Abstract
Osteoporosis is a major healthcare problem that continues growing as the population ages. Sufferers become increasingly susceptible to fractures, which compromise physical and emotional health and increase healthcare costs. Bisphosphonates are the most widely used medicines for the treatment and prevention of postmenopausal osteoporosis. However, therapeutic adherence is suboptimal, meaning that outcomes demonstrated in clinical trials are not realized in the real world. It is anticipated that reducing dosing frequency may facilitate medication intake and thereby improve adherence. Ibandronate is a potent nitrogen-containing bisphosphonate specifically developed for administration with long dose-free intervals. The comprehensive ibandronate preclinical development program has demonstrated dose-dependent improvements or preservation of bone quality and strength. The feasibility of intermittent dosing using the same total dose level as continuous dosing was also confirmed. In postmenopausal osteoporosis, once-monthly oral ibandronate has been shown to be therapeutically equivalent and even superior to daily oral ibandronate, which has demonstrated antifracture efficacy for vertebral and nonvertebral fractures, bone mineral density gains at the spine and hip, and reduction in bone resorption to premenopausal levels. Once-monthly oral ibandronate is also associated with excellent safety and tolerability, and promises to further improve therapeutic adherence to bisphosphonate treatment, thereby enhancing therapeutic outcomes.
Keywords: monthly, ibandronate, osteoporosis, bisphosphonate
Introduction
With the rapidly rising average age of the population, osteoporosis has become a major healthcare problem. The disease, often a consequence of the menopause, is characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequential increase in fragility and susceptibility to fractures (NIH 2000). Although the disease is silent for years without or with only little physical restraint for the patient, it results in substantial morbidity and mortality once the first fracture has occurred. The health and financial impact of the rising incidence of osteoporosis is of increasing significance. More than 2 million individuals in the US will experience osteoporosis-related fractures in 2005, resulting in medical costs estimated to be more than US$16.9 billion (Kuehn 2005). This figure is expected to increase over the following decades due to demographic changes within the population.
Bisphosphonates are the most widely used prescription medicines for the treatment and prevention of postmenopausal osteoporosis. The nitrogen (N)-containing bisphosphonates alendronate and risedronate have demonstrated antifracture efficacy when administered orally daily. Their weekly regimens are now the most frequently prescribed. Whilst having proven efficacy and demonstrated tolerability and safety similar to placebo in controlled clinical trials, these compounds are cumbersome to take since patients need to follow stringent dosing requirements. Due to the very low and highly variable oral bioavailability of bisphosphonates, they have to be taken early in the morning after an overnight fasting state, followed by a post-dose fasting period of at least 30 minutes. In order to facilitate the esophageal passage of the tablets and to prevent the frequent gastrointestinal (GI) side effects due to local irritation of the GI mucosa, patients also need to remain upright after intake of the tablets. This is a significant hurdle for patients to continue treatment to the degree necessary to achieve the demonstrated outcomes of clinical trials. Consequently, patient adherence to therapy can be assumed to be less than optimal in daily life outside the controlled clinical trial environment. There is an increasing body of evidence which clearly highlights poor adherence to oral bisphosphonate regimens (Cramer et al 2004; Ettinger et al 2004; Bartl et al 2005; Cowell, Corner, et al 2005; Cowell, Fulford-Smith, et al 2005). Approximately half of all patients abandon their treatment within 6 months of starting therapy, and for daily regimens, only 15%–30% of patients remain on treatment at the end of the first year. Such adherence cannot translate into the protection promised by clinical trial results. One way to address this is to facilitate the intake of the bisphosphonates by reducing the dosing frequency (SG 2004). A first step has been taken with the development of weekly regimens, which have clearly resulted in an improvement in therapeutic adherence, but the overall rates of patients staying on weekly treatment are still insufficient (Cramer et al 2004; Bartl et al 2005), indicating the medical need for further improvement.
Ibandronate is a highly potent N-containing bisphosphonate which has been developed with the aim of improving the therapeutic effectiveness of bisphosphonate treatment. Ibandronate combines the known efficacy of the bisphosphonate class with good safety and tolerability and more convenient long dose-free intervals, thus optimizing the dosing frequency. This has resulted in the recent approval of a unique once-monthly tablet of ibandronate for the treatment and prevention of postmenopausal osteoporosis in the US and the EU. This paper comprehensively reviews the efficacy and safety of ibandronate from the preclinical through to the clinical development stages.
Ibandronate
Ibandronate is a highly potent N-containing bisphosphonate, which binds to bone mineral surface and inhibits osteoclasts, the cells which are responsible for bone resorption. Compared with other bisphosphonates, a high antiresorptive potency means that lower doses are needed to achieve the same efficacy with regard to inhibition of bone resorption. The combination of its antiresorptive potency and the excellent safety profile provides the basis for ibandronate to be administered with increased doses and extended dosing intervals without compromising tolerability.
Structure–activity relationship
Bisphosphonates are structural analogues of pyrophosphate, in which the oxygen atom of the phosphorous-oxygen-phosphorous (P-O-P) bond is replaced by a carbon atom resulting in a phosphorous-carbon-phosphorous (P-C-P) bond. Like all biologically active bisphosphonates, ibandronate contains a core P-C-P structure (Figure 1) essential for affinity to bone mineral and inhibition of bone resorption (Russell et al 1999; Rogers et al 2000; Rogers 2003). Modification of the side-chain substituents determines the therapeutic characteristics of the different bisphosphonates. On its R1 lateral chain, ibandronate contains a hydroxyl group, which enhances the strength of skeletal binding and prevention of hydroxyapatite crystal growth (van Beek 1994). The R2 side chain is the major determinant of antiresorptive potency (Fleisch 1998); at R2 ibandronate contains one of the most potent structural features, a tertiary nitrogen group. Due to its structural attributes, ibandronate was found to be approximately 2, 10, 50, and 500 times more potent than risedronate, alendronate, pamidronate, and clodronate, respectively (Mühlbauer et al 1991).
Figure 1.
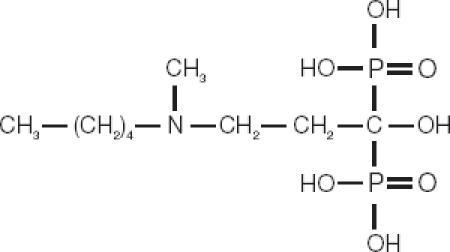
The chemical structure of ibandronate.
Bisphosphonates are subdivided into 2 groups with different molecular mechanisms of action (Russell et al 1999; Rogers et al 2000; Rogers 2003). In the first group are etidronate and clodronate, which do not contain nitrogen in their molecule. After incorporation by osteoclasts during the bone resorption process, non-N-containing bisphosphonates either form cytotoxic metabolites or inhibit protein tyrosine phosphatases. Bisphosphonates in the potent, N-containing group (including pamidronate, alendronate, risedronate, ibandronate, and zoledronate) inhibit the mevalonate pathway in osteoclasts, in particular the farnesyl pyrophosphate synthase. This prevents post-translation lipid modification (ie, prenylation) of small guanosine triphosphatase signaling proteins required for osteoclast function and cytoskeletal integrity. Both mechanisms of action lead to apoptosis. Based on their mode of action, the terminology for such bone antiresorptive drugs was recently proposed to be changed to “anticatabolic” drugs (Riggs and Parfitt 2005).
Pharmacokinetics
As with other bisphosphonates, ibandronate is poorly absorbed by the GI tract after oral administration. Its oral bioavailability in humans was estimated to be 0.63%, and in rats and dogs ≤ 1% (Barrett et al 2004; Bauss and Russell 2004). Once they have entered the circulation, about 40%–60% of the bisphosphonate binds tightly to the bone surface, while the remaining drug is excreted unchanged by the kidneys. Ibandronate is not metabolized and uptake by soft tissues is less than 2% of the systemic dose. Protein binding is low (about 85%–87%) and there is no inhibition or induction of the major cytochrome P450 isoforms, thus drug–drug interaction is unlikely. Further details on the pharmacokinetic properties of ibandronate in humans have been reviewed and previously summarized (Barrett et al 2004).
Bone-binding kinetics
Due to strong binding to the bone surface, the effects of the systemically available amount of a bisphosphonate are almost exclusively related to its concentration in bone rather than serum levels. When subcutaneously (SC) administered daily over 1 year to rats, the concentration of ibandronate in bone was found to be linearly related to the systemic dose, suggesting linear kinetics in the tested dose range of 0.2–25 μg/kg/day, resulting in a total cumulative dose of approximately 0.07–9.1 mg/kg (Bauss, Lalla, et al 2002). Even after lifelong (2 years) oral ibandronate administration with doses up to 15 mg/kg/day, bone uptake of ibandronate in rats was linear with the dose, and no gender-related differences were observed in adult animals (Endele et al 2005). A dose-dependent uptake into rat bone has also been demonstrated for single doses of alendronate up to 5 mg/kg (Lin et al 1992).
Bones consisting of predominantly trabecular bone, like vertebrae, have a 2–3 times greater uptake of ibandronate than those consisting of primarily cortical bone, like long bones (Bauss, Lalla, et al 2002; Smith et al 2003). A higher uptake by trabecular bone in comparison to cortical bone is also reported for alendronate (Lin et al 1991). The release kinetics from bone also seem to be similar for all bisphosphonates, and the half-life for elimination from rat bone is approximately 1 year depending on the type of bone (trabecular vs cortical bone). In humans the elimination half-life from bone was calculated to be approximately 10 years for alendronate (Kahn et al 1997). These bone kinetic characteristics provide the most important component of intermittent treatment with ibandronate and serve as the basis for understanding its long-lasting effects on bone.
Preclinical support for the development of ibandronate regimens with extended between-dose intervals
Bone loss in estrogen-depleted (ovariectomized [OVX]) rats and in postmenopausal women share many characteristics, including pathophysiological changes and skeletal response to different therapies (Kalu 1991; Frost and Jee 1992). Consequently, drug approval authorities request the OVX rat model as 1 of 2 animal models to be used for the preclinical investigation of drugs intended for the prevention and treatment of postmenopausal osteoporosis (FDA 1994; CPMP 2001). However, in addition to the OVX rat, which is regarded to be a modeling species, larger animals such as OVX dogs and monkeys serve as a remodeling species, are required. The World Health Organization (WHO) recently acknowledged the value of these animal models as being highly predictive for drug action in human postmenopausal osteoporosis with regard to bone mass, remodeling, bone architecture, and strength (WHO 1998). The more practical aspects related to the WHO guidelines have previously been reviewed (Bonjour et al 1999). The preclinical development of ibandronate is fully aligned with drug approval authority and WHO expectations.
Preclinical efficacy and total dose concept of ibandronate
Bone mass and turnover
The efficacy of intermittent administration of ibandronate has been demonstrated in 3 estrogen-depleted animal species (rat, dog, and monkey). In these species, ibandronate dose dependently prevented or restored bone loss and depressed increased bone turnover due to estrogen depletion, as assessed by bone densitometry, mineral analyses in bone ash, histomorphometry, and biochemical parameters of bone turnover. A comprehensive review article by Bauss and Russell (2004) provides the rationale for intermittent dosing of ibandronate, summarizes the nonclinical osteoporosis program, and compares the results with those published for other bisphosphonates (Bauss and Russell 2004).
Several dose-finding studies have been carried out to determine the optimal dosing of ibandronate. One such dose-finding study in aged OVX rats confirmed that a daily SC dose of 1 μg/kg/day ibandronate optimally prevented bone loss (optimal in that results obtained with this dose were not statistically different from those of age-matched sham-operated controls), while higher doses resulted in a plateau (Bauss, Wagner, et al 2002). A follow-up study in the same model compared the daily dose of 1 μg/kg/day with 2 intermittent cycles (on/off weeks = 1/2, 1/4, and 1/6) with all doses resulting in the same cumulative total dose. Daily and all intermittent ibandronate regimens provided equivalent results with regard to prevention of bone loss and bone architecture. This total dose concept has also been proven valid when 1-year treatment with daily (0.2–25 μg/kg/day) and intermittent (25 and 125 μg/kg every 25 days) dosing was initiated after considerable bone loss has been demonstrated (Bauss, Lalla, et al 2002).
A study in fully-grown ovariohysterectomized (OHX) beagle dogs found the optimal SC dose to prevent all estrogen deficiency-related changes on bone mass and architecture to be, as in rats, 1 μg/kg/day (Monier-Faugere et al 1993). Lower doses were not effective and higher doses resulted in a plateau. In the same experimental model, treatment with ibandronate was started 4 months after OHX when significant trabecular bone loss and increased trabecular separation (p ≤ 0.05 for both) had already been demonstrated, as well as significant increases in bone turnover and activation frequency (Monier-Faugere et al 1999). Daily (5 of 7 days) and intermittent (on/off weeks = 2/11) ibandronate administration at ≥ 4.1 μg/kg/day prevented further bone loss while the highest dose 14 μg/kg/day reversed bone loss completely to baseline values irrespective of whether the continuous or cyclical regimen was used. All doses induced a significant decrease in bone turnover and activation frequency.
Preclinical evidence of preventive treatment with ibandronate was established in studies using OVX monkeys. Intravenous (IV) ibandronate was administered the day after surgery with 10, 30, or 150 μg/kg given every 30 days for a duration of 16 months (Smith et al 2003; Müller et al 2004). The interval reflects the bone remodeling interval in monkeys (approximately 1/3 of humans) and thus, 16 months is equivalent to 4 years of treatment in humans. OVX induced a decrease in spine and femoral bone mineral density (BMD) and a decrease in trabecular BMD in the proximal tibia and distal radius. As compared with OVX-controls, ibandronate revealed significant dose-related suppression of activation frequency and the rate of bone turnover, and prevented the OVX-induced loss of bone mass in trabecular bone, with a somewhat lesser effect on cortical bone. In general, ibandronate prevented all OVX-induced changes dose-dependently with the 10 μg/kg dose expressing suboptimal effects, the 30 μg/kg dose being the optimal dose (not different from controls) at clinically relevant sites, and the 150 μg/kg dose showing further increases in bone mass and strength.
In all species tested, there was consistency regarding normalization of bone turnover, prevention of bone loss, trabecular separation (an indicator for trabecular connectivity and architecture), and normal bone mineralization. Data from preclinical studies suggest the total cumulative dose administered over a period of time is more important for efficacy than the treatment schedule itself. Although a total dose concept was demonstrated by SC administration only, the similar ibandronate concentrations in bone following SC and oral administration after correction for bioavailability suggest the total cumulative dose over time will also be important for the oral regimen (Hoffman-La Roche, Basel, Switzerland data on file).
Bone quality
Improved bone strength is an essential prerequisite for long-term clinical use of bisphosphonates and is influenced by several factors, including BMD, bone microarchitecture, and bone defect repair. When assessed by biomechanical tests (compression of predominantly cancellous bone; 3-point bending of cortical bone; torsion of long bones), bone quality was maintained or improved, even when using doses of ibandronate far in excess of any therapeutically intended dose. Bone strength also positively correlated with bone density and architecture. The consistent positive correlation between bone mass or BMD with bone strength is in accordance with the reported inverse relationship of fracture incidence and BMD (Wasnich and Miller 2000; Hochberg et al 2002).
In an interventional 12-month study in OVX rats, 3-point bending tests in femur shafts and compression tests in lumbar vertebrae were used (Bauss, Lalla, et al 2002). The clear reduction in femoral bone strength between sham- and OVX-controls (p ≤ 0.05) was dose-dependently prevented by ibandronate, with the daily dose of 0.2 μg/kg being optimal. In general, daily administration and cyclical intermittent administration (all resulting in the same total dose) produced equivalent results. Correlation between lumbar ultimate load to failure (Fmax) and dual-energy X-ray absorptiometry (DXA) or peripheral quantitative computed tomography (pQCT) was r = 0.88 (p ≤ 0.0001 for both), while DXA and pQCT results correlated by r = 0.89 (p ≤ 0.0001). Correlation of femoral Fmax versus cortical bone density was 0.61 (p ≤ 0.0001). Increased trabecular separation (p ≤ 0.0001) was fully prevented by all doses.
In the monkey study (Smith et al 2003; Müller et al 2004), biomechanical strength testing was performed on the whole vertebrae, vertebral cores, ulna, humerus cortical beams, and femoral necks and significant correlations between BMD and bone strength were determined. Ibandronate dose-dependently prevented the OVX-induced loss of bone strength in all bones. The 30 μg/kg dose was deemed to be the optimal dose based upon the most relevant parameters. At 30 μg/kg, the biomechanical properties of cancellous bone were normal with a somewhat lesser effect on cortical bone. Importantly, a 5-fold higher dose (150 μg/kg) had no adverse consequences on biomechanical properties and showed a further increase in bone mass and strength. Similarly, when alendronate was administered to OVX monkeys, no significant changes were observed in the femoral neck with regard to biomechanical properties whereas BMD was increased (although less pronounced than compared with ibandronate) (Balena et al 1993). The smaller effect of both bisphosphonates on bone sites with predominantly cortical bone is consistent with the lesser uptake of both drugs by bones consisting of predominantly cortical bone when compared with those consisting of predominantly trabecular bone (Lin et al 1992; Bauss and Russell 2004).
In the 2-year carcinogenicity study in intact rats (Lalla et al 1998), bone quality was investigated in vertebrae and femurs after daily dosing of up to 15 mg/kg. Compressive strength and stiffness were significantly higher in ibandronate-treated animals, whereas no changes occurred in strain or modulus of elasticity. A highly significant correlation was found between maximal strength in vertebral bodies and BMD measured either by DXA (r = 0.86) or pQCT (r = 0.85) (both p < 0.0001). Femur analyses revealed increased bone strength in cortical as well as in cancellous bone.
In a long-term study in skeletally mature intact dogs, administration of ibandronate at a dose that is therapeutically active in OVX dogs (1 μg/kg/day) (Monier-Faugere et al 1993) did not impair bone repair or any other biomechanical parameters (Bauss et al 2004). In this study, animals were administered SC ibandronate either daily or in two cyclical intermittent regimens (1 week “on” and 2 or 6 weeks “off”) to provide the same total dose at the end of the study. Healing of drill hole defects (tibia), which simulates the first stage of fracture healing as well as the internal remodeling of bone (femur) that occurs in the later stages of fracture healing, was neither qualitatively nor quantitatively influenced by ibandronate. Biomechanical analyses in vertebrae, indentation tests in the metaphyses, and 3-point bending of cortical beams did not produce significant differences in any group.
Bone safety
In preclinical studies no adverse effects on bone mineralization and biomechanical properties have been observed with ibandronate, even at doses several orders of magnitude above therapeutically active levels.
Unlike etidronate, no impairment of bone mineralization is expected for the more potent N-containing bisphosphonates. In OHX dogs administered ibandronate, no osteomalacia and no overt defective mineralization of osteoid was observed at 100-fold higher dose than the preventive dose of 1 μg/kg/day (Monier-Faugere et al 1993), which indicates a relatively wide therapeutic margin. When ibandronate was repeatedly administered in intact growing rats, mineralization was not affected in woven or lamellar bone, or in cartilage (Mühlbauer et al 1991). The highest ibandronate dose tested in this respect (1.0 mg P/kg/day SC, corresponding to 5.14 mg free acid equivalents/kg) was found to be > 5000 times the therapeutic dose used in osteoporosis (Bauss, Lalla, et al 2002; Bauss, Wagner, et al 2002).
The highest cumulative dose of ibandronate administered SC in OVX rats (9.125 mg/kg) was associated with normal bone quality (Bauss, Lalla, et al 2002). Assuming an average weight of 65 kg for an osteoporotic woman, this total dose equates to 593 mg. Thus, using a clinical ibandronate regimen (3 mg every 3 months IV) (Recker et al 2004), which results in a 12 mg annual exposure, the same total ibandronate exposure in patients would require a treatment period of about 50 years. A similar calculation for oral dosing, using the total maximal oral exposure in rats (approximately 9.7 mg/kg), which provided maintained or increased bone quality (Lalla et al 1998), would correspond to a treatment period of 350 years for a once-monthly oral 150 mg regimen in osteoporotic women (assuming similar bioavailability over time).
These preclinical findings support the conclusion that bone quality will most likely not be adversely affected during long-term treatment with ibandronate in humans, even with doses exceeding the therapeutic range by multiples.
Clinical development: dose-finding for daily administration of ibandronate
A phase II dose-finding study for the oral daily administration of ibandronate represented the first step in the clinical development program (Ravn et al 1996; Schimmer and Bauss 2003). The study included 180 postmenopausal women (time since menopause > 10 years) with low bone mass as determined by a distal forearm BMD T-score < –1.5. Patients were randomized to receive either placebo, or daily doses of oral ibandronate of 0.25 mg, 0.5 mg, 1.0 mg, 2.5 mg, or 5 mg. All patients received 1000 mg calcium daily. Dose-proportional increases in lumbar spine BMD were observed after 1 year, which were all significantly different from placebo with the exception of the lowest dose tested (0.25 mg). The top of the dose-response was reached with the 2.5 mg daily dose; the higher 5.0 mg dose did not show additional benefits. Daily ibandronate 2.5 mg produced increases in lumbar spine BMD relative to baseline of 4.6%, 1.9% at the total proximal femur, 3.1% at the trochanter, and 2.5% at the femoral neck. For this dose, highly significant (p < 0.001) changes in lumbar spine BMD compared with baseline were observed as early as 3 months. The dose-relationship was also apparent in the reduction of biochemical markers of bone turnover. Urinary C-telopeptide of the α-chain of type I collagen (CTX) was reduced by 74% and serum osteocalcin by 33% relative to baseline after 1 year of treatment with 2.5 mg daily oral ibandronate. Significant reductions of these markers were observed as early as the first measurement point (3 months, p < 0.001). On the basis of these results 2.5 mg was determined to represent the optimum therapeutic dose for the daily oral administration of ibandronate.
Feasibility of ibandronate administration with longer (>2 months) dose-free intervals
Based on the potency and safety characteristics of ibandronate, the intermittent administration of this bisphosphonate with longer dose-free intervals has been the main focus of the clinical development program. Consequently, a second phase II study was established to explore and compare the daily regimen with a new intermittent regimen featuring a greater than 2 month dose-free interval in order to assess the clinical feasibility of such usage (Riis et al 2001). This comparative, equivalence trial randomized 240 postmenopausal women with a BMD T-score < –2.5 at either the lumbar spine or the hip into three treatment groups: placebo and either daily oral ibandronate 2.5 mg or 20 mg every other day for 12 doses every 3 months, a regimen which thus comprised a dose-free interval of 9–10 weeks. This intermittent regimen was composed to deliver, in principle, the same cumulative dose over a 3-month period as the daily administration of 2.5 mg (approximately 225 mg for daily and 240 mg for intermittent). All patients received 500 mg calcium and 400 IU vitamin D supplementation. The trial succeeded in demonstrating statistically equivalent BMD gains at the lumbar spine after 2 years (5.6% vs 5.5% for the daily and intermittent regimens, respectively). Also the hip BMD gains were nominally identical (3.4% both regimens).
Markers of bone resorption and bone formation were reduced to a similar degree with both regimens. Typically, the steady state of reduction of markers of bone resorption with bisphosphonate treatment is seen after 3–6 months. Mean reduction of urinary N-telopeptide of the α-chain of type I collagen (NTX), for example, was 58% after 6 months for daily ibandronate and 50% for intermittent ibandronate. Similarly, mean relative reduction of this marker after 1 year was 58% for daily and 50% for the intermittent regimen (Riis et al 2001; Roche data on file). Mean relative reduction of serum osteocalcin for daily and intermittent ibandronate was 35% and 32%, respectively, after 6 months, and 42% and 38%, respectively, after 1 year.
Ibandronate was well tolerated and both regimens showed an adverse event profile comparable with placebo. This is particularly noteworthy for the intermittent regimen, since it provided an overall larger dose within a given time frame (240 mg within 24 days as opposed to 60 mg within 24 days with the daily dose) but did not result in any meaningful difference of adverse events compared with placebo (Riis et al 2001; Schimmer and Bauss 2003).
Antifracture efficacy with daily and intermittent oral ibandronate
Osteoporotic fractures are the individually, clinically, and economically most relevant pathological element of osteoporosis. For an antiosteoporotic medicine to be made available to physicians and patients, antifracture efficacy in postmenopausal osteoporosis must be demonstrated. Drug approval authority guidelines state that the assessment of the effect of a new drug regimen on the incidence of new vertebral fractures is of primary importance in judging efficacy (FDA 1994). Consequently, trials investigating the effect on new morphometric vertebral fractures after 3 years of treatment are required for efficacy demonstration. Such trials have been conducted for all currently approved N-containing bisphosphonates (alendronate, risedronate, and ibandronate) (Black et al 1996; Harris et al 1999; Chesnut et al 2004). All of these trials were performed using a daily administration of the respective bisphosphonate, as was also the case for ibandronate with its daily form receiving approval and registration for the treatment and prevention of postmenopausal osteoporosis.
However, antifracture efficacy for current weekly regimens of alendronate or risedronate has not been demonstrated, and approval of these regimens was granted based on the demonstration of therapeutic equivalence of weekly and daily regimens for the surrogate marker BMD at the lumbar spine. This was derived from the biological and pharmacological understanding that the administration of a single dose of a bisphosphonate within a period of up to 2 weeks represents a continuous and not an intermittent treatment (Bone et al 2000; Cremers et al 2005].
In the case of ibandronate, specifically aiming at dose-free intervals longer than weekly, such an extrapolation would not be appropriate, and therefore direct demonstration of antifracture efficacy with a truly intermittent regimen was pursued in addition to the daily form. Thus, the phase III fracture trial of ibandronate is uniquely different from other phase III osteoporosis trials in that it included not only a daily regimen, but also, as proof of principle, the intermittent regimen explored in phase II studies with a dose-free interval of 9–10 weeks (20 mg every other day for 12 doses every 3 months).
Antifracture efficacy of ibandronate – vertebral fractures
A total of 2946 patients were included in the phase III trial (BONE – Oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe) and randomized to placebo, 2.5 mg daily oral ibandronate, or 20 mg intermittent oral ibandronate (Chesnut et al 2004). Calcium and vitamin D were supplemented at doses of 500 mg and 400 IU, respectively. The inclusion criteria specified a lumbar spine BMD T-score < –2.0 in at least 1 vertebra and at least 1 prevalent vertebral fracture, and were thus very similar to other trials with N-containing bisphosphonates or other antiresorptive compounds (Black et al 1996; Ettinger et al 1999; Harris et al 1999). In this context, it is of interest to compare the actual fracture risk across such trials. Despite very similar inclusion criteria a wide variability of fracture risk can be observed (Figure 2) (Kanis et al 2003). The population recruited for the BONE study was shown to have the lowest fracture risk of all these osteoporosis trials to date. Nevertheless, ibandronate proved to be highly effective and daily ibandronate reduced the incidence of new morphometric vertebral fractures significantly from 9.6% in the placebo group to 4.7% (p = 0.0003). This translated into a relative risk reduction of 62% (p = 0.0001). Ibandronate demonstrated a very consistent effect with relative risk reductions of 58% (95% confidence interval [CI]: 83, –2) after 1 year, 61% (95% CI: 77, 33) after 2 years and 62% (95% CI: 75, 41) after 3 years (Figure 3) (Chesnut et al 2004). Analysis of the fractures using a semiquantitative (Genant et al 1993, 1996) rather than a morphometric classification, corroborated the early onset of the effect and the remarkable consistency for the clinically most relevant moderate and severe vertebral fractures. Relative risk reductions of 59% each after 1 year (p = 0.0164), 2 years (p = 0.0004), and 3 years (p < 0.0001) were observed (Figure 4) (Felsenberg et al 2005). Significant reductions compared with placebo were also observed for new and worsening vertebral fractures (62%, p = 0.0001) as well as clinical (symptomatic) vertebral fractures (49%, p = 0.0117).
Figure 2.
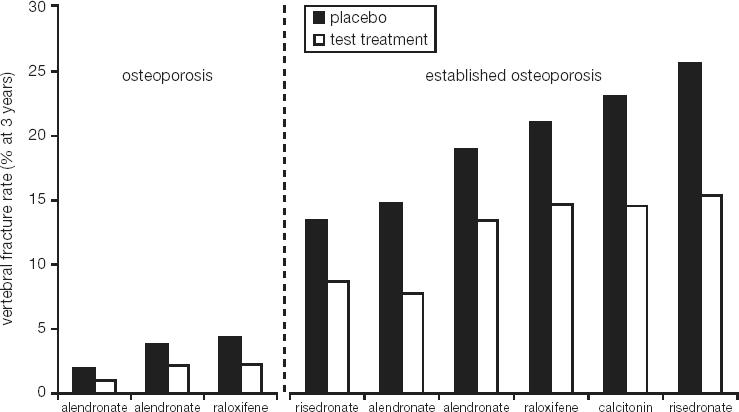
Variability of vertebral fracture rates in recent phase III studies of osteoporosis and established osteoporosis (Kanis et al 2003).
Figure 3.
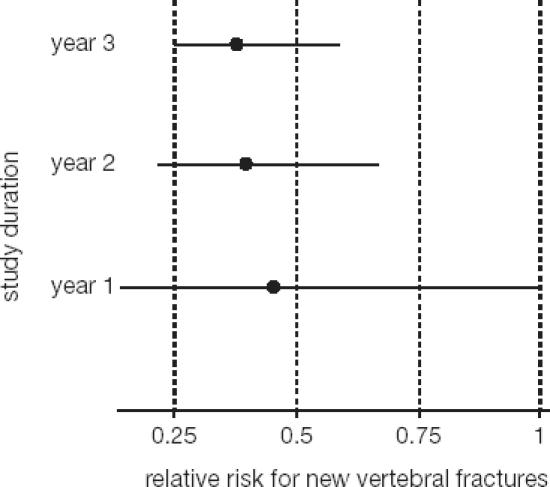
Cumulative effect of oral daily ibandronate on new vertebral fractures during each year of study (adapted from Chesnut et al 2004).
Figure 4.
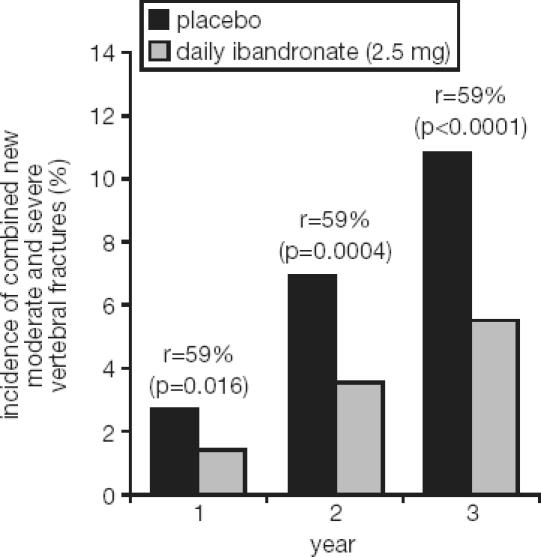
Relative-risk reductions for new moderate and severe vertebral fractures at years 1, 2, and 3 (Felsenberg et al 2005). Reprinted from Bone, In press. Felsenberg et al. 2005. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2 and 3 years in postmenopausal women with osteoporosis. Copyright © 2005, with permission from Elsevier.
Ibandronate also showed consistent risk reductions in new vertebral fractures across a wide risk spectrum as determined in post-hoc subgroup analyses. This was demonstrated for the subgroups of the US vs the European study population (Chesnut et al 2005), and also for additional risk levels determined by number of baseline vertebral fractures, baseline BMD T-score, and history of clinical fractures (Figure 5) (Ettinger et al 2002).
Figure 5.
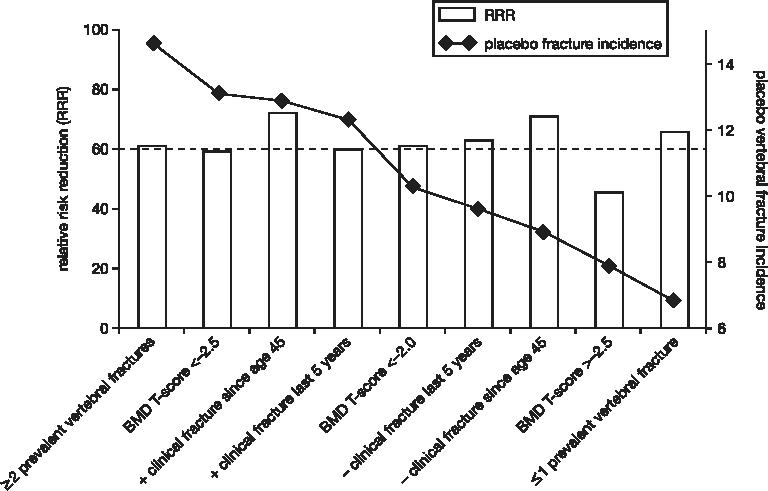
Reduction in relative risk with oral daily ibandronate (± 95% CI) of first new incident vertebral fracture through year 3 across various risk levels (intent-to-treat population) (adapted from Ettinger et al 2002).
The BONE trial also explored the primary endpoint of new morphometric vertebral fractures for the intermittent regimen with a dose-free interval of 9–10 weeks. This ibandronate regimen succeeded in reducing the incidence of new vertebral fractures to 4.9% (p = 0.0005) compared with placebo (9.6%), which translates into a relative risk reduction of 50% (p = 0.0006). The difference between the daily and the intermittent regimen was not statistically significant (p = 0.2785). This was the first prospective demonstration of antifracture efficacy for a bisphosphonate regimen other than daily and proof of principle that fracture prevention is possible with ibandronate administered with longer dose-free intervals.
Antifracture efficacy of ibandronate – nonvertebral fractures
Clinical osteoporotic fractures (including clinical vertebral and all nonspine fractures except fingers and toes) and nonvertebral fractures (including all of the former without vertebral fractures) were explored as secondary endpoints in the study. Like other similarly designed osteoporosis trials, the BONE study was adequately statistically powered to demonstrate between group differences for the primary endpoint of new vertebral fractures (FDA 1994; CPMP 2001), but not for nonvertebral fractures. To date, very few trials have been designed and statistically powered to detect an effect on nonvertebral fractures as a primary endpoint. The Hip Intervention Program study, to date the only trial specifically designed to study the effect on hip fractures, investigated the effects of risedronate and included almost 10 000 patients (6197 received risedronate, 3134 received placebo) (McClung et al 2001), while a recently published study on the effect of strontium ranelate on nonvertebral fractures randomized 5091 (2554 received strontium ranelate, 2537 received placebo) patients (Reginster, Seeman, et al 2005). Otherwise, effects on nonvertebral fractures or individual peripheral fractures for the N-containing bisphosphonates risedronate and alendronate could be shown through enlarged analysis populations, with increased statistical power, by pooled meta-analyses (Cranney, Wells, et al 2002; Cranney, Tugwell, et al 2002; Harrington et al 2004; Papapoulos et al 2005).
Efficacy on nonvertebral fractures has not been consistently demonstrated in the overall study population of individual osteoporosis trials (Marcus et al 2002; Delmas and Seeman 2004), which were usually designed and powered to detect treatment effects on vertebral fractures. In numerous studies, no significant effects on nonvertebral fractures have been observed, for example, with the licensed N-containing bisphosphonates alendronate and risedronate (Liberman et al 1995; Black et al 1996; Cummings et al 1998; Reginster et al 2000). However, in this situation, subgroup analyses exploring patients at higher risk than the average overall population have been shown to be helpful to elucidate and characterize the effect of a compound on such fractures. For example, the effect of alendronate on all clinical osteoporotic fractures in a study with more than 4400 patients with low BMD, but without prevalent vertebral fractures, could not be demonstrated at a statistically significant level in the overall population (Cummings et al 1998), but in patients with lower BMD at the femoral neck (T-score < –2.5), the drug did show a significant risk reduction. In the Hip Intervention Program study (n = 9331) risedronate reduced the risk of hip fractures by 40% in the subgroup of patients with low femoral neck BMD (T-score < –3.0) plus at least 1 risk factor for hip fracture or BMD T-score < –4.0, but not in the older (≥ 80 years of age) patients in this study, which were primarily selected on the basis of clinical risk factors for hip fracture alone (McClung et al 2001). Similarly, strontium ranelate showed a significant effect on nonvertebral fractures in a study designed and powered (n = 5091) to assess this endpoint (Reginster, Seeman, et al 2005), but not hip fractures. However, again in patients with lower femoral neck BMD (T-score < –3.0) which showed a higher incidence of hip fractures, the compound did demonstrate a significant effect in a post-hoc analysis.
In parallel with these observations, the effect on clinical osteoporotic and nonvertebral fractures was also not significant with ibandronate in the BONE study (Chesnut et al 2004), in which enrolled patients had also a very low fracture risk level compared with those in other trials. A significant effect on nonvertebral fractures of consistent magnitude was observed in post-hoc analyses, however, in subgroups of patients at higher risk and thus with a higher incidence of these fractures. A 69% relative risk reduction was seen in patients with low BMD as defined by a femoral BMD T-score below –3.0 (p = 0.013 vs placebo). Also in patients with a lumbar spine BMD T-score < –2.5 and a history of clinical fractures in the previous 5 years, a significant 60% relative risk reduction of nonvertebral fractures was shown (p = 0.0371) (Roche and GSK data on file). These criteria correspond with the majority of patients typically treated for postmenopausal osteoporosis in many European countries.
Effects of daily oral ibandronate on bone turnover and BMD
The ibandronate antifracture efficacy described above is based on the common mode of action of all bisphosphonates: the reduction of bone resorption through their direct effects on osteoclasts. Daily ibandronate reduced markers of bone resorption (urinary CTX and urinary NTX) significantly to a steady state level of approximately 65%–70% (Delmas, Recker, et al 2004), and serum osteocalcin to a level of approximately 40%.
This sustained reduction resulted in substantial and significant gains in BMD at the lumbar spine (6.5%) and the total hip (3.4%) after 3 years (p < 0.0001 at all sites compared with placebo). It is also important to characterize the effect of a bisphosphonate on the reduction of bone turnover and subsequent BMD increase. Based on the common mechanism of action, these surrogate parameters can provide additional information about antifracture efficacy, which may not be possible to obtain through the primary fracture endpoint of the study. For such scientific extrapolations it is necessary to demonstrate a clear relationship between treatment-induced BMD gains and fracture effect. Such a relationship has been demonstrated for ibandronate using a surrogate marker analysis which has quantified the fraction of fracture effect explained solely by BMD (Wasnich et al 2003).
Class efficacy of nitrogen-containing bisphosphonates
The N-containing bisphosphonates like ibandronate, alendronate, or risedronate share a common mechanism of action and many large clinical trials have demonstrated consistent and similar effects on reduction of bone turnover, increases in BMD, and reduction in vertebral fractures. Effects on nonvertebral fractures, including hip fractures, were shown in a less consistent way as indicated above, mostly due to the fact that the individual osteoporosis trials were not designed and powered to demonstrate effects on these fractures. Meta-analyses and the use of surrogate markers for efficacy, ie, BMD and biochemical markers of bone turnover together with the demonstration of a clear relationship between bisphosphonate-induced increases in BMD and reduction in bone resorption markers to antifracture efficacy for vertebral as well as nonvertebral fractures (Wasnich and Miller 2000; Hochberg et al 2002), corroborate the understanding of a similar effect of this class on all these fractures.
The magnitude of treatment effect between the individual bisphosphonates may show some degree of variability, particularly for BMD and bone turnover reduction (Rosen et al 2005a), and this variability likely depends on several factors such as potency, dose, and bone-binding characteristics. Different effect sizes for BMD and bone marker reduction, as recently shown for alendronate and risedronate (Rosen et al 2005a), do appear to matter when individual patients rather than group levels are considered by means of responder analyses. Here, larger treatment effects translate into more patients responding favorably to treatment, which is likely to result in an improved protection from osteoporotic fractures for these patients.
Effects of daily oral ibandronate in the prevention of postmenopausal osteoporosis
Oral daily ibandronate has also been explored for the prevention of osteoporosis in postmenopausal women at risk for developing osteoporosis. For this purpose a phase II/III trial was conducted in 653 women with either normal bone mass or women with osteopenia. Three doses were tested in this trial, 0.5 mg, 1.0 mg, and 2.5 mg administered daily over 2 years with the primary endpoint of relative change in lumbar spine BMD. The study demonstrated dose-dependent effects of ibandronate, with the 2.5 mg dose showing not only prevention of bone loss but significant increases of BMD at the lumbar spine and the hip (McClung et al 2004). Correspondingly, dose-dependent decreases in markers of bone resorption and formation were observed.
Based on the described studies and the demonstration of its robust characteristics, daily oral ibandronate at a dose of 2.5 mg was approved for the treatment and prevention of postmenopausal osteoporosis by the health authorities in the US and Europe in 2003.
Development of once-monthly oral ibandronate
Dose range determination for once-monthly ibandronate
The development of a single once-monthly tablet of ibandronate for the treatment of osteoporosis had to integrate the outcomes and insights of the ibandronate development program in order to define monthly doses that would deliver optimal efficacy relative to this particular dose-free interval. The impact of extending the dose-free interval beyond daily was observed in the preclinical development program (Bauss and Russell 2004). This reflected a slight decline in effect size, which was positively related to the length of the dose-free interval. Also in clinical trials of alendronate or risedronate (Figure 6), this effect decrease was apparent when moving from daily to weekly intervals, though again not statistically significant or clinically relevant (Schnitzer et al 2000; Brown et al 2002; Harris et al 2002; Rizzoli et al 2002). In the BONE trial, this effect was noted when comparing treatment effect magnitudes of the daily regimen with the intermittent regimen with a dose-free interval of more than 2 months. Given the large variability of the effect size in humans, a clear need existed to optimize the treatment effect size for a once-monthly regimen of ibandronate by increasing the dose to compensate for this commonly observed loss of effect over time. Using a 4-compartment model combined with an indirect response pharmacodynamic model (Pillai et al 2004), the effect of monthly dosing intervals of various doses of ibandronate could be simulated (Gieschke and Reginster 2004). This laid the foundation for the decision of dose increases of up to 100% compared with the cumulative monthly dose of the daily regimen resulting in once-monthly doses of ibandronate of 100 mg and 150 mg being tested clinically.
Figure 6.
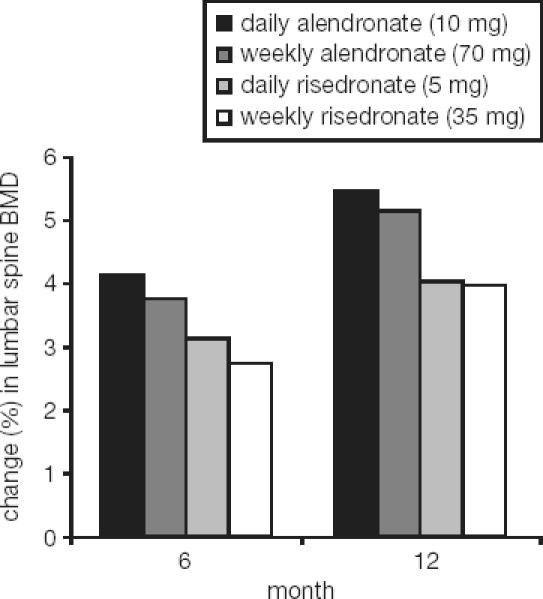
Impact of extending the dose-free interval from daily to weekly on efficacy for alendronate and risedronate (Schnitzer et al 2000; Brown et al 2002).
Clinical feasibility of once-monthly ibandronate dosing – the MOPS study
Following the comprehensive characterization of efficacy and safety of daily as well as intermittent ibandronate, the development of a unique once-a-month tablet was started in order to further reduce the dosing frequency compared with established daily and weekly regimens and thus support patients and physicians towards long-term improvement of adherence to treatment.
The principal feasibility of a once-monthly ibandronate dose regarding safety and tolerability and the effect on bone turnover was explored in a phase I pilot double-blind, placebo-controlled study (MOPS – Monthly Oral Pilot Study) (Reginster, Wilson, et al 2005). The study randomized 144 postmenopausal women aged 55–80 years and > 3 years postmenopause. Three once-monthly doses were investigated in the study: 50 mg, 100 mg, and 150 mg without additional calcium and vitamin D supplementation over a study duration of 3 months. Monthly ibandronate was well tolerated with a safety and tolerability profile comparable with placebo, despite the higher doses used. This was particularly noteworthy for GI tolerability, which again was similar to placebo.
Monthly ibandronate also showed dose-dependent decreases in serum CTX, a marker of bone resorption. Analysis of the area under the effect curve (AUEC over days 1–90) for the median relative change (% × days) in both, serum and urinary CTX revealed a dose-dependent effect of monthly ibandronate, with the 150 mg dose showing the most pronounced treatment effect size. Based on these outcomes and the very good safety and tolerability observed for the monthly doses in this trial, the development was moved forward to a phase III comparative trial aiming to demonstrate therapeutic equivalence to the daily regimen with its proven antifracture efficacy.
Demonstration of therapeutic equivalence of once-monthly ibandronate to daily – the MOBILE study
Once antifracture efficacy has been established, usually for the daily administration of a bisphosphonate as done for the class of N-containing bisphosphonates ibandronate, alendronate, and risedronate, further modified administration regimens with the respective molecule can achieve regulatory approval based on the demonstration of therapeutic equivalence of the new regimen with the established regimen. This therapeutic equivalence can be based on a surrogate parameter for antifracture efficacy, in this case BMD at the lumbar spine. Examples for this procedure include the introduction of the weekly regimens for alendronate and risedronate. For both regimens, no antifracture efficacy data have been generated, but trials comparing the weekly regimens with the respective daily regimen were sufficient to obtain regulatory approval. Therapeutic equivalence may be based on statistical equivalence (Schnitzer et al 2000) or noninferiority (Brown et al 2004).
The same approach was taken for monthly ibandronate, namely comparing the new once-monthly regimens with established daily ibandronate which has demonstrated antifracture efficacy for vertebral and nonvertebral fractures. For this purpose, the MOBILE study (Monthly Oral iBandronate In LadiEs) randomized 1609 postmenopausal women with baseline BMD T-scores at the lumbar spine below –2.5 into 4 treatment arms: 2.5 mg daily, 100 mg monthly administered as 50 mg single doses over 2 consecutive days, 100 mg monthly on a single day, and 150 mg monthly on a single day (Miller et al 2005). All patients also received 500 mg calcium and 400 IU vitamin D. The primary endpoint was the relative change in lumbar spine BMD after 1 year. The study analyzed additional secondary endpoints like BMD at the hip sites, reduction in serum CTX, a marker of bone resorption and responders to treatment for various cut-offs of BMD increases and bone resorption maker reductions. Therapeutic equivalence of monthly to daily regimens was assessed by a noninferiority analysis using a prespecified, conservatively defined noninferiority margin.
The study succeeded in clearly meeting the primary endpoint by demonstrating noninferiority for both monthly doses (Figure 7). Moreover, the compensatory dose increase of the monthly regimen resulted in even larger BMD increases compared with the daily regimen, which, in the case of the 150 mg dose, were statistically superior. The observed BMD increases were dose-dependent in their magnitude. In a similar fashion, dose-dependent BMD increases were also observed at all hip sites, with the 150 mg dose showing the largest gains again. Noninferiority, based on 95% CIs, was shown for the 150 mg dose at all hip sites. In addition, this dose also demonstrated superiority at all hip sites, except the femoral neck.
Figure 7.
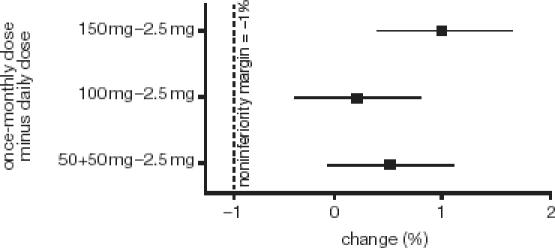
Forest plot of the difference in the means (95% confidence interval) of the mean percent change from baseline in lumbar spine (L2–L4) bone mineral density between the monthly and daily oral ibandronate regimens after 1 year (per protocol population) (Miller et al 2005).
Further to BMD increases assessed at study group level, analyses were also performed at the individual patient level using responder analyses. Several responder definitions were applied, for preservation of BMD or increases above baseline; for increases above 6% at the lumbar spine, for increases above 3% at the total hip, and for combined spine and hip BMD increases above baseline. For all these responder analyses the 150 mg once-monthly dose showed the largest percentage of patients falling above the targets; thus the 150 mg dose consistently provided treatment benefits for the largest number of patients.
Serum CTX, a marker of bone resorption was decreased rapidly and subsequently sustained at the lower level throughout the study. While the 100 mg dose achieved similar reductions to the daily ibandronate dose, the 150 mg dose improved reduction by, approximately, a further 10% of the median levels. Given the reported linear relationship between bone marker reduction and in particular nonvertebral antifracture efficacy (Hochberg et al 2002; Eastell et al 2003; Rosen et al 2005b), the demonstrated efficacy performance of the 150 mg monthly dose indicates the potential for greater patient benefits.
Safety and tolerability (BONE and MOBILE)
The safety and tolerability of oral ibandronate have been monitored continuously during all of the trials included within the clinical development program. Such data have therefore been collected for ∼3500 patients, providing an extensive safety profile (Ravn et al 1996; Riis et al 2001; Chesnut et al 2004; Miller et al 2005).
The adverse event profile for daily and intermittent ibandronate regimens studied in the phase II studies was similar to placebo; this is notable given the 8-fold higher single dose administered for the intermittent regimen. The incidence of GI adverse events was also comparable with placebo, even in patients with a history of GI disturbance (Ravn et al 1996; Riis et al 2001; Schimmer et al 2003).
A large patient population (n = 2929) was included in the BONE study and has therefore allowed a detailed evaluation of the safety and tolerability profile of daily and intermittent oral ibandronate (Chesnut et al 2004). The overall analysis indicated that both regimens were well tolerated and the safety profiles were similar to placebo. In summary, 89%–92% of patients experienced at least one adverse event, only 18%–20% had an adverse event that was considered to be treatment-related, and ≤ 25% experienced an adverse event that was classified as serious (22%–25%). A subgroup analysis by age indicated that elderly patients (aged ≥ 70 years) were not at any greater risk of adverse events (Ettinger et al 2005). The number of patients withdrawing from the study due to a treatment-related adverse event was comparable in the placebo (8.1%) and ibandronate groups (7.5% and 7.2% for the daily and intermittent groups, respectively).
Ibandronate administration was not associated with an increased incidence of GI adverse events either in the overall population or a subgroup of patients at increased risk for upper GI events (Chesnut et al 2004). Approximately 30% of the overall population had a history of upper GI disorders, and approximately 60% were taking concomitant nonsteroidal antiinflammatory drugs (NSAIDs). The number of dyspepsia, gastroenteritis, and nausea events, within these subgroups, was similar for placebo and the two treatment groups (15%–20%, 6%–9%, and 7%–9%, respectively in patients with a history of upper GI disorders; 11%–15%, 6%–7%, and 4%–7%, respectively in patients taking NSAIDs) (Rosen et al 2003).
The safety and tolerability of the once-monthly regimens were assessed throughout the MOBILE study (Miller et al 2005). The safety population of all patients taking at least 1 dose of study medication with one follow-up data point, equaled 1583 women. The overall incidence of adverse events was comparable between the daily and once-monthly treatment groups: 67%–70% of patients in each group experienced at least one adverse event. Approximately 1 third of patients had an adverse event that was considered treatment-related (27%–33%), with only 6 patients having drug-related serious adverse events. The number of patients withdrawing from the study due to a treatment-related adverse event was similar in the once-monthly and daily groups (5%–7%).
When GI adverse events were considered separately there was no difference in the overall incidence between the groups (18%, 15.9%, 21.7%, and 16.9% in the 2.5 mg, 50 + 50 mg, 100 mg, and 150 mg groups, respectively). A subgroup analysis was also completed in this study, including patients with a history of upper GI disorders or taking concomitant NSAIDs. With 1 exception, the number of patients experiencing upper GI adverse events was comparable across the treatment groups (37%–46% in patients with a history of upper GI disorders; 18%–25% in patients taking NSAIDs). The number of patients with a history of upper GI disorder experiencing an upper GI adverse event was lower in the 150 mg group (19.6%).
The data from the BONE and MOBILE studies confirmed that oral ibandronate is well tolerated in women with postmenopausal osteoporosis. It was also demonstrated that there is no safety disadvantage between the daily ibandronate dose (2.5 mg) and the larger single doses (150 mg) required for once-monthly dosing.
Taken together, the consistently greater treatment effects of the 150 mg once-monthly dose compared with the 100 mg dose and lack of any safety disadvantage, led to marketing applications for the 150 mg dose being filed with the US and European health authorities and subsequently approval was received in March and September 2005, respectively.
Conclusion
Ibandronate is a new N-containing bisphosphonate which has been developed specifically for administration with long dose-free intervals and which has recently been approved in the US and Europe for a unique once-monthly oral regimen. The comprehensive preclinical development program for ibandronate in estrogen-depleted rats, dogs, and monkeys has demonstrated the feasibility and therapeutic equivalence of intermittent dosing compared with continuous dosing for ibandronate for the same total cumulative dose administered over a given time period. Ibandronate was shown to dose-dependently improve or preserve bone mass, bone turnover, architecture, and bone strength. Bone quality and bone safety have been maintained or improved even with doses far exceeding the therapeutic range. These studies, which followed the guidelines of drug approval authorities for development of antiosteoporotic drugs, provided a sound basis for intermittent treatment of women with postmenopausal osteoporosis. The once-monthly regimen of ibandronate can be expected to deliver at least the robust antifracture efficacy documented in the BONE study (Chesnut et al 2004) for vertebral and nonvertebral fractures. Antifracture efficacy has also been demonstrated for the first time with a bisphosphonate for intermittent treatment, with ibandronate administered with a dose-free interval of more than 2 months. Once-monthly ibandronate has been shown to be therapeutically equivalent and even superior to daily ibandronate for BMD gains at the lumbar spine and the hip with good safety and tolerability. This convenient once-monthly regimen, which is linked with strong patient preference over a weekly administration (Simon et al 2005), promises to further support and improve therapeutic adherence to bisphosphonate treatment.
References
- Balena R, Toolan BC, Shea M, et al. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993;92:2577–86. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Worth E, Bauss F, et al. Ibandronate: A clinical pharmacological and pharmacokinetic update. J Clin Pharmacol. 2004;44:951–65. doi: 10.1177/0091270004267594. [DOI] [PubMed] [Google Scholar]
- Bartl R, Goette S, Hadji P, et al. Persistence and compliance with daily- and weekly-administered bisphosphonates for osteoporosis in Germany [abstract p195] Osteoporos Int. 2005;16(Suppl 3):S45. [Google Scholar]
- Bauss F, Lalla S, Endele R, et al. The effects of treatment with ibandronate on bone mass, architecture, biomechanical properties and bone concentration of ibandronate in ovariectomized aged rats. J Rheumatol. 2002;29:2200–8. [PubMed] [Google Scholar]
- Bauss F, Russell RGG. Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing. Osteoporos Int. 2004;15:423–33. doi: 10.1007/s00198-004-1612-7. [DOI] [PubMed] [Google Scholar]
- Bauss F, Schenk RK, Hört S, et al. New model for simulation of fracture repair in full-grown beagle dogs: Model characterization and results from a long-term study with ibandronate. J Pharm Tox Methods. 2004;50:25–34. doi: 10.1016/j.vascn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bauss F, Wagner M, Hothorn LH. Total administered dose of ibandronate determines its effects on bone mass and architecture in ovariectomized aged rats. J Rheumatol. 2002;29:990–8. [PubMed] [Google Scholar]
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- Bone HG, Adami S, Rizzoli R, et al. Weekly administration of alendronate: rationale and plan for clinical assessment. Clin Ther. 2000;22:15–28. doi: 10.1016/s0149-2918(00)87974-6. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Ammann P, Rizzoli R. Importance of preclinical studies in the development of drugs for treatment of osteoporosis: a review related to the 1998 WHO guidelines. Osteoporos Int. 1999;9:379–93. doi: 10.1007/s001980050161. [DOI] [PubMed] [Google Scholar]
- Brown JP, Kendler DL, McClung MR, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int. 2002;71:103–11. doi: 10.1007/s00223-002-2011-8. [DOI] [PubMed] [Google Scholar]
- Chesnut CH, Ettinger MP, Miller PD, et al. Ibandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONE. Curr Med Res Opin. 2005;21:391–401. doi: 10.1185/030079905X30752. [DOI] [PubMed] [Google Scholar]
- Chesnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–9. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- [CPMP] Committee for Proprietary Medicinal Products. London: The European Agency for the Evaluation of Medicinal Products; Human Medicines Evaluation Unit; 2001. Note for guidance on postmenopausal osteoporosis in women. [Google Scholar]
- Cowell W, Corner A, Fulford-Smith A, Poultney S. Analysis of nonadherence with bisphosphonate treatment for osteoporosis in UK patients [abstract p13] J Bone Miner Res. 2005b;20:1299. [Google Scholar]
- Cowell W, Fulford-Smith A, Poultney S. Adherence with bisphosphonate treatment for osteoporosis in UK patients [abstract] Bone. 2005a;36(Suppl 2):S604. [Google Scholar]
- Cramer JA, Amonkar MM, Hebbron A, et al. Does dosing regimen impact persistence with bisphosphonate therapy among post-menopausal osteoporotic women? J Bone Miner Res. 2004;19(Suppl 1):S448. [Google Scholar]
- Cranney A, Tugwell P, Adachi J, et al. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:517–23. doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- Cranney A, Wells G, Willan A, et al. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–16. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005;44:551–70. doi: 10.2165/00003088-200544060-00001. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–8. doi: 10.1007/s00198-004-1602-9. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with antiresorptive therapy. Bone. 2004;34:599–604. doi: 10.1016/j.bone.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Eastell R, Barton I, Hannon RA, et al. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–6. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- Endele R, Loew H, Bauss F. Analytical methods for the quantification of ibandronate in body fluids and bone. J Pharm Biomed Anal. 2005;39:246–56. doi: 10.1016/j.jpba.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Ettinger M, Emkey R, Schimmer R, et al. Efficacy of oral ibandronate in postmenopausal osteoporosis: incidence of vertebral fractures by subgroup. J Bone Miner Res. 2002;17(Suppl 1):S206. [Google Scholar]
- Ettinger MP, Felsenberg D, Harris ST, et al. The safety and tolerability of oral daily and intermittent ibandronate are not influenced by age. J Rheumatol. 2005;32:1968–74. [PubMed] [Google Scholar]
- Ettinger M, Gallagher R, Amonkar M, et al. Medication persistence is improved with less frequent dosing of bisphosphonates, but remains inadequate. Arthritis Rheum. 2004;15(Suppl):S513. [Google Scholar]
- [FDA] United States Food and Drug Administration. Rockville, MD: FDA; 1994. Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. [Google Scholar]
- Felsenberg D, Miller P, Armbrecht G, et al. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2 and 3 years in postmenopausal women with osteoporosis. Bone. 2005;37:651–4. doi: 10.1016/j.bone.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- Frost HM, Jee WSS. On the rat model of human osteoporosis. Bone Miner. 1992;18:227–236. doi: 10.1016/0169-6009(92)90809-r. [DOI] [PubMed] [Google Scholar]
- Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996;11:984–96. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- Gieschke R, Reginster JY. Successful prediction of the effect of once-monthly oral ibandronate on a marker of bone resorption using Clinical Trial Simulation (CTS) Osteoporos Int. 2004;15(Suppl 1):S97. [Google Scholar]
- Harrington JT, Ste-Marie LG, Brandi ML, et al. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–35. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Li Z, et al. Two-year efficacy and tolerability of risedronate once a week for the treatment of women with postmenopausal osteoporosis. Curr Med Res Opin. 2004;20:757–64. doi: 10.1185/030079904125003566. [DOI] [PubMed] [Google Scholar]
- Hochberg MC, Greenspan S, Wasnich RD, et al. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87:1586–92. doi: 10.1210/jcem.87.4.8415. [DOI] [PubMed] [Google Scholar]
- Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–92. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Alexandre J-M, Bone HG, et al. Study design in osteoporosis: a European perspective. J Bone Miner Res. 2003;18:1133–8. doi: 10.1359/jbmr.2003.18.6.1133. [DOI] [PubMed] [Google Scholar]
- Khan SA, Kanis JA, Vasikaran S, et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:1700–7. doi: 10.1359/jbmr.1997.12.10.1700. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Better osteoporosis management a priority: Impact predicted to soar with aging population. JAMA. 2005;293:2453–8. doi: 10.1001/jama.293.20.2453. [DOI] [PubMed] [Google Scholar]
- Lalla S, Hothorn LA, Haag N, et al. Lifelong administration of high doses of ibandronate increases bone mass and maintains bone quality of lumbar vertebrae in rats. Osteoporos Int. 1998;8:97–103. doi: 10.1007/BF02672503. [DOI] [PubMed] [Google Scholar]
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- Lin JH, Duggan DE, Chen I-W, et al. Physiological disposition of alendronate, a potent antiosteolytic bisphosphonate, in laboratory animals. Drug Metab Dispos. 1991;19:926–32. [PubMed] [Google Scholar]
- Lin JH, Chen IW, Duggan DE. Effects of dose, sex, and age on the disposition of alendronate, a potent antiosteolytic bisphosphonate, in rats. Drug Metab Dispos. 1992;20:473–8. [PubMed] [Google Scholar]
- Marcus R, Wong M, Heath H, 3rd, et al. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16–37. doi: 10.1210/edrv.23.1.0453. [DOI] [PubMed] [Google Scholar]
- McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- McClung MR, Wasnich RD, Recker R, et al. Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res. 2004;19:11–18. doi: 10.1359/JBMR.0301202. [DOI] [PubMed] [Google Scholar]
- Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–22. doi: 10.1359/JBMR.050313. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Friedler RM, Bauss F, et al. A new bisphosphonate, BM 21.0955, prevents bone loss associated with cessation of ovarian function in experimental dogs. J Bone Miner Res. 1993;8:1345–55. doi: 10.1002/jbmr.5650081109. [DOI] [PubMed] [Google Scholar]
- Monier-Faugere MC, Geng Z, Paschalis EP, et al. Intermittent and continuous administration of the bisphosphonate ibandronate in ovariohysterectomized beagle dogs: effects on bone morphometry and mineral properties. J Bone Miner Res. 1999;14:1768–78. doi: 10.1359/jbmr.1999.14.10.1768. [DOI] [PubMed] [Google Scholar]
- Mühlbauer RC, Bauss F, Schenk R, et al. BM 21.0955, a potent new bisphosphonate to inhibit bone resorption. J Bone Miner Res. 1991;6:1003–11. doi: 10.1002/jbmr.5650060915. [DOI] [PubMed] [Google Scholar]
- Müller R, Hannan M, Smith SY, et al. Intermittent ibandronate preserves bone quality and bone strength in the lumbar spine after 16 months of treatment in the ovariectomized cynomolgus monkey. J Bone Miner Res. 2004;19:1787–96. doi: 10.1359/JBMR.040809. [DOI] [PubMed] [Google Scholar]
- [NIH] National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17:1–36. [PubMed] [Google Scholar]
- Papapoulos SE, Quandt SA, Liberman UA, et al. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005;16:468–74. doi: 10.1007/s00198-004-1725-z. [DOI] [PubMed] [Google Scholar]
- Pillai G, Gieschke R, Goggin T, et al. A semimechanistic and mechanistic population PK–PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br J Clin Pharmacol. 2004;58:618–31. doi: 10.1111/j.1365-2125.2004.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn P, Clemmesen, Riis BJ, et al. The effect on bone mass and bone markers of different doses of ibandronate: a new bisphosphonate for prevention and treatment of postmenopausal osteoporosis: a 1-year, randomized, double-blind, placebo-controlled dose-finding study. Bone. 1996;19:527–33. doi: 10.1016/s8756-3282(96)00229-3. [DOI] [PubMed] [Google Scholar]
- Recker RR, Reid DM, Sambrook P, et al. Intermittent intravenous ibandronate injection regimens provide at least equivalent efficacy to daily oral ibandronate: 1-year results from DIVA. Arthritis Rheum. 2004;50(Suppl):4095. [Google Scholar]
- Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–22. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Wilson KM, Dumont E, et al. Monthly oral ibandronate is well tolerated and efficacious in postmenopausal women: results from the monthly oral pilot study. J Clin Endocrinol Metab. 2005;90:5018–24. doi: 10.1210/jc.2004-1750. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–84. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- Riis BJ, Ise J, Von Stein T, et al. Ibandronate: a comparison of oral daily dosing versus intermittent dosing in postmenopausal osteoporosis. J Bone Miner Res. 2001;16:1871–8. doi: 10.1359/jbmr.2001.16.10.1871. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Greenspan SL, Bone G, et al. Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res. 2002;17:1988–96. doi: 10.1359/jbmr.2002.17.11.1988. [DOI] [PubMed] [Google Scholar]
- Roche Roche data on file. 2005. Unpublished.
- Roche GSK. Roche and GSK data on file. 2005. Unpublished.
- Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–58. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–78. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Delmas PD, Emkey R, et al. Favorable upper gastrointestinal safety profile of ibandronate in patients with a history of gastrointestinal disorders or receiving concomitant NSAIDs [abstract] Arthritis Rheum. 2003;48(Suppl 9):102. [Google Scholar]
- Rosen CJ, Hochberg MC, Bonnick SL, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005a;20:141–51. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Hochberg MC, Bonnick SL, et al. How to interpret surrogate markers of efficacy in osteoporosis. J Bone Miner Res. 2005b;20:1263–4. doi: 10.1359/JBMR.050217. [DOI] [PubMed] [Google Scholar]
- Russell RGG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9(Suppl 2):S66–S80. doi: 10.1007/pl00004164. [DOI] [PubMed] [Google Scholar]
- Schimmer RC, Bauss F. Effect of daily and intermittent use of ibandronate on bone mass and bone turnover in postmenopausal osteoporosis: a review of three phase II studies. Clin Ther. 2003;25:19–34. doi: 10.1016/s0149-2918(03)90005-1. [DOI] [PubMed] [Google Scholar]
- Schnitzer T, Bone HG, Crepaldi G, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 2000;12:1–12. [PubMed] [Google Scholar]
- [SG] Surgeon General. Bone health and osteoporosis: a report of the Surgeon General [online] 2004 Accessed 13 Jan 2005 URL: http://www.hhs.gov/surgeongeneral/library/bonehealth/content.html. [PubMed]
- Simon JA, Beusterien KM, Kline Leidy N, et al. Women with postmenopausal osteoporosis express a preference for once-monthly versus once-weekly bisphosphonate treatment. The Female Patient. 2005 in press. [Google Scholar]
- Smith SY, Recker RR, Hannan M, et al. Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone. 2003;32:45–55. doi: 10.1016/s8756-3282(02)00923-7. [DOI] [PubMed] [Google Scholar]
- van Beek E, Hoekstra M, van de Ruit M, et al. Structural requirements for bisphosphonate actions in vitro. J Bone Miner Res. 1994;9:1875–82. doi: 10.1002/jbmr.5650091206. [DOI] [PubMed] [Google Scholar]
- Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85:231–6. doi: 10.1210/jcem.85.1.6267. [DOI] [PubMed] [Google Scholar]
- Wasnich R, Miller PD, Chesnut CH, et al. Changes in bone mineral density as a predictor for vertebral fracture efficacy with ibandronate: results from a phase III fracture study. J Bone Miner Res. 2003;18(Suppl 2):S160. [Google Scholar]
- [WHO] World Health Organization. Geneva: WHO; 1998. Guidelines for preclinical evaluation and clinical trials in osteoporosis. [Google Scholar]


