Abstract
Sunscreen products are widely used to protect the skin from sun-related damage. Previous studies have shown that some sunscreen chemicals are absorbed across the skin to the systemic circulation. The current study shows that absorption into the skin of sunscreen chemicals applied to the face is up to four times greater than that of the same product applied to the back. This has implications for the way sunscreen products are formulated and may allow the use of less potent products on the face compared with the rest of the body. The effect of formulation vehicles on the release and skin penetration of the common sunscreen agent benzophenone-3 (common name oxybenzone) was also assessed. Penetration of benzophenone-3 across excised human epidermis and high-density polyethylene (HDPE) membrane was measured using in vitro Franz-type diffusion cells. Penetration and epidermal retention was measured following application of infinite and finite (epidermis only) doses of benzophenone-3 in five vehicles: liquid paraffin, coconut oil, 50:50 ethanol:coconut oil, aqueous cream BP, and oily cream BP. Highest benzophenone-3 skin retention was observed for the ethanol:coconut oil combination. Maximal and minimal benzophenone-3 fluxes were observed from liquid paraffin and coconut oil, respectively. The alcohol-based vehicle exhibited low benzophenone-3 release from the vehicle but high skin penetration and retention.
Keywords: sunscreen, skin penetration, vehicle effect, formulation, skin stripping
Introduction
Sunscreen products are widely used by adults and children to prevent sunburn, photo-aging, and skin cancer. They are routinely applied to the arms and face for everyday protection or to larger body surface areas during swimming and beach-related activities. In addition, many cosmetics and hair products contain sunscreens, which often results in the daily application of sunscreens without the user making a conscious decision to use a sunscreen.
Sunscreens are applied topically to reduce the harmful effects of solar radiation by the ability of their chemical structure to absorb, scatter, or reflect ultraviolet radiation (UVR), thereby reducing the amount of UVR penetrating the skin. Topical sunscreen agents can be classified into one of two groups: physical blockers, such as titanium dioxide and zinc oxide, or chemical UV absorbers. Chemical sunscreens are further classified into UVA and UVB absorbers according to their spectrum of activity; for example, benzophenone-3 absorbs mainly in the UVB region, although its activity does extend into the UVA range (Patel et al 1992). Consequently, sunscreen products generally contain at least two chemical sunscreens to achieve a high sun protection factor (SPF) and broad spectrum of UVR absorption. Physical sunscreens are used in a limited number of sunscreen products as their inclusion creates a more opaque product, which is not acceptable to all users. A recent investigation demonstrated that the physical sunscreen titanium dioxide does not penetrate the skin (Schulz et al 2002). Development of micro- or nanoparticulate titanium dioxide and zinc oxide (such as Z-Cote,1 BASF Corporation,2 and ZinClear, Advanced Nanotechnology Ltd3), which are transparent, may provide more elegant sunscreen formulations. There has also been the recent suggestion that titanium dioxide contributes to significant photodegradation of the chemical sunscreen benzophenone-3 (Serpone et al 2002). This interaction creates free radicals that cause product destabilization and low SPF values and could damage tissues.
Systemic absorption of some chemical sunscreens following topical application to the skin has been reported (Benson 2000; Fernandez et al 2000; Potard et al 2000; Fernandez et al 2002; Chatelain et al 2003). Benzophenone-3 has also been detected in the plasma, tissues, and urine of animals (Okereke et al 1994). Hayden et al (1997) reported that following topical application of a commercial sunscreen product, 1%–2% of the applied dose of benzophenone-3 and its metabolites was excreted in the urine of human volunteers. This was confirmed by Sarveiya et al (2004), who also reported excretion of up to 1% benzophenone-3 and its metabolites following application of a sunscreen product to human volunteers. In both studies, benzophenone-3 was the only sunscreen detected in the urine, despite the presence of a number of other sunscreen chemicals in the applied product. Of the physicochemical characteristics of benzophenone-3, in particular its partition coefficient (calculated values for octanol:water partition coefficient have been reported as log P = 2.63 and 3.58 [Agrapidis et al 1987; Treffel and Gabard 1996]) is consistent with the optimal log P for maximum flux of solutes through the skin reported by Roberts and Walters (1998), and benzophenone-3 is thus more favorable for skin penetration than the other more lipophilic sunscreen chemicals. The more lipophilic sunscreens would be expected to diffuse into the lipid-rich stratum corneum, but only slowly partition into the more aqueous viable epidermis and deeper tissues.
A number of properties may influence skin penetration of sunscreen chemicals. These include the anatomical site of application, age, skin condition, seasonal variations, and the physicochemical properties of the sunscreen chemical and the vehicle in which it is formulated. The anatomical site to which a topical formulation is administered has been shown to influence skin penetration of a number of drugs (Roberts et al 1982; Wester and Maibach 1999). Sunscreen products are most frequently applied to the face, yet testing of products for SPF and other properties is normally undertaken on the back. It is possible that these testing procedures underestimate facial skin absorption.
Several studies have shown that the vehicle in which the sunscreen is applied to the skin may influence dermal absorption. For example, in a study of five different emulsion types, the presence of lamellar liquid crystals and silicone in emulsions with aqueous and oily external phases, respectively, reduced the penetration rate of the sunscreen octyl methoxycinnamate (ethylhexyl methoxycinnamate), whereas the emulsifier-free oil-in-water emulsion showed increased epidermal penetration (Lazar et al 1996). This increase may be explained by the presence of propylene glycol, which can act as an absorption enhancer. The water-in-oil emulsion provided greatest retention of octyl methoxycinnamate in the stratum corneum, suggesting that preparations with oily external phases may provide advantages with respect to low penetration rates and high substantivity to the stratum corneum. In addition, regardless of what vehicle was used, percutaneous absorption was related to the concentration of filter in the emulsions.
Other studies have shown that the permeation of benzophenone-3 through the horny layer was increased by incorporation into white petrolatum and isopropyl lanolate (Nannipieri et al 1990), and that the in vitro skin penetration of octyldimethyl-PABA (para-aminobenzoic acid) was greater from an alcoholic formulation than from a lotion (Kenney et al 1995). In addition, in a comparison of an oilin-water emulsion-gel and petroleum jelly, the emulsiongel provided greater penetration into, and retention of three UV filters (benzophenone-3, ethylhexyl methoxycinnamate [common name octyl methoxycinnamate], and ethylhexyl salicylate [common name octyl salicylate]) in the epidermis, whereas the latter favored diffusion through the skin rather than retention (Treffel and Gabard 1996). In comparing in vitro human epidermal and polyethylene membrane penetration and retention of benzophenone-3 from a range of single phase solvents, it was reported that benzophenone-3 penetration is minimized by topical application in vehicles with a vehicle solubility parameter (δv) substantially different from the solubility parameter of the membrane (Jiang et al 1998). However, it is also noted that finite vehicle conditions give a more realistic assessment of sunscreen retention and penetration through skin than do infinite vehicle dosing conditions (Cross et al 2001).
The influence of anatomical site (face versus back) on the skin penetration of sunscreen chemicals from a commercial product applied to human volunteers is investigated. In addition, the skin penetration of benzophenone-3 following application as an infinite and finite dose is investigated using an in vitro human skin model. The influence of vehicle formulation on skin retention and penetration is demonstrated for a number of commonly used vehicles and formulations. Comparison of the penetration across epidermal and HDPE membranes is used to permit assessment of possible vehicle–skin interactions.
Methods
Materials
Benzophenone-3 and bovine serum albumin (BSA, fraction V) were supplied by Sigma-Aldrich Chemical Co (Sydney, NSW, Australia), emulsifying ointment BP, glycerol BP, and wool alcohol ointment BP were purchased from David Craig & Co (Brisbane, QLD, Australia), light liquid paraffin was the gift of Aloe Vera Industries Pty Ltd (Loganholme, QLD, Australia), coconut oil was purchased from The Oil Garden (Brisbane, QLD, Australia), 2-phenoxyethanol and magnesium sulfate were purchased from Ajax Chemicals (Sydney, NSW, Australia), analytical grade absolute ethanol was supplied by Merck Pty Ltd (Kilsyth, VIC, Australia), and HDPE 20 μm membrane was donated by Beaver Plastics (QLD) Pty Ltd (Coopers Plains, QLD, Australia). High performance liquid chromatography (HPLC) grade methanol was used for HPLC analysis, and all other chemicals used in the study were of analytical grade.
In vivo study protocol
The study was approved by the University of Manitoba and St Boniface Hospital Ethics Committees, and written informed consent was obtained from all volunteers (5 male, 7 female, age range 22–61 years in the main study). The study was a crossover design with sunscreen application to the face or back on day 1, followed by application to the other site on day 8 of the study. A commercially available sunscreen lotion (SPF 30) containing 8% (w/v) homosalate, 7.5% (w/v) octyl methoxycinnamate, 6% (w/v) benzophenone-3, and 5% (w/v) octyl salicylate was applied at a rate of 2 mg/cm2 to an equal-sized area (112 cm2) on the face or back of the volunteers. This application rate is greater than OECD and COLIPA (the European Cosmetic Toiletry and Perfumery Association) recommendation of an applied dose between 0 and 10 mg/cm2 for skin penetration in vitro assessment and application rate used in assessment of SPF of 2 mg/cm2 (DHHS and FDA 1993; Stokes and Diffey 1999). However, in a preliminary study, when instructed to “apply the sunscreen liberally as if they were on the beach”, this was the application rate used by the volunteers. The sunscreen lotion remained unoccluded for 8 hours before being removed by washing the site. An area of the skin was immediately tape stripped by application and removal of Scotch crystal clear tape (3 cm × 1.9 cm). The tapes were applied to the treated areas by application of a consistent pressure along the tape. The stratum corneum was sequentially stripped 16 times on the back and 6 times on the face, and the strips were grouped for analysis by HPLC with UV absorption detection (Sarveiya et al 2004) (group 1, strip 1; group 2, strips 2–6; group 3, strips 7–11; group 4, strips 12–16). Tape stripping is a relatively noninvasive technique which permits layers of the stratum corneum to be collected from the treated area. It has been previously reported that complete removal of the stratum corneum was not possible even after 30–40 strippings (Hojyo-Tomoka and Kligman 1972), and a certain barrier function in the tissue so treated remains (Malkinson 1958; Feldmann and Maibach 1965). The stripping procedure was not normalized, since the inconsistent cohesion of the corneocyte layers means that reproducible amounts of stratum corneum (within and between subjects) cannot be removed (Rougier et al 1987). Blood samples were taken from all volunteers at pre-application baseline and at a suitable steady-state time after application (7.5 hours), and the urine output over 48 hours after application was collected. The 7.5-hour time-point was determined on the basis of a preliminary study in three volunteers with multiple blood samples over a 48-hour period after application of sunscreen (Sarveiya et al 2004). Sunscreen content in all samples was analyzed by HPLC.
In vitro study protocol
Sunscreen formulation preparation
Five formulation vehicles were prepared with 2% benzophenone-3, which was incorporated into the oil phase in each case. The formulation vehicles investigated were light liquid paraffin (LP), coconut oil (CO), 50:50 ethanol:coconut oil (EC), aqueous cream BP (AC), and oily cream BP (OC). Creams were prepared from first principles as described in the British Pharmacopoeia with benzophenone-3 incorporated in the oil phase during preparation.
Membrane diffusion studies
Approval was obtained from the University of Queensland and Wesley Hospital Medical Ethics Committees for collection and use of human skin. Human epidermal tissue (abdominal region of a single female) was obtained by blunt dissection of full-thickness skin and heat separation (Kligman and Christophers 1963). Epidermis was air-dried and stored at –20 °C.
Prior to use, human epidermis was thawed at room temperature and HDPE membranes were rinsed with distilled water before mounting between donor and receptor chambers of horizontal Franz-type diffusion cells. The surface area available for diffusion was 1.18 cm2 and the receptor chamber volume approximately 3.4 mL. BSA (4%) in phosphate-buffered saline (pH 7.4) was placed in the receptor chambers (Dal Pozzo et al 1991) and stirred throughout the experimental period with magnetic stirring bars. Diffusion cells were equilibrated at 37 ± 0.1 C in a blacked-out water bath (to protect sunscreen from potential photo-degradation) for at least 1 hour prior to application of the benzophenone-3 formulations. An aliquot of each formulation (200 mg/cm2) was applied to the epidermal surface under occlusion (by placing a cover-slip on the donor compartment) for the infinite dose studies and 20 mg/cm2 of each formulation applied without occlusion for the finite dose studies. For HDPE membrane, 1 g of each formulation was applied under occlusion. The contents of the receptor chamber were removed and replaced periodically for 8 or 24 hours following application to HDPE or epidermal membranes, respectively. A 100-μL sample from each receptor phase volume was mixed with 200 μL acetonitrile:methanol (95:5), refrigerated for 15 minutes then centrifuged at 10 000 g for 10 minutes to precipitate the protein prior to HPLC assay. At the end of each study the membrane was wiped with soft tissue and distilled water. Benzophenone-3 remaining in the epidermis (Rs, μg) was extracted twice with methanol and quantified by HPLC. This procedure had been previously validated as providing a recovery of 96.7%. Five or six replicates were conducted for the epidermis (infinite or finite dose, respectively) and four for the HDPE membrane.
HPLC analysis
Sunscreen content in all in vitro and in vivo samples was quantified by HPLC assay with UV detection (Sarveiya et al 2004). Samples were prepared for analysis as described. Excellent linearity was obtained over the range 0.1–5.0 μg/mL for all sunscreens. Lower limits of detection and quantitation, calculated as greater than 3 or 10 times the baseline noise in the assay, respectively, were 0.8 and 2 ng for benzophenone-3, 0.3, and 1 ng for octyl methoxycinnamate, and 2 and 4 ng for octyl salicylate and homosalate. Extraction procedures from BSA receptor fluids (0.5 mg/mL), plasma (0.5 mg/mL), urine (5 mg/mL), and skin were validated at > 98%, 97%, 86%, and 98% recovery, respectively.
Data analysis
A plot of the total amount penetrated (μg/cm2) versus time was prepared for infinite dose applications on epidermis and HDPE and for the finite dose application to epidermis. The slope of the linear portion gave the flux Js or Jm (μg/cm2•h) for epidermis and HDPE, respectively. Membrane flux was assumed to be related to the permeability coefficient (Kp, cm/h) and the concentration gradient of the solute across the membrane (ΔC, μg/cm3):
| (1) |
Kp values are useful for comparing penetration profiles for solutes from a range of formulation vehicles as they relate to the rate of diffusion of the solute within the membrane adjusted for concentration differences. In the infinite dose study, negligible donor depletion occurred and receptor sink conditions were maintained; therefore, ΔC is defined by the initial benzophenone-3 vehicle concentration (Cv).
The ratio of Kps obtained from epidermis and Kpm from HDPE was used to evaluate the effect of benzophenone-3 or vehicle on skin permeability, as described previously (Jiang et al 1997), and noted briefly:
| (2) |
since ΔCs = ΔCm,
An apparent diffusion parameter ds of solute in the epidermis is defined as the ratio of solute flux to that retained in the epidermis (Rs) at steady state, while an apparent partition parameter κs may be defined from Rs and Cv as (Jiang et al 1998):
| (3) |
Statistical methods
Differences between the flux and the intercept of the amount versus time plots, for infinite dose application in each of the vehicles, were assessed using multiple regression with pairwise comparisons. For the finite dosing study, differences between the maximum amount of benzophenone-3 penetrated to the receptor were assessed by comparing the means of each formulation. This utilized oneway ANOVA (analysis of variance) followed by Tukey's HSD post-hoc test.
Results and discussion
In vivo study
Figure 1 shows a substantial amount of all sunscreen chemicals in the stratum corneum of the back after 8 hours, a finding which is consistent with previously published in vitro research (Treffel and Gabard 1996; Jiang et al 1999). Greater amounts of sunscreen were present in the superficial layers (ranging from approximately 4% to 10% of the applied dose in groups 1 and 2 combined) than in deeper layers. This is a desirable outcome, as the target activity of sunscreens is in the superficial layers of the skin where they will absorb potentially damaging UVR to minimize penetration to deeper viable tissues where damage may occur.
Figure 1.
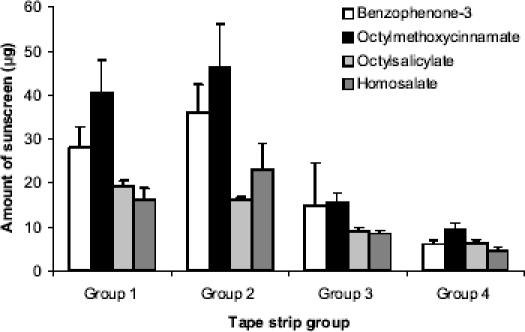
Amount of sunscreen in the stratum corneum of the back after 8 hours (mean ± SE; n = 12); group 1, strip 1; group 2, strips 2–6; group 3, strips 7–11; group 4, strips 12–16.
Figure 2 shows a comparison of the distribution of sunscreens in the stratum corneum of the face and back of the volunteers. Approximately two to four times the amount of sunscreen was present in the superficial stratum corneum layers of the face compared with the back. The difference in absorption between the anatomical sites was statistically significant for benzophenone-3, octyl salicylate, and homosalate, but not for octyl methoxcinnamate. The percentage of applied dose of sunscreen in the six superficial layers of the stratum corneum was approximately 10%, 18%, 18%, and 25% for homosalate, octyl methoxycinnamate, benzophenone-3, and octyl salicylate, respectively. This finding is consistent with previous studies that have demonstrated anatomical differences in the skin penetration of other chemicals (Rougier et al 1986). Experimental protocols for skin stripping vary, with some scientists discarding strip 1 data as non-penetrated material. In the current study the skin surface was washed prior to skin stripping to remove non-penetrated sunscreen. Nevertheless, the first strips were analyzed and plotted separately to allow interpretation including and excluding the first strip (Figures 1 and 2).
Figure 2.
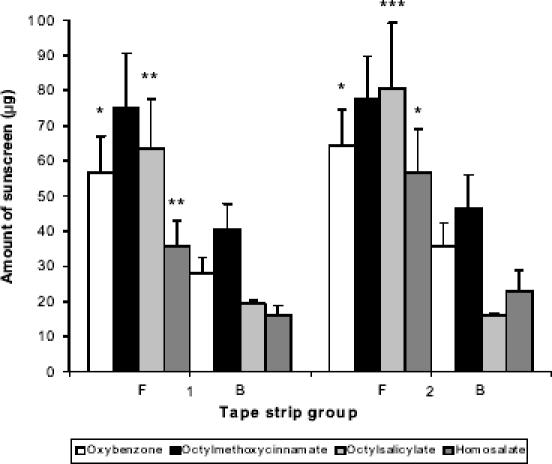
Amount of sunscreen in the stratum corneum of the back (B) and face (F) after 8 hours (mean ± SE; n = 12); 1 and 2 denotes group 1 (strip 1) and group 2 (strips 2–6) from the respective areas. * p < 0.05, ** p < 0.02, *** p < 0.01, comparisons between anatomical sites for each group.
Further, sunscreens were not detected in the plasma or urine samples of the volunteers in the present study. Such an outcome is desirable, as it is undesirable that sunscreen chemicals be absorbed either into viable tissues within the skin or into the systemic circulation. These findings appear to be at variance with our previous work in which benzophenone-3 and/or metabolites were detected in the plasma and urine (Hayden et al 1997; Sarveiya et al 2004). However, only a small surface area of skin was used for application in this study, whereas in previous studies, application occurred to extensive areas of the body. Consequently, in this study the sunscreen chemicals or metabolite levels were below the detectable limits of the HPLC assay.
Infinite dose in vitro study
Membrane and epidermal penetration and retention data for application of an infinite dose of benzophenone-3 in a range of formulation vehicles are shown in Table 1, with the penetration versus time profiles shown in Figure 3. Comparison of the five formulation vehicles showed that significant differences existed between all five formulations with respect to penetration of benzophenone-3 across HDPE membrane following application of an infinite dose in a range of formulation vehicles. LP showed the highest flux, followed by OC > AC > 50:50 EC > CO. In the case of benzophenone-3 penetration across epidermal membrane, LP again had greatest flux, followed by 50:50 EC, though
Table 1.
Penetration of benzophenone-3 from each of the vehicles studied following application of an infinite dose to human epidermis or high-density polyethylene (HDPE) membrane
| Vehicle | |||||
|---|---|---|---|---|---|
| Parametera | LP | CO | EC | AC | OC |
| Js | 9.25 | 1.64 | 7.95 | 5.26 | 6.16 |
| Jm | 54.22 | 9.15 | 12.56 | 14.26 | 42.53 |
| Rsb | 30.2 ± 6.1 | 14.8 ± 10.6 | 60.0 ± 8.4 | 24.0 ± 1.6 | 23.5 ± 2.3 |
| Kps | 2.3 × 10–3 | 4.1 × 10–4 | 2.0 × 10–3 | 1.2 × 10–3 | 1.65 × 10–3 |
| Kpm | 2.7 × 10–3 | 4.73 × 10–4 | 6.3 × 10–4 | 7.1 × 10–4 | 2.1 × 10–3 |
| Kps/Kpm | 0.84 | 0.86 | 3.17 | 1.73 | 0.77 |
| ds | 0.61 | 0.09 | 0.27 | 0.44 | 0.53 |
| κs | 7.5 × 10–4 | 9.4 × 10–4 | 1.5 × 10–3 | 6.0 × 10–4 | 5.87 × 10–4 |
Units: J = μg/cm2 • h; R = μg; d = cm–2 • h–1; κ = cm–3.
Mean ± SEM of 5 replicates.
Abbreviations: AC, aqueous cream; CO, coconut oil; EC, ethanol:coconut oil; LP, liquid paraffin; OC, oily cream; m, HDPE membrane values; s, epidermis values; J, flux; Rs, amount remaining in epidermis; Kp, permeability coefficient; d, diffusion; κ, apparent partition.
Figure 3.
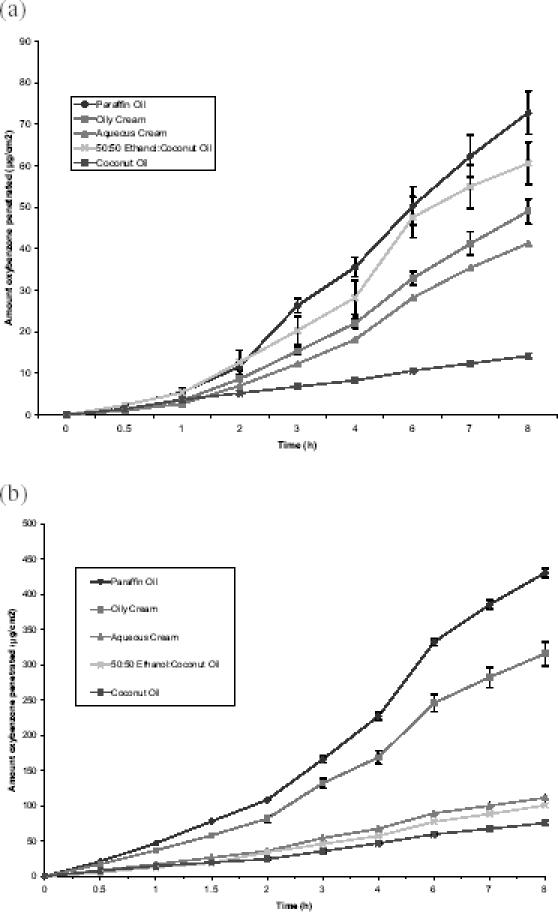
Penetration profiles of benzophenone-3 across (a) human epidermis and (b) high-density polyethylene (HDPE) membranes following application of a finite dose of benzophenone-3 in a range of vehicles. Data represent mean ± SEM of 5 and 4 replicates for the epidermis and HDPE, respectively.
the difference between these two vehicles was not significant (p = 0.087). The order of benzophenone-3 flux for the remaining vehicles was OC > AC > CO. Significant differences (p < 0.05) existed between the benzophenone-3 fluxes across epidermis for all formulation vehicles except LP and EC. The ratio of apparent permeability coefficient of benzophenone-3 through epidermis (Kps), to apparent permeability coefficient of benzophenone-3 through polyethylene (Kpm) for each formulation, is noted in Table 1. This shows that Kps/Kpm was greatest for 50:50 EC (3.17), followed by AC (1.73), with the other vehicles having similar data (≅0.8).
Measurement of benzophenone-3 penetration following application of an infinite dose of solute to a membrane permits assessment of steady-state flux and apparent permeability coefficient (as noted in Table 1) and thereby allows evaluation of vehicle effects on membrane penetration. Formulation vehicle significantly influences the penetration of benzophenone-3 across HDPE membrane, with flux greatest from LP > OC > AC > EC > CO. Flux across HDPE represents the partitioning of benzophenone-3 from the vehicle to the receptor and thereby provides a means of comparing release of benzophenone-3 from each of the vehicles. The difference in benzophenone-3 release from the two oils reflects their solubility of 29 mg/mL in LP and 133 mg/mL in CO (Jiang et al 1998), showing that the slow release rate of benzophenone-3 from CO is due to its higher affinity to this solvent. Benzophenone-3 has low solubility in ethanol (≈2%); therefore, the slow release of benzophenone-3 from EC is most probably due to its affinity to the CO. In the case of the cream/emulsion formulations, benzophenone-3 is likely to exist predominantly in the oil phase, reflecting its octanol/water partition coefficient (log P = 2.63–3.58: Agrapidis et al 1987; Treffel and Gabard 1996). As OC is a water-in-oil emulsion, benzophenone-3 will be predominantly in the external phase, whereas it will be in the internal phase of the oil-in-water emulsion AC. Thus benzophenone-3 release from AC requires a partitioning step from oil to water phase prior to release, which is likely to contribute to the slower release rate compared with OC. This may be less relevant in the clinical situation where the sunscreen formulation is rubbed into the skin. Comparing flux across HDPE and epidermis, as expected, flux across epidermis was less than HDPE because of the barrier to penetration presented by the epidermal membrane. The pattern of benzophenone-3 flux from each of the vehicles was similar for both membranes with the exception of the EC vehicle, which showed a relatively higher penetration across epidermis compared with the other vehicles. This difference is attributed to vehicle–skin interactions as described below.
Percutaneous absorption involves two processes, namely partitioning of the solute from its vehicle to the skin, followed by permeation of the solute through the skin. The vehicle can potentially influence both processes via vehicle–solute interactions and vehicle–skin interactions. Vehicle–solute interactions will influence the rate and extent to which the solute is released from the vehicle, while vehicle–skin interactions result in penetration enhancement or retardation (Roberts 1991). In this study, penetration of benzophenone-3 across HDPE membrane provides a measure of vehicle–solute interaction (ie, benzophenone-3 release from the vehicle), as the vehicles do not interact with the synthetic membrane to influence penetration (Jiang et al 1997, 1998). In contrast, flux of benzophenone-3 across skin can be influenced by both vehicle–solute interactions, which affect benzophenone-3 release from the vehicle, and vehicle–skin interactions. In order to elucidate which interactions are occurring for each of the vehicles, a ratio of apparent permeability coefficient for benzophenone-3 across skin and HDPE membrane was calculated (Kps/Kpm; Table 1).
Kps/Kpm for LP, CO, and OC are similar and close to 1, which suggests that these vehicles do not interact with the skin to influence penetration. Comparison of penetration across skin and HDPE following application of benzophenone-3 in the mixed EC vehicle shows a marked increase in Kps/Kpm (3.2 compared with 0.86 for CO alone), suggesting that this vehicle is enhancing penetration of benzophenone-3 across the epidermis. This was not unexpected, as the penetration-enhancing effect of ethanol has been demonstrated previously for other compounds and is thought to be due to extraction of stratum corneum lipids (Scheuplein and Blank 1973; Barry 1987). Mixed ethanol and oil vehicles are used for some commercial sunscreen spray formulations.
The Kps/Kpm for AC (1.73) also suggests that this vehicle enhances skin penetration of benzophenone-3. The cause of this penetration enhancement is unclear, but it may be suggested that AC increases hydration of the skin. Evidence for increased skin penetration by hydration has been well reported in the scientific literature (McKenzie and Stoughton 1962), and it has been suggested that oil-in-water emulsions, such as AC, may donate water to the stratum corneum to increase hydration (Barry 1983). In addition, AC contains surfactants (sodium lauryl sulfate and cetostearyl alcohol), which may enhance skin penetration (Tupker et al 1990). In contrast, OC which is a water-in-oil emulsion, did not appear to influence skin penetration, although it has been suggested that this vehicle may increase stratum corneum hydration by retarding water loss because of its occlusive nature (Barry 1983). However, in the infinite dose study an occluded diffusion cell system was used, so any occlusive influence of the vehicle would not be seen.
Finite dose in vitro study
Penetration data obtained following application of a finite dose of benzophenone-3 in each of the formulation vehicles to epidermal membrane are summarized in Table 2, with the penetration versus time profiles shown in Figure 4. The percentage of applied dose absorbed ranged between 1.97% from CO and 9.97% from LP. Comparison of the maximum amount of benzophenone-3 penetrated showed that LP and CO significantly differed from each other and the remaining formulations, but no other comparisons were significantly different.
Table 2.
Penetration of benzophenone-3 from each of the vehicles studied following application of a finite dose to human epidermis
| Vehicle | |||||
|---|---|---|---|---|---|
| LP | CO | EC | AC | OC | |
| Maximum amount penetrated at 8 h (μg/cm2 ± SEM; n = 6) | 46.6 ± 3.9 | 9.3 ± 0.6 | 22.9 ± 0.9 | 20.9 ± 0.8 | 30.3 ± 2.4 |
| % absorbeda | 9.97 | 1.97 | 4.86 | 4.43 | 6.41 |
| Rs (μg ± SEM) | 9.2 ± 1.9 | 9.5 ± 1.9 | 133.2 ± 19.3 | 9.7 ± 0.8 | 16.1 ± 4.8 |
| % retainedb | 1.94 | 2.00 | 28.22 | 2.04 | 3.42 |
Percentage of dose applied penetrating across epidermis to receptor over 8-hour application period.
Percentage of dose applied retained in epidermis at 8 hours after application.
Abbreviations: AC, aqueous cream; CO, coconut oil; EC, ethanol:coconut oil; LP, liquid paraffin; OC, oily cream; Rs, amount remaining in epidermis.
Figure 4.
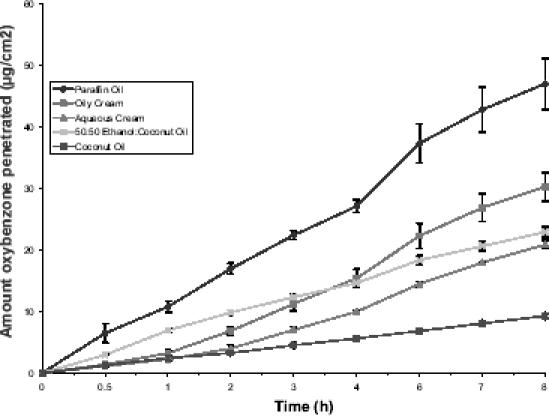
Penetration profiles of benzophenone-3 across human epidermis following application of a finite dose of benzophenone-3 in a range of vehicles. Data represent mean ± SEM of 6 replicates.
Application of a finite dose of formulation to skin permits a closer approximation of “in-use” conditions. Defining an appropriate finite dose can be problematic. A dose of 2 mg/cm2 has been recommended for assessment of the SPF of sunscreen formulations (Lowe et al 1997). However, for adequate sun protection, sunscreen manufacturers and health promotion organizations recommend that sunscreens should be applied liberally with frequent reapplication throughout a period of sun exposure and swimming. A pilot study was conducted in which five adult volunteers were asked to apply sunscreen as they would normally do on the beach. A mean application rate of 20 mg/cm2 was determined. As a result, this was used as the rate of application in the finite dose study. When each of the formulations was applied at this rate, the percentage of applied dose absorbed ranged from 1.9% for CO to 9.3% for LP, the difference being significant for these two formulations only. This is a substantial level of absorption given that a sunscreen generates its therapeutic effect on the skin surface and superficial layers and ideally should not penetrate to deeper tissues or the blood circulation. Previous studies have reported up to 10% of an applied finite dose absorbed from commercial sunscreen formulations (Jiang et al 1999) and 5% from petroleum jelly (Treffel and Gabard 1996). The order of percentage absorbed for the formulations was: LP > OC > EC > AC > CO. When comparing penetration following infinite and finite dose application to epidermis, there is a reversal in the 50:50 EC and OC formulations. From Figure 4 it appears that initially the flux of benzophenone-3 from EC is higher than from OC, but from approximately 2 hours the flux decreases. This most likely occurs because initially the ethanol present has an enhancement effect on benzophenone-3 penetration but as the ethanol evaporates from the donor formulation this effect is lost and benzophenone-3 flux reduces to that seen for CO alone. This may also contribute to the much higher skin retention of benzophenone-3 following application of the EC formulation, which is of the order of 10 times that obtained from the other formulations (67 μg for EC compared with 5–8 μg for the other formulations), as discussed below. We have previously reported that different profiles between finite dose and infinite doses can occur when formulations with different viscosities are used (Cross et al 2001). Greater penetration is evident with finite doses of high viscosity formulations than with lower viscosity products. The reverse occurs with infinite dose formulations.
Epidermal retention
With the application of an infinite dose the amount of benzophenone-3 remaining in the epidermis (Rs) at the completion of the experiment was shown not to be significantly different between the five formulations (Table 1). However, following application of a finite dose of benzophenone-3, all formulations showed similar retention with the exception of 50:50 EC, which showed significantly higher retention within the epidermis (Table 2; p < 0.05). It is interesting to note that despite the application of a smaller amount of benzophenone-3, the amount retained in the epidermis is greater following application of the finite dose than the infinite dose (66.6 ± 9.6 and 30.0 ± 2.4 μg, respectively). It is postulated that ethanol is increasing penetration of benzophenone-3 into the stratum corneum but as it evaporates it leaves the benzophenone-3 in CO, a vehicle for which it has high affinity. Thus, flux across the epidermis decreases to that seen for the CO vehicle, leaving a high loading of benzophenone-3 within the stratum corneum. As the ethanol evaporates from the vehicle, this will also concentrate the sunscreen in the remaining vehicle, which would also increase benzophenone-3 skin retention. In a clinical setting, this benzophenone-3 may then penetrate into the viable epidermis and deeper tissues or blood supply over time.
Conclusions
We conclude that sunscreen chemicals applied to the skin are substantially retained in the superficial layers of the stratum corneum, thereby optimizing sunscreen efficacy and minimizing potential interaction between these chemicals and viable tissues. Absorption and retention of sunscreens in the stratum corneum of the face is higher than the back and arms (Sarveiya et al 2004). This anatomical difference in skin absorption is in accordance with literature on a range of chemicals. We have also shown that release and skin penetration of the sunscreen benzophenone-3 was influenced by the formulation vehicle in which it was applied to the membrane. With the exception of the alcohol-based formulation, the penetration profile was the same for both infinite and finite dosing. The alcohol:coconut oil based formulation provided low benzophenone-3 release from the vehicle but high benzophenone-3 penetration across epidermis, most probably due to the enhancer effects of ethanol on the stratum corneum barrier. When applied as a finite dose, mimicking the real-life situation, the effect of the alcohol was less pronounced as it evaporated from the formulation.
Acknowledgments
The authors thank Mary-Lou Nocente, David Collins, and the volunteers for their contribution to this research. Financial support from the Canada Foundation for Innovation, Manitoba Infrastructure Fund, HillTop Research Inc, Queensland Cancer Fund, University of Manitoba Graduate Fellowship (VS), Leslie F Buggey Graduate Fellowship (VS), and Curtin Research Fellowship (HB) is acknowledged.
Notes
Use of trade names is for product identification only and does not imply endorsement.
References
- Agrapidis PL, Nash RA, Shaath NA. Effect of solvents on the ultraviolet absorbance of sunscreens. J Soc Cosmet Chem. 1987;38:209–21. [Google Scholar]
- Barry BW. Dermatological formulations: percutaneous absorption. New York: Marcel Dekker; 1983. [Google Scholar]
- Barry BW. Mode of action of penetration enhancers in human skin. J Control Rel. 1987;6:85–7. [Google Scholar]
- Benson HAE. Assessment and clinical implications of absorption of sunscreens across skin. Am J Clin Dermatol. 2000;1:217–24. doi: 10.2165/00128071-200001040-00003. [DOI] [PubMed] [Google Scholar]
- Chatelain E, Gabard B, Surber C. Skin penetration and sun protection factor of five UV filters: effect of the vehicle. Skin Pharmacol Appl Skin Physiol. 2003;16:28–35. doi: 10.1159/000068291. [DOI] [PubMed] [Google Scholar]
- Cross SE, Jiang R, Benson HAE, et al. Can increasing the viscosity of formulations be used to reduce the human skin penetration of the sunscreen oxybenzone? J Invest Dermatol. 2001;117:147–50. doi: 10.1046/j.1523-1747.2001.01398.x. [DOI] [PubMed] [Google Scholar]
- Dal Pozzo A, Liggeri E, Delucca C, et al. Prediction of skin penetration of highly lipophilic compounds; in vitro model with a modified receptor phase. Int J Pharm. 1991;70:219–23. [Google Scholar]
- [DHHS] Department of Health and Human Services, FDA, USA. Sunscreen drug products for over-the-counter human use; tentative final monograph; proposed rule. Federal Register. 1993. pp. 28194–302.
- Feldmann RJ, Maibach HI. Penetration of 14C hydrocortisone through normal skin: the effect of stripping and occlusion. Arch Dermatol. 1965;91:661–6. doi: 10.1001/archderm.1965.01600120093023. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Marti-Mestres G, Mestres JP, et al. LC analysis of benzophenone-3 in pigskin and in saline solution: application to determination of in vitro skin penetration. J Pharm Biomed Anal. 2000;22:393–402. doi: 10.1016/s0731-7085(99)00277-0. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Nielloud F, Fortune R, et al. Benzophenone-3: rapid prediction and evaluation using non-invasive methods of in vivo human penetration. J Pharm Biomed Anal. 2002;28:57–63. doi: 10.1016/s0731-7085(01)00630-6. [DOI] [PubMed] [Google Scholar]
- Hayden CG, Roberts MS, Benson HA. Systemic absorption of sunscreen after topical application. Lancet. 1997;350:863–4. doi: 10.1016/S0140-6736(05)62032-6. [DOI] [PubMed] [Google Scholar]
- Hojyo-Tomoka MT, Kligman AM. Does cellophane tape stripping remove the horny layer? Arch Dermatol. 1972;106:767–8. [PubMed] [Google Scholar]
- Jiang R, Benson HAE, Cross SE, et al. In vitro human epidermal and polyethylene membrane penetration and retention of the sunscreen benzophenone-3 from a range of solvents. Pharm Res. 1998;15:1863–8. doi: 10.1023/a:1011958006973. [DOI] [PubMed] [Google Scholar]
- Jiang R, Roberts MS, Collins DM, et al. Absorption of sunscreens across human skin: an evaluation of commercial products for children and adults. Br J Clin Pharmacol. 1999;48:635–7. doi: 10.1046/j.1365-2125.1999.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Roberts MS, Prankerd RJ, et al. Percutaneous absorption of sunscreen agents from liquid paraffin: self-association of octyl salicylate and effects on skin flux. J Pharm Sci. 1997;86:791–6. doi: 10.1021/js960523y. [DOI] [PubMed] [Google Scholar]
- Kenney GK, Sakr A, Lichtin JL, et al. In vitro skin absorption and metabolism of Padimate-O and a nitrosamine formed in Padimate-O-containing cosmetic products. J Soc Cosmet Chem. 1995;46:117–27. [Google Scholar]
- Kligman A, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:70–3. doi: 10.1001/archderm.1963.01590240026005. [DOI] [PubMed] [Google Scholar]
- Lazar GM, Baillet A, Fructus AE, et al. Evaluation of in vitro percutaneous absorption of UV filters used in sunscreen formulations. Drug and Cosmetic Industry. 1996;May:50–62. [Google Scholar]
- Lowe NJ, Shaath NA, Pathak MA. Sunscreens: development, evaluation and regulatory aspects. New York: Marcel Dekker; 1997. [Google Scholar]
- Malkinson FD. Studies on the percutaneous absorption of C14 labeled steroids by use of the gas-flow cell. J Invest Dermatol. 1958;31:19–28. doi: 10.1038/jid.1958.71. [DOI] [PubMed] [Google Scholar]
- McKenzie AW, Stoughton RB. Method for comparing percutaneous absorption of steroids. Arch Dermatol. 1962;86:608–10. [Google Scholar]
- Nannipieri E, Carelli V, DiColo G, et al. Vehicle influence on the permeation of a highly lipophilic molecule: in vitro technique to evaluate skin vehicle interactions. Int J Cosmet Sci. 1990;12:21–31. [Google Scholar]
- Okereke CS, Abdel-Rhaman MS, Friedman MA. Disposition of benzophenone-3 after dermal administration in male rats. Toxicol Lett. 1994;73:113–22. doi: 10.1016/0378-4274(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Patel NP, Highton A, Moy RL. Properties of topical sunscreen formulations. A review. J Dermatol Surg Oncol. 1992;18:316–20. doi: 10.1111/j.1524-4725.1992.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Potard G, Laugel C, Schaefer H, et al. The stripping technique: in vitro absorption and penetration of five UV filters on excised fresh human skin. Skin Pharmacol Appl Skin Physiol. 2000;13:336–44. doi: 10.1159/000029941. [DOI] [PubMed] [Google Scholar]
- Roberts MS. Structure-permeability considerations in percutaneous absorption. In: Scott RC, Guy RH, Hadgraft J, editors. Prediction of percutaneous penetration. London: IBC Technical Services; 1991. pp. 210–28. [Google Scholar]
- Roberts MS, Favretto WA, Meyer A, et al. Topical bioavailability of methyl salicylate. Aust NZ J Med. 1982;12:303–5. doi: 10.1111/j.1445-5994.1982.tb02485.x. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Walters K. The relationship between structure and barrier function of skin. In: Roberts MS, Walters K, editors. Dermal absorption and toxicity assessment. New York: Marcel Dekker; 1998. pp. 1–42. [Google Scholar]
- Rougier A, Dupuis D, Lotte C, et al. Regional variation in percutaneous absorption in man: measurement by the stripping method. Arch Dermatol Res. 1986;278:465–9. doi: 10.1007/BF00455165. [DOI] [PubMed] [Google Scholar]
- Rougier A, Lotte C, Maibach HI. In vivo percutaneous penetration of some organic compounds related to anatomic site in humans: predictive assessment by the stripping method. J Pharm Sci. 1987;76:451–4. doi: 10.1002/jps.2600760608. [DOI] [PubMed] [Google Scholar]
- Sarveiya V, Risk S, Benson HAE. Liquid chromatographic assay for common sunscreen agents: application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J Chromatogr B. 2004;803:225–31. doi: 10.1016/j.jchromb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Scheuplein RJ, Blank IH. Mechanism of percutaneous absorption. IV. Penetration of nonelectrolytes (alcohols) from aqueous solutions and from pure liquids. J Invest Dermatol. 1973;60:286–96. doi: 10.1111/1523-1747.ep12723090. [DOI] [PubMed] [Google Scholar]
- Schulz J, Hohenberg H, Pflucker F, et al. Distribution of sunscreens on skin. Adv Drug Del Rev. 2002;54(Suppl 1):S157–63. doi: 10.1016/s0169-409x(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Serpone N, Salinaro A, Emeline AV, et al. An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents. Photochem Photobiol Sci. 2002;1:970–81. doi: 10.1039/b206338g. [DOI] [PubMed] [Google Scholar]
- Stokes RP, Diffey BL. The feasibility of using fluorescence spectroscopy as a rapid, non-invasive method for evaluating sunscreen performance. J Photochem Photobiol B: Biol. 1999;50:137–43. doi: 10.1016/S1011-1344(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Treffel P, Gabard B. Skin penetration and sun protection factor of ultra-violet filters from two vehicles. Pharm Res. 1996;13:770–4. doi: 10.1023/a:1016012019483. [DOI] [PubMed] [Google Scholar]
- Tupker RA, Pinnagoda J, Nater JP. The transient and cumulative effect of sodium lauryl sulphate on the epidermal barrier assessed by transepidermal water loss: inter-individual variation. Acta Derm Venereol. 1990;70:1–5. [PubMed] [Google Scholar]
- Wester RC, Maibach HI. Regional variation in percutaneous absorption. In: Bronaugh RL, Maibach HI, editors. Percutaneous absorption; drugs-cosmetics-mechanisms-methodology. New York: Marcel Dekker; 1999. pp. 107–16. [Google Scholar]


