Abstract
We investigated how the nervous system processes ambiguous cues from the otolith organs by measuring roll tilt perception elicited by two motion paradigms. In one paradigm (tilt), eight subjects were sinusoidally tilted in roll with the axis of rotation near ear level. Stimulus frequencies ranged from 0.005 to 0.7 Hz, and the peak amplitude of tilt was 20°. During this paradigm, subjects experienced a sinusoidal variation of interaural gravitational force with a peak of 0.34 g. The second motion paradigm (translation) was designed to yield the same sinusoidal variation in interaural force but did not include a roll canal cue. This was achieved by sinusoidally translating the subjects along their interaural axis. For the 0.7-Hz translation trial, the subjects were simply translated from side to side. A centrifuge was used for the 0.005- to 0.5-Hz translation trials; the subjects were rotated in yaw at 250°/s for 5 min before initiating sinusoidal translations yielding an interaural otolith stimulus composed of both centrifugal and radial acceleration. Using a somatosensory task to measure roll tilt perception, we found substantial differences in tilt perception during the two motion paradigms. Because the primary difference between the two motion paradigms was the presence of roll canal cues during roll tilt trials, these perceptual differences suggest that canal cues influence tilt perception. Specifically, rotational cues provided by the semicircular canals help the CNS resolve ambiguous otolith cues during head tilt, yielding more accurate tilt perception.
INTRODUCTION
The otolith organs are the vestibular system’s graviceptors/ linear accelerometers. Like all linear accelerometers, otolith organs respond to specific gravito-inertial force (GIF), which is the sum of the specific force associated with gravity and the specific inertial force due to linear acceleration. Specific force is force per unit mass (units of m/s2); for simplicity, “force” is used to refer to specific force throughout the rest of the paper. Mathematically, the relationship between gravity, linear acceleration, and gravito-inertial force is f = g + fi = g − a, where f represents gravito-inertial force per unit mass, g represents gravitational force per unit mass, and fi represents inertial force per unit mass, which is just the negative of linear acceleration (fi = −a). Despite this inherent physical ambiguity, humans perceive both tilt (Dichgans et al. 1972; Graybiel and Clark 1965; Merfeld and Zupan 2003; Stockwell and Guedry 1970) and translation (Benson et al. 1986; Israël and Berthoz 1989; Melvill Jones and Young 1978; Parker et al. 1979). The fact that we perceive both tilt and translation demonstrates that the nervous system includes neural processes that at least partially resolve the ambiguous otolith cues.1
Two recent papers (Merfeld et al. 2005a,b) reported human tilt and translation responses during a set of three different motion paradigms: roll tilt alone (tilt), interaural translation alone (translation), and combined tilt and translation (tilt&translation). Results of the above studies suggested that the translational vestibulo-ocular reflex (VOR) responses were governed by a different mechanism than that used to elicit perception of translation or perception of tilt. Specifically, horizontal eye movement responses were consistent with simple filtering of the interaural otolith cues. This finding is consistent with the contributions of simple filters to the processing of sensory cues (Mayne 1974; Merfeld et al. 1996; Paige and Tomko 1991; Seidman et al. 1998; Telford et al. 1997). Specifically, the horizontal VOR responses were independent of the canal rotational cues because the responses were nearly the same when the subject was tilted or translated. The same studies reported that both the perception of tilt and the perception of translation depended on the canal cues that were present when the subject was tilted but were not present when the subject was translated. These perceptual findings are consistent with the contributions of internal models to the neural processing of ambiguous sensory cues (Angelaki et al. 1999; Dichgans et al. 1972; Glasauer 1992, 1995; Mayne 1974; Merfeld 1995; Merfeld and Young 1995; Merfeld et al. 1999; Stockwell and Guedry 1970).
It is important to note that the perceptual measures discussed in the preceding text were obtained via post hoc verbal reports of perceived motion. Our earlier findings have been criticized because the measures were “just” verbal reports. Although verbal reports, which are a form of magnitude estimation (Stevens 1974), are reliable and, hence, commonly used to measure vestibular tilt perception (Clement et al. 2001; Kaptein and Van Gisbergen 2003; Merfeld et al. 2005a,b), they assay a different quantity than psychophysical techniques and are not as well-anchored as measurements made via commonly used psychophysical methods like the subjective visual vertical/horizontal (Jaggi-Schwarz and Hess 2003; Jaggi-Schwarz et al. 2003; Kaptein and Van Gisbergen 2003, 2005; Mast and Jarchow 1996; Merfeld et al. 2001; Mittelstaedt 1992) or a somatosensory task (Clement et al. 2002; Merfeld et al. 2001; Wade and Curthoys 1997; Zupan and Merfeld 2003). In addition, psychophysical measures have better spatial and temporal resolution, are not reported retrospectively, and are direct (i.e., not mediated by language). Perhaps even more importantly, phase information was unavailable from the verbal reports that we reported earlier. Therefore we performed the same motion protocols used for an earlier study (Merfeld et al. 2005a) but used a somatosensory task instead of verbal reports to record tilt perception. As before, we used two motion paradigms that provide identical interaural otolith cues but different canal cues—sinusoidal roll tilt about a head-centered rotation axis (tilt) and sinusoidal interaural linear acceleration without the presence of roll canal cues (translation). These tests were performed across a broad range of frequencies (0.01–0.7 Hz) and, hence, also provide the first broad band characterization of human tilt perception using a psychophysical task.
Methodological descriptions are brief because general methods are identical to those published earlier (Merfeld et al. 2005a). Details, especially those regarding the motion stimulation, can be found in the earlier paper. Eight healthy subjects (7 males and 1 female) aged between 22 and 60 yr participated after being prescreened. Six subjects were naïve regarding testing goals and device motion capabilities. All subjects signed informed consent prior to participation. All eight subjects successfully completed the tilt testing, but only six successfully completed translation testing. One subject could not complete the translation testing due to motion sickness, and the data from a second were not analyzable due to a technical problem with the somatosensory bar. During testing, each subject sat securely in a chair that was identical for both experimental paradigms. Each subject’s head, trunk, and legs were secured. All trials were performed in darkness. The order of trials was randomized for each subject to minimize the influence of any potential order effects. To minimize potential cognitive influences on the responses, subjects were not shown the motion devices beforehand and were seated in the chair as quickly as possible so that they could not determine what motions were possible.
In one motion paradigm, labeled “tilt,” the subjects were sinusoidally roll-tilted with the center of rotation near ear level. Stimulus frequencies ranged from 0.005 to 0.7 Hz, and the peak amplitude of tilt was 20°. During this paradigm, subjects experienced sinusoidal roll canal cues due to the roll angular velocity as well as sinusoidal interaural otolith cues that were almost exclusively due to tilt with respect to gravity.
In the “translation” paradigm, subjects were seated on a chair mounted on a linear acceleration device and translated side-to-side at the same stimulus frequencies as those employed for the tilt paradigm. Translation trials were designed to provide identical interaural otolith cues (0.34 g) as the tilt trials without dynamic roll canal cues. For the highest stimulus frequency (0.7 Hz), the subjects were simply sinusoidally translated from side to side with a peak displacement of 17.3 cm. Centrifugation at a constant angular velocity was necessary to provide the appropriate interaural otolith stimulus for all other frequencies, (0.005–0.5 Hz) due to the limited length of the translational device. Details of the protocol have been described previously (Merfeld et al. 2005a). The peak amplitude of the steady-state, radial, translation oscillation ranged between 11.5 and 17.5 cm, depending on the stimulus frequency, and was chosen such that the interaural linear acceleration, which consisted of both radial and centripetal components, had a peak amplitude of 0.34 g, matching the interaural gravitational force experienced during a 20° roll tilt.
An aluminum tube (30.5 cm long) was placed ~35 cm from the subject’s midriff. This tube was attached to a precision potentiometer and rotated by the subject in a plane parallel to their coronal plane (Merfeld et al. 2001). Prior to data collection, all subjects were instructed how to perform each of two different somatosensory roll tilt indication tasks, one discrete and one continuous. In addition, each subject performed a subjective calibration task in which they tilted the bar in the dark to an angle, randomly commanded by the experimenter, between ±40° at 10° intervals. This calibration methodology has been described previously (Clement et al. 2002). This personal calibration value was then applied to all subsequent somatosensory measures for each subject.
For the discrete task, subjects were asked to offset the bar back-and-forth at least twice (by ≥20°) before aligning the bar to their perceived earth-horizontal. The subjects then pressed a push-button located at the end of the bar to mark their setting. This task is similar to that previously reported by Wade and Curthoys (1997). Offsetting the bar was required to help minimize the influence of previous settings (Merfeld et al. 2001). Subjects were asked to make as many settings as they could while maintaining high accuracy. Subjects performed this discrete task with an average setting frequency of ~0.5 Hz.
Because the discrete task could not be performed fast enough to provide adequate data at higher stimulus frequencies, motion trials at frequencies between 0.05 and 0.7 Hz were also performed with a continuous somatosensory bar task (Merfeld et al. 2001). For the continuous task, subjects continuously aligned the bar with their perceived earth-horizontal.
Analog data were acquired at a rate of 60 Hz. The tilt angle of the somatosensory bar and state of the push-button (used for the discrete task) were recorded. The tilt angle of the subject’s seat (measured with a potentiometer placed on the rotation shaft) was recorded for the tilt paradigm, while the radial position of the subject’s seat (measured via a potentiometer placed on the linear motion drive shaft) was recorded for the translation paradigm.
Steady-state sinusoidal data were fit, using linear regression least mean square methods, to the equation, x(t) = B + Ac cos(2πft) + As sin(2πft), where B is the DC bias, Ac is the amplitude of the cosine component, and As is the amplitude of the sine component. This fit was performed on a cycle-by-cycle basis. Reliable sine fit results were obtained only for data that had at least four points per cycle for the discrete task or at least half of a response cycle for the continuous task; data that did not meet these criteria are not reported.
The somatosensory bar indications of tilt showed a substantial sinusoidal component at the stimulus frequency that was almost in phase with the stimulus (Figs. 1, 2, and 3B). A phase shift of 0° means that the reported tilt was in the appropriate direction (i.e., a tilt to the left during the tilt paradigm and an acceleration to the right during the translation paradigm produced reports of tilt to the left). The discrete somatosensory task was performed fast enough to provide reliable fits of the data for stimulus frequencies up to and including 0.1 Hz. Figure 1 shows the discrete responses of one subject at 0.005 and 0.05 Hz. Consistent with the overall mean across subjects, this subject indicated slightly greater perceived tilt during the tilt paradigm than during the translation paradigm. Furthermore, the tilt indications had about the same amplitude for stimuli at 0.005 and 0.05 Hz for both tilt and translation paradigms.
FIG. 1.
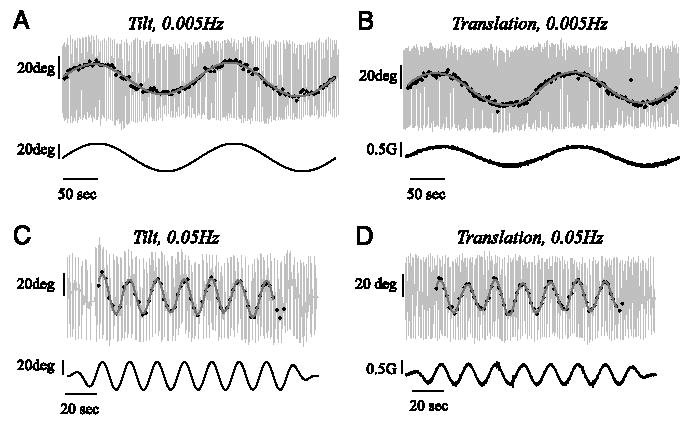
Examples of discrete somatosensory bar tilt settings provided by a single subject during the 0.005-Hz tilt paradigm (A), 0.005-Hz translation paradigm (B), 0.05-Hz tilt paradigm (C), and 0.05-Hz translation paradigm (D). Large light gray vertical lines show the subject offsetting the bar to the left and right, as instructed, before providing a discrete tilt indication (black dots). Discrete tilt indications are only shown for the steady-state part of the motion stimulus. Cycle-by-cycle fits are shown in gray. Motion stimuli (tilt angle for the tilt paradigm and interaural acceleration for the translation paradigm) are shown on the bottom row of each graph. Note that for these 2 stimulus frequencies, the tilt responses provided during the tilt paradigm (A and C) were nearly equal in amplitude to those provided during the translation paradigm (B and D).
FIG. 2.
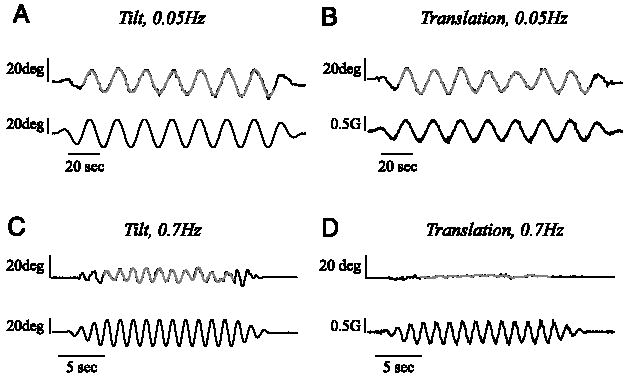
Examples of continuous somatosensory bar tilt settings provided by a single subject during 0.05-Hz tilt paradigm (A), 0.05- Hz translation paradigm (B), 0.7-Hz tilt paradigm (C), and 0.7-Hz translation paradigm (D). Cycle-by-cycle fits of tilt responses at steady-state are shown in gray. Motion stimulus is shown on the bottom row of each graph. Note that there is almost no indication of perceived tilt during the 0.7-Hz translation stimulus (D), whereas a substantial indication of perceived tilt is present during the 0.7-Hz tilt stimulus (C).
FIG. 3.
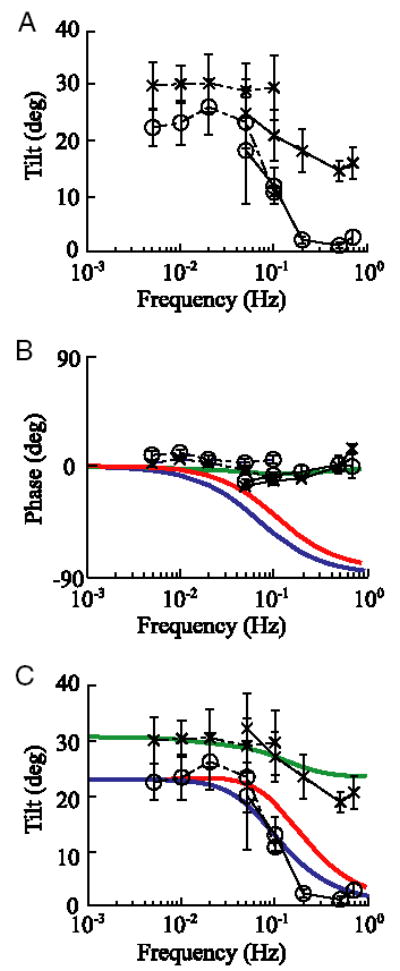
Average somatosensory tilt responses (amplitude and phase) as a function of frequency during the translation (○, n = 6 subjects) and tilt (×, n = 8 subjects) paradigms. Error bars represent SE. A: mean tilt amplitude was calculated by computing the vector average across subjects. B: mean phase was calculated by normalizing the data from each subject to have gain of 1 before performing a vector average, from which phase was calculated φ = tan−1(As/Ac). As and Ac are the mean sine and cosine components, respectively. The normalization process was performed so that the phase of responses from subjects with smaller tilt indications (e.g., 10–15°) would have the same influence on the average phase data as the phase from subjects with larger tilt indications (e.g., 30–40°). C: because previous studies have shown that continuous tilt measures underestimate tilt (Merfeld et al. 2001), we rescaled the continuous data (—) to match the amplitude of the discrete task data (···) at overlapping frequencies (0.05 and 0.1 Hz). To accomplish this, we calculated the average ratio of the mean discrete tilt indications divided by the mean continuous tilt indications at overlapping frequencies and used this ratio of 1.09 and 1.29 for each translation and tilt trial, respectively, as a scaling factor to multiply all continuous tilt settings. A first order low-pass filter (blue) was fit to the amplitude of the tilt data during the translation paradigm. A cut-off frequency of 0.07 Hz provided the best fit to the amplitude data. Internal model predictions of the phase (B) and amplitude (C) of the tilt response during tilt (green) and translation (red) paradigms are also shown. To provide a direct comparison between the tilt data and model predictions, the modeled nondimensional tilt gain (the ratio of peak estimated interaural gravitational force divided by the peak interaural gravito-inertial force) was scaled to best match the data with the scaling factor of 30.7 and 24.0° for translation and tilt paradigms, respectively.
Figure 2 shows perceptual tilt data provided by the same subject performing the continuous somatosensory task during motion stimuli at 0.05 Hz (providing overlap with the discrete data shown in Fig. 1) and 0.7 Hz. Again, consistent with the overall mean across subjects, the tilt response for this subject during the 0.05-Hz stimulus was slightly smaller during the translation paradigm (Fig. 2B) than during the tilt paradigm (Fig. 2A). Also consistent with previous studies (Merfeld et al. 2001), the bar indications of tilt were a little greater for the discrete task (Fig. 1, C and D) than for the continuous task (Fig. 2, A and B) with an average ratio of 1.09 and 1.29 over all subjects for tilt and translation trials, respectively. While a substantial tilt response was still evident for this subject during the tilt paradigm motion stimulus at 0.7 Hz (Fig. 2C), little or no tilt indication was present during the translation stimulation at the same frequency (Fig. 2D).
The tilt response for the tilt trials (×) was almost constant, though it did show a decrease around 0.05 Hz, consistent with modeling predictions (Merfeld and Zupan 2002), before leveling off again at higher frequencies (Fig. 3A). As for the verbal responses reported in Merfeld et al. (2005a); we observed an overestimation of tilt at the lower frequencies, which was discussed in detail in Merfeld et al. (2005a). The amplitude of the tilt response during the translation trials (○) showed characteristics consistent with a low-pass filter, being roughly constant at lower frequencies and falling substantially at frequencies > 0.1 Hz (Fig. 3A). These findings are qualitatively similar to verbal tilt reports reported previously (Merfeld et al., 2005a), although the response decline appears more precipitous for the somatosensory task. Inconsistent with simple low-pass filtering of otolith signals, which would show substantial phase changes over the frequency range of 0.005 to 0.7 Hz, tilt perception was always about 0° in- phase with the stimulation (Fig. 3B). This indicates that the subjects’ tilt perceptions were tightly phase-locked to the stimulation, independent of frequency.
It is worth noting that the discrete and continuous tilt indications were significantly different from one another at the overlapping frequencies (P < 0.01), which indicates that the tasks, although qualitatively very similar, do not provide identical subjective tilt measures. The continuous task resulted in slightly, but significantly, smaller tilt indications than the discrete task, probably because previous and present settings affect future settings (Merfeld et al. 2001). The discrete task eliminates this influence of previous settings by having the subjects rapidly oscillate the bar back and forth between each pair of settings. The continuous task data were scaled so that they had the same magnitude as the discrete task data at the frequencies of overlap (i.e., at 0.05 and 0.1 Hz, Fig. 3C). The general trends remain identical to those shown in Fig. 3A.
We have shown that substantial differences in tilt perception were measured for the translation and tilt stimuli. Because the two motion paradigms provide drastically different canal cues (i.e., absence of canal cues during translation vs. presence of canal cues during tilt), while providing identical interaural otolith cues, the results suggest that rotational cues from the semicircular canals have a substantial influence on tilt perception. This is primarily evident at frequencies where the canals provide accurate rotational cues (>0.05 Hz). These findings are consistent with previously published modeling predictions, based on internal models and sensory fusion (Merfeld and Zupan 2002; Merfeld et al. 2005a), that showed substantial difference in tilt responses for the translation and tilt conditions especially at frequencies >0.1 Hz.
Another potential explanation is differences in the force variations along the long body axis (z axis) during tilt and translation. However, we think that z-axis force variations are unlikely to be the predominant influence for small angles of tilt because 1) these force variations are substantially smaller (varying between 1.0 and 0.94 g) than the interaural force variations (varying between 0.0 and 0.34 g); 2) subjects accurately estimate tilt for small tilt angles when the z-axis force variations are subthreshold (Merfeld et al. 2005b); and 3) the z-axis force variations are identical for both low and high frequencies, while the response difference is observed only at higher frequencies, when the canals cues provide good rotation information.
This study was, in part, motivated by an earlier classic study (Stockwell and Guedry 1970) that showed that perceptions of roll tilt changed much more rapidly during an actual roll tilt (similar to the roll tilt used in our tilt condition) than during fixed-radius centrifugation (Clark and Graybiel 1963; Graybiel and Brown 1951; Graybiel and Clark 1965). This difference in time course led Stockwell and Guedry (1970) to conclude that roll rotation cues from the canals were used to help estimate roll tilt. In hindsight, however, it appears that a comparison to the fixed-radius centrifugation findings was not a good baseline because a recent study (Merfeld et al. 2001) showed that the yaw rotation cues present during fixed-radius centrifugation caused a substantial lag in the perception of roll tilt; the sensation of roll tilt built up much more slowly when yaw canal cues were present during fixed-radius centrifugation than in the absence of yaw rotation cues during a variable radius paradigm (Merfeld et al. 2001). Because yaw canal cues were not present at the time the translational stimuli were delivered in the translation paradigm used herein, the findings presented resolve these issues left open by the earlier Stockwell and Guedry study. However, our study supports the same basic conclusion, namely, that roll rotation cues from the vertical canals have a substantial and relatively direct influence on roll tilt perception. This is consistent with our own recently published findings (Merfeld et al. 2005a,b) and with the findings of an earlier study (Glasauer 1995) that, based on similar tilt perception findings during translation, concluded that the nonlinear mechanisms must be contributing. But the contribution of nonlinear mechanisms does not prove that low-pass filtering of otolith afferent signals could not contribute.
In fact, consistent with low-pass filtering of otolith afferent signals and with earlier studies (Glasauer 1995; Seidman and Paige 1996), the amplitude of tilt responses during translation decreases as frequency increases (Fig. 3A). However, the phase of tilt responses during translation is qualitatively different from the phase predicted via a low-pass filtering model. In addition, both the phase and amplitude of tilt responses are qualitatively different from predictions made by low-pass filtering otolith afferent signals when the canals are dynamically activated during tilt.
In comparison, both the amplitude and phase of tilt responses during tilt demonstrate frequency characteristics that match predictions made by a recent model (Merfeld et al. 2005a) of canal-otolith interactions (Fig. 3, B and C). Similarly, the amplitude of tilt responses during translation also nicely matches model predictions (Fig. 3C). However, the frequency characteristics of the phase response during translation do not match the predictions from our current internal model (Fig. 3B). This discrepancy shows that our understanding of these sensory interactions is incomplete. For example, it is possible that other influences that are not modeled, like vibration and/or auditory cues (Yong et al. 2001), might contribute to this multi-sensory interaction. In conclusion, the data unambiguously demonstrate the influence of canal cues on perception of roll tilt, which is a concept incorporated in internal models. However, our current internal model did not predict one of the four measures of tilt perception reported here (specifically, the phase of tilt perception during the translation paradigm). Therefore the data also demonstrate a limitation in our understanding of the mechanisms underlying roll tilt perception.
Acknowledgments
The authors thank T. Bennett and V. Stallings for technical contributions, M. Marsden and P. Cunningham for administrative assistance, and Drs. Lionel Zupan and Rick Lewis for commenting on early versions of the manuscript. Experiments were performed at the Legacy Neurotology Research Laboratory.
Footnotes
It is important to note that the influence of other cues to help elicit perceptions of tilt and translation is consistent with Einstein’s equivalence principle, which simply states that no single sensor can measure a difference between linear acceleration and gravity. Specifically, the use of other cues, sensory or non-sensory (e.g., efferent copy or cognitive), or other forms of processing (e.g., simple low-pass or high-pass filtering) to help process these ambiguous cues are not prohibited by Einstein’s equivalence principle.
GRANTS
The authors acknowledge support from National Institute on Deafness and Other Communication Disorders Grants DC-004158 to D. M. Merfeld and S. Park and DC-00205 to C. Gianna-Poulin, S. Wood, and F. O. Black as well as National Aeronautics and Space Administration Grant NAW9-1254 to F. O. Black.
References
- Angelaki D, McHenry M, Dickman JD, Newlands S, Hess B. Computation of inertial motion: Neural strategies to resolve ambiguous otolith information. J Neurosci. 1999;19:316–327. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57:1088–1096. [PubMed] [Google Scholar]
- Clark B, Graybiel A. Contributing factors in the perception of the oculogravic illusion. Am J Psychol. 1963;76:18–27. [PubMed] [Google Scholar]
- Clement G, Maciel F, Deguine O. Perception of tilt and ocular torsion of normal human subjects during eccentric rotation. Otol Neurotol. 2002;23:958–966. doi: 10.1097/00129492-200211000-00025. [DOI] [PubMed] [Google Scholar]
- Clement G, Moore ST, Raphan T, Cohen B. Perception of tilt (somatogravic illusion) in response to sustained linear acceleration during space flight. Exp Brain Res. 2001;138:410–418. doi: 10.1007/s002210100706. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Held R, Young LR, Brandt T. Moving visual scenes influence the apparent direction of gravity. Science. 1972;178:1217–1219. doi: 10.1126/science.178.4066.1217. [DOI] [PubMed] [Google Scholar]
- Glasauer S. Interaction of semicircular canals and otoliths in the processing structure of the subjective zenith. Ann NY Acad Sci. 1992;656:847–849. doi: 10.1111/j.1749-6632.1992.tb25272.x. [DOI] [PubMed] [Google Scholar]
- Glasauer S. Linear acceleration perception: frequency dependence of the hilltop illusion. Acta Otolaryngol Suppl. 1995;520:37–40. doi: 10.3109/00016489509125184. [DOI] [PubMed] [Google Scholar]
- Graybiel A, Brown R. The delay in visual reorientation following exposure to a change in direction of resultant force on a human centrifuge. J Gen Psychol. 1951;45:143–150. [Google Scholar]
- Graybiel A, Clark B. Validity of the oculogravic illusion as a specific indicator of otolith function. Aerosp Med. 1965;36:1173–1181. [Google Scholar]
- Israël I, Berthoz A. Contribution of the otoliths to the calculation of linear displacements. J Neurophysiol. 1989;62:247–263. doi: 10.1152/jn.1989.62.1.247. [DOI] [PubMed] [Google Scholar]
- Jaggi-Schwarz K, Hess BJ. Influence of dynamic tilts on the perception of earth-vertical. Exp Brain Res. 2003;149:340–350. doi: 10.1007/s00221-002-1343-y. [DOI] [PubMed] [Google Scholar]
- Jaggi-Schwarz K, Ortega M, Hess BJ. Reciprocal error behavior in estimated body position and subjective visual vertical. Exp Brain Res. 2003;150:122–125. doi: 10.1007/s00221-003-1430-8. [DOI] [PubMed] [Google Scholar]
- Kaptein RG, Van Gisbergen JA. Interpretation of a discontinuity in the sense of verticality at large body tilt. J Neurophysiol. 2003 doi: 10.1152/jn.00804.2003. [DOI] [PubMed] [Google Scholar]
- Kaptein RG, Van Gisbergen JA. Nature of the transition between two modes of external space perception in tilted subjects. J Neurophysiol. 2005;93:3356–3369. doi: 10.1152/jn.01137.2004. [DOI] [PubMed] [Google Scholar]
- Mast F, Jarchow T. Perceived body position and the visual horizontal. Brain Res Bull. 1996;40:393–397. 398. doi: 10.1016/0361-9230(96)00132-3. [DOI] [PubMed] [Google Scholar]
- Mayne R. A systems concept of the vestibular organs. In: Kornhuber H, editor. Handbook of Sensory Physiology. Vestibular System. Psychophysics, Applied Aspects and General Interpretations. part 2. VI. Berlin-New York: Springer-Verlag; 1974. pp. 493–580. [Google Scholar]
- Melvill Jones G, Young LR. Subjective detection of vertical acceleration: a velocity dependent response? Acta Otolaryngol. 1978;85:45–53. doi: 10.3109/00016487809121422. [DOI] [PubMed] [Google Scholar]
- Merfeld DM. Modeling the vestibulo-ocular reflex of the squirrel monkey during eccentric rotation and roll tilt. Experimental Brain Research. 1995;106:123–134. doi: 10.1007/BF00241362. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol. 2005a;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined tilt and translation. J Neurophysiol. 2005b;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld D, Teiwes W, Clarke A, Scherer H, Young L. The dynamic contributions of the otolith organs to human ocular torsion. Exp Brain Res. 1996;110:315–321. doi: 10.1007/BF00228562. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Young LR. The vestibulo-ocular reflex of the squirrel monkey during eccentric rotation and roll tilt. Exp Brain Res. 1995;106:111–122. doi: 10.1007/BF00241361. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan LH. Neural processing of gravitoinertial cues in humans. III. Modeling tilt and translation responses. J Neurophysiol. 2002;87:819–833. doi: 10.1152/jn.00485.2001. [DOI] [PubMed] [Google Scholar]
- Merfeld D, Zupan L. Influence of rotational cues on the neural processing of gravito-inertial force. In: Harris L, Jenkin L, editors. Levels of Perception. New York: Springer-Verlag; 2003. pp. 341–373. [Google Scholar]
- Merfeld DM, Zupan LH, Gifford CA. Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol. 2001;85:1648–1660. doi: 10.1152/jn.2001.85.4.1648. [DOI] [PubMed] [Google Scholar]
- Merfeld D, Zupan L, Peterka R. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Somatic versus vestibular gravity reception in man. Ann NY Acad Sci. 1992;656:124–139. doi: 10.1111/j.1749-6632.1992.tb25204.x. [DOI] [PubMed] [Google Scholar]
- Paige G, Tomko D. Eye movement responses to linear head motion in the squirrel monkey. II. Visual-vestibular interactions and kinematic considerations. J Neurophysiol. 1991;65:1183–1196. doi: 10.1152/jn.1991.65.5.1183. [DOI] [PubMed] [Google Scholar]
- Parker DE, Wood DL, Gulledge WL, Goodrich RL. Self-motion magnitude estimation during linear oscillation: changes with head orientation and following fatigue. Aviat Space Environ Med. 1979;50:1112–1121. [PubMed] [Google Scholar]
- Seidman S, Telford L, Paige G. Tilt perception during dynamic linear acceleration. Exp Brain Res. 1998;119:307–314. doi: 10.1007/s002210050346. [DOI] [PubMed] [Google Scholar]
- Seidman SH, Paige GD. Perception and eye movement during low-frequency centripetal acceleration. Ann NY Acad Sci. 1996;781:693–695. doi: 10.1111/j.1749-6632.1996.tb15762.x. [DOI] [PubMed] [Google Scholar]
- Stevens SS. Perceptual Magnitude and Its Judgement. New York: Academic; 1974. [Google Scholar]
- Stockwell C, Guedry F. The effect of semicircular canal stimulation during tilting on the subsequent perception of the visual vertical. Acta Oto-Laryngologica. 1970;70:170–175. doi: 10.3109/00016487009181874. [DOI] [PubMed] [Google Scholar]
- Telford L, Seidman S, Paige G. Dynamics of squirrel monkey linear vestibuloocular reflex and interactions with fixation distance. J Neurophysiol. 1997;78:1775–1790. doi: 10.1152/jn.1997.78.4.1775. [DOI] [PubMed] [Google Scholar]
- Wade S, Curthoys I. The effect of ocular torsional position on perception of the roll-tilt of visual stimuli. Vision Res. 1997;37:1071–1078. doi: 10.1016/s0042-6989(96)00252-0. [DOI] [PubMed] [Google Scholar]
- Yong N, Seidman SH, Paige GD. Multi-modal was underlying the perception of translational motion. 31st Annual meeting of the Society for Neuroscience; San Diego. 2001. p. 298.17. [Google Scholar]
- Zupan LH, Merfeld DM. Neural processing of gravito-inertial cues in humans. IV. Influence of visual rotational cues during roll optokinetic stimuli. J Neurophysiol. 2003;89:390–400. doi: 10.1152/jn.00513.2001. [DOI] [PubMed] [Google Scholar]


