, in allergic dermatitis. Br J Pharmacol. 2001;134:1498–504. doi: 10.1038/sj.bjp.0704397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz AC, Issekutz TB. The role of E-selectin, P-selectin, and very late activation antigen-4 in T lymphocyte migration to dermal inflammation. J Immunol. 2002;168:1934–9. doi: 10.4049/jimmunol.168.4.1934. [DOI] [PubMed] [Google Scholar]
- Jung U, Ley K. Mice lacking two or all three selectins demonstrate overlapping and distinct functions for each selectin. J Immunol. 1999;162:6755–62. [PubMed] [Google Scholar]
- Kaila N, Thomas BE. Design and synthesis of sialyl LewisX mimics as E- and P-selectin inhibitors. Med Res Rev. 2002;22:566–601. doi: 10.1002/med.10018. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Auger WR, Marsh JJ, et al. The use of cylexin (CY-1503) in prevention of reperfusion lung injury in patients undergoing pulmonary thromboendarterectomy. Am J Respir Crit Care Med. 2000;162:14–20. doi: 10.1164/ajrccm.162.1.9712142. [DOI] [PubMed] [Google Scholar]
- Khor SP, McCarthy K, DuPont M, et al. Pharmacokinetics, pharmacodynamics, allometry, and dose selection of rPSGL-Ig for phase I trial. J Pharmacol Exp Ther. 2000;293:618–24. [PubMed] [Google Scholar]
- Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–22. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- Kunstfeld R, Lechleitner S, Groger M, et al. HECA-452+ T cells migrate through superficial vascular plexus but not through deep vascular plexus endothelium. J Invest Dermatol. 1997;108:343–8. doi: 10.1111/1523-1747.ep12286483. [DOI] [PubMed] [Google Scholar]
- Labow MA, Norton CR, Rumberger JM, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–20. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Ley K. Functions of selectins. Results Probl Cell Differ. 2001;33:177–200. doi: 10.1007/978-3-540-46410-5_10. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–36. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb Haemost. 2003;89:213–20. [PubMed] [Google Scholar]
- Ludwig RJ, Schultz JE, Boehncke WH, et al. Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J Invest Dermatol. 2004;122:830–6. doi: 10.1111/j.0022-202X.2004.22318.x. [DOI] [PubMed] [Google Scholar]
- Maly P, Thall A, Petryniak B, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin biosynthesis. Cell. 1996;86:643–53. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Marinier A, Martel A, Bachand C, et al. Novel mimics of sialyl Lewis X; design, synthesis and biological activity in a series of 2- and 3-malonate substituted galactoconjugates. Bioorg Med Chem. 2001;9:1395–427. doi: 10.1016/s0968-0896(01)00015-3. [DOI] [PubMed] [Google Scholar]
- McEver RP, Beckstead JH. GMP-140, a platelet alpha granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Waibel-Palade bodies. J Clin Invest. 1989;84:92–9. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuffer JJ, Horuk R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci. 2002;23:459–67. doi: 10.1016/s0165-6147(02)02064-3. [DOI] [PubMed] [Google Scholar]
- Picker LJ, Kishimoto TK, Smith CW, et al. ELAM-1 is an adhesion molecule for skin-homing T-cells. Nature. 1991;349:796–9. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Picker LJ, Michie SA, Rott LS, et al. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-425 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–68. [PMC free article] [PubMed] [Google Scholar]
- Reinhardt PH, Elliott JF, Kubes P. Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood. 1997;89:3837–46. [PubMed] [Google Scholar]
- Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–28. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- Rossiter H, van Reijsen F, Mudde GC, et al. Skin disease-related T cells bind to endothelial selectins: expression of cutaneous lymphocyte antigen (CLA) predicts E-selectin but not P-selectin binding. Eur J Immunol. 1994;24:205–10. doi: 10.1002/eji.1830240132. [DOI] [PubMed] [Google Scholar]
- Schön MP, Krahn T, Schön M, et al. Efomycine M, a new specific inhibitor of selectin, impairs leukocyte adhesion and alleviates cutaneous inflammation. Nat Med. 2002;8:366–72. doi: 10.1038/nm0402-366. [DOI] [PubMed] [Google Scholar]
- Schön MP, Zollner TM, Boehncke WH. The molecular basis of lymphocyte recruitment to the skin: clues for pathogenesis and selective therapies of inflammatory disorders. J Invest Dermatol. 2003;121:951–62. doi: 10.1046/j.1523-1747.2003.12563.x. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Newman W, Gopal TV, et al. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991;113:1203–12. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai A, Shinoda K, Sasaki K, et al. High-level expression and purification of a recombinant human alpha(1,3)fucosyltransferase in baculovirus-infected insect cells. Prot Exp Purif. 1997;10:379–85. doi: 10.1006/prep.1997.0751. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Shitara K, Yoshihara Y, et al. Panosialins, inhibitors of an alpha 1,3 fucosyltransferase Fuc-VII, suppress the expression of selectin ligands on U937 cells. Glycoconj J. 1998;15:1079–83. doi: 10.1023/a:1006953626578. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Thatte J, Smith ML, et al. A CD2-green fluorescence protein-transgenic mouse reveals very late antigen-4-dependent CD8+ lymphocyte rolling in inflamed venules. J Immunol. 2001;166:7520–6. doi: 10.4049/jimmunol.166.12.7520. [DOI] [PubMed] [Google Scholar]
- Smith CH, Barker JN, Morris RW, et al. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–82. [PubMed] [Google Scholar]
- Smithers N, Kelly VA, Witham SJ, et al. Expression of a secreted form of human alpha(1,3)fucosyltransferase VII from insect cells. Biochem Soc Trans. 1997;25:426. doi: 10.1042/bst025426s. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Nalesnik MA, Rilo HR, et al. ICAM-1 and E-selectin expression in lesional biopsies of psoriasis patients responding to systemic FK 506 therapy. Autoimmunity. 1993;15:215–23. doi: 10.3109/08916939309019930. [DOI] [PubMed] [Google Scholar]
- Todderud G, Nair X, Lee D, et al. BMS-190394, a selectin inhibitor, prevents rat cutaneous inflammatory reactions. J Pharmacol Exp Ther. 1997;282:1298–304. [PubMed] [Google Scholar]
- Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–7. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994;91:7390–7. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Chambers JD, McEvoy LM, et al. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta-2 integrins in vivo. Proc Natl Acad Sci U S A. 1991;88:7538–42. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Weninger W, Ulfman LH, Cheng G, et al. Specialized contributions by alpha(1,3)fucosyltransferase-IV and Fuc-TVII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–76. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- Yvin JC, Alban S, Franz G. Anti-inflammatory and healing medicine based on laminarin sulphate. 2002. In PCT Int Appl Patent nr. WO 2002036132 (10.05.2002): France.
- Zhao LC, Edgar JB, Dailey MO. Characterization of the rapid proteolytic shedding of murine L-selectin. Dev Immunol. 2001;8:267–77. doi: 10.1155/2001/91831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zollner TM, Podda M, Pien C, et al. Proteasome inhibition reduces superantigen-mediated T cell activation and the severity of psoriasis in a SCID-hu model. J Clin Invest. 2002;109:671–9. doi: 10.1172/JCI12736. [DOI] [PMC free article] [PubMed] [Google Scholar]


