Abstract
Currently there is considerable interest among oncologists to find anticancer drugs in Chinese herbal medicine (CHM). In the past, clinical data showed that some herbs possessed anticancer properties, but western scientists have doubted the scientific validity of CHM due to the lack of scientific evidence from their perspective. Recently there have been encouraging results, from a western perspective, in the cancer research field regarding the anticancer effects of CHM. Experiments showed that CHM played its anticancer role by inducing apoptosis and differentiation, enhancing the immune system, inhibiting angiogenesis, reversing multidrug resistance (MDR), etc. Clinical trials demonstrated that CHM could improve survival, increase tumor response, improve quality of life, or reduce chemotherapy toxicity, although much remained to be determined regarding the objective effects of CHM in human in the context of clinical trials. Interestingly, both laboratory experiments and clinical trials have demonstrated that when combined with chemotherapy, CHM could raise the efficacy level and lower toxic reactions. These facts raised the feasibility of the combination of herbal medicines and chemotherapy, although much remained to be investigated in this area.
Keywords: Chinese herbal medicine, Anticancer
BACKGROUND OF CHINESE HERBAL MEDICINE (CHM)
CHM is one branch of traditional Chinese medicine (TCM). TCM and western medicine have different ethnic and social backgrounds. The main difference between TCM and western medicine rests how to deal with illness. TCM, focusing on holism and naturalism, combines Chinese medical experience with Chinese culture. In general, Chinese practitioners focus on the “yin” and “yang” balance in the body, which is in accordance with the law of nature. The body will be healthy if it keeps balance between “yin” and “yang”. Diseases will occur if the balance is disrupted.
CHM is empirically based and has a history of over 4000 years. The biological ingredients of herbal remedies are extracted from natural substances: plants, animal parts, shells, insects and even stones and minerals. The drugs used in CHM often result from a combination of multiple ingredients, called formula, to ensure effective actions on different targets simultaneously (Attele et al., 1999). The composite formulas usually have greater efficacy than single ingredients. This may be due to the synergistic interactions of the ingredients (Xue and Roy, 2003). In Chinese herbalism, every herb has its own properties. Chinese herbalists believe that illness can be effectively treated by combining herbs based on their various characteristics and the patient’s overall status. The composition of the formula and the dosage of the individual ingredients depend on the signs and symptoms of the patient, which is the basic principle of CHM. These formulas can be modified to fit specific individuals more closely. This theory is in accordance with the idea of “individual therapy” in western medicine. CHM is very popular in China, and has been historically used in treating chronic diseases, such as constipation, dermatitis, insomnia, among other conditions.
ANTICANCER ROLE OF CHM
Recently, modern biomolecular science has contributed to the interpretation of the anticancer effects of CHM. As more information becomes available, CHM is becoming recognized worldwide. The gap between East and West is narrowing. Although the anticancer role of CHM needs further investigation, the achievements in this field do give us some important information.
Literature searches were conducted through PubMed until Feb. 2005. Additional manual searches were carried out on relevant medical journals. Search items were “herb”, “anticancer mechanism”, “traditional Chinese medicine”, and “cancer therapy”. No restrictions were placed on the language of publication. Only primary data or data that superseded earlier work were included. Case reports were not included. Anticancer clinical trials using CHM in patients and studies investigating anticancer mechanisms of CHM from a western perspective were included. The anticancer effects of CHM have been reported in many Chinese journals. Studies lacking controls were excluded. Two authors independently assessed the quality of the studies, and extracted data.
Laboratory evidence emerging recently
Experiments in test tubes and animals explored the mechanisms for the anticancer role of CHM (Table 1). It was demonstrated that CHM plays its anticancer role by inducing apoptosis and differentiation, enhancing the immune system, inhibiting angiogenesis, reversing multidrug resistance (MDR), etc. Many herbal remedies played their anticancer roles through multiple mechanisms (Ikezoe et al., 2003a; 2003b; Cheng et al., 2003a; Hong-Fen et al., 2001; Hsieh et al., 2002; Wartenberg et al., 2003).
Table 1.
Anticancer effects of CHM (laboratory experiments)
| Type of cancer | Model | Herb | Mechanism | Ref. |
| HNSCC | SCC-25 and KB cell lines, four nude mice with s.c. inoculation of KB cells | Scutellaria baicalensis | Inhibition of cell growth in vitro and in vivo, inhibition of PGE2 synthesis via suppression of COX-2 expression | Zhang et al., 2003 |
| KB, KBv200 cell lines | Asiaticoside | Induction of apoptosis and enhancement of the anti-tumor activity of vincristine | Huang et al., 2004 | |
| Leukemia | U937 cell line | Mylabris phalerlata, Scutellaria barbata | Induction of apoptosis | Huh et al., 2003; Cha et al., 2004 |
| NB4, HL60 cell lines | Red orpiment | Induction of apoptosis | Zhong et al., 2003 | |
| AKR/J mice | Echinacea purpurea | Enhancement of nonspecific immune or cellular immune systems (or of both) | Hayashi et al., 2001 | |
| CCRF-CEM, CEM/E1000, CEM/VLB (100) cell lines | Artesunate (ART), Bufalin | ART significantly increased daunorubicin accumulation in CEM/E1000 cells, but not in CEM/VLB (100) or CCRF-CEM parental cells. Bufalin caused a small, but significant increase in daunorubicin accumulation in CEM/VLB (100) and CEM/E1000 cells | Efferth et al., 2002 | |
| NB4 cell line | Arsenic trioxide | Induction of apoptosis | Chen et al., 1996 | |
| HL-60 cell line | Hydrolysable tannis from Eugenia jambos L. | Induction of apoptosis | Yang et al., 2000 | |
| HL-60, NB4, U937 and THP-1 cell lines | PC-SPES | Inhibition of growth, induction of differentiation and apoptosis | Ikezoe et al.,2003a | |
| Colorectal carcinoma | CoLo205 cell line | Magnolol | Induction of apoptosis | Lin et al., 2002 |
| Mice bearing colon26/clone 20 carcinoma cells | Coptidis rhizome and Berberine | Reduction of IL-6 mRNA levels and protein levels in tumors and sera | Iizuka et al., 2002 | |
| Gastric cancer | MGC-803 cell line | Isoliquiritigenin | Induction of apoptosis | Ma et al., 2001 |
| AGS cell line | Astragali (AR) | Cytostatic | Lin et al., 2003 | |
| MNK45 and KATO-III cell lines | Anemarrhena asphodeloides | Induction of apoptosis | Takeda et al., 2001 | |
| Hepatic cancer | Hep-G2 cell line | Magnolol | Induction of apoptosis | Lin et al., 2002 |
| SMMC-7221 cell line | Isoverbascoside | Induction of differentiation | Chen et al., 2002a | |
| Lung cancer | A549 cell line | Bupleuri radix | Inhibition of telomerase activity and activation of apoptosis | Cheng et al., 2003a |
| Lung cancer cells | Triptolide | Induction of apoptosis in combination with Apo2L/TRAIL | Frese et al., 2003 | |
| Breast cancer | 95-D cell line | Acutiaporberine | Induction of apoptosis | Chen et al., 2002b |
| F344 rats | Anticancer-number-one | Increasement of NK cell activity and inhibition of tumor metastasis | Hong-Fen et al., 2001 | |
| MCF-7 cell line | Rosemary | Reversing MDR | Plouzek et al., 1999 | |
| MCF-7 cell line | Tea and tea polyphenlos | Suppression of fatty acid synthase (a key enzyme in lipogenesis) | Yeh et al., 2003 | |
| MCF-7 and MCF-7/ADM cell lines | Asiaticoside | Enhancement of the anti-tumor activity of vincristine | Huang et al., 2004 | |
| Ovarian cancer | SKOV3, CAOV3 and OVCAR-3 cell lines | Scutellaria barbatae | Induction of apoptosis | Powell et al., 2003 |
| Prostate cancer | LNCaP cell lines | PC-SPES | Activation of the JNK/c-Jun/AP-1 signal pathway resulting in growth arrest and apoptosis of prostate cancer cells | Ikezoe et al., 2003b |
| Prostate carcinoma cells | Equiguard | Down-regulation of expression of androgen receptor and prostate-specific antigen, induction of apoptosis | Hsieh et al., 2002 | |
| Embryoid bodies and multicellular DU-145 prostate tumor spheroids | Baicalein, Epicatechin, Berberine, Acteoside | Down-regulation of MMP expression, inhibition of angiogenesis | Wartenberg et al., 2003 | |
| Glioma | Rat C6 glioma cells | Saikosaponin a, b | Induction of differention | Tsai et al., 2002 |
| Mice that injected with LZEJ-C3 cells subcutaneously | Dang-gui-bu-xai-tang | Increasement of the population of CTLs and NK cells, and down-regulation of activated T helper cells (CD4+/CD25+) in spleen and TDLN | Hsieh et al., 2003 |
Note: HNSCC, head and neck squamous cell carcinoma; PGE2, prostaglandin E2; COX-2, cyclooxygenase 2; EC: endotheliocytes; NK, natural killer; MDR, multidrug resistance; MMP, matrix metalloproteinase; CTLs, cytotoxic T lymphocytes; TDLN, tumor-draining lymph nodes. Anticancer-number-one, Panax ginseng, Poria cocos, Atractylodes macrocephala, Anglica sinensis, A. membranaceus, Curcuma zedoaria, Scutellaria baicalensis, Phellodenron chinense, Coptis chinensis, Glycyrrhiza uralensis, Crataegus pinnatifida, Hordeum õulgare, Salõia miltiorrhiza, Schisandra chinensis, Hedyotis diffusa, Ophiopogon japonicus, Lobelia chinesis lour, Scutellaria barbaba, Massa fermentata medicinalis; PC-SPES, Reishi mushroom, Baikal skullcap, Rabdosia, Dyer’s woad, Chrysanthemum, Saw palmetto, Panax ginseng, and Licorice; Dang-gui-bu-xai-tang, Radix Angelicae sinensis and Radix Astragali membranaceus
1. Characters of anticancer effect of herbal remedies
The synergistic effects of various components in Scutellaria baicalensis extract [baicalin (80%), wogonoside (16%), baicalein (2%), wogonin (1%) and other compounds in trace amounts] were demonstrated (Zhang et al., 2003). Both the extract and its pure compound-baicalein inhibited cancer cell growth (in vitro). The extract could inhibit prostaglandin E2 (PGE2) production, whereas its pure compound, baicalein, could not. Another pure compound wogonin significantly inhibited cyclooxygenase 2 (COX-2) activity in lipopolysaccharide (LPS)-stimulated macrophages. The various components in the herb act on different anticancer pathways such as COX-dependent and COX-independent pathways.
Some herbs were most effective on cancer of a certain type. It was demonstrated that artesunate (ART) was most active against leukemia and colon cancer cell lines [mean GI50 values: (1.11±0.56) μmol/L and (2.13±0.74) μmol/L, respectively]. Non-small cell lung cancer (NSCLC) cell lines showed the highest mean GI50 value [(25.62±14.95) μmol/L] indicating the lowest sensitivity towards ART (Efferth et al., 2002). This may be due to the herb’s affinity for specific organs.
There exists an interesting phenomenon that some herbs might be selective for cancer cells instead of normal cells. It was reported that Scutellaria baicalensis demonstrated a strong inhibition in two tested human head and neck squamous cell carcinoma (HNSCC) cell lines, while no growth inhibition of HaCaT cells (a non-tumorigenic cell line) was observed (Zhang et al., 2003). The butanol fraction of Mylabris phalerlata was specifically cytotoxic on human monocytic leukemic U937 cells rather than on peripheral blood mononuclear lymphocytes (Huh et al., 2003). The herb formula PC-SPES inhibited proliferation of HL-60, NB4, U937 and THP-1 human leukemia cells with ED 50 of 0.17, 0.09, 0.18, 0.32 μl/ml respectively. On the contrary, PC-SPES (0.1 μl/ml) stimulated growth of normal myeloid committed stem cells by 1.4 fold of control (P=0.03) (Ikezoe et al., 2003a). It was demonstrated that magnolol at concentrations of 3~10 μmol/L inhibited DNA synthesis and decreased cell number in cultured human cancer cells (COLO-205 and Hep-G2) in a dose-dependent manner but not in non-transformed human cells such as keratinocytes, fibroblasts, and human umbilical vein endothelial cells (HUVEC). When magnolol concentration was increased to 100 μmol/L, apoptosis was observed in COLO-205 and Hep-G2 cells, but not in cultured human fibroblasts and HUVEC (Lin et al., 2002). Triptolide was also demonstrated to sensitize lung cancer but not normal human bronchial epithelial cells to Apo2L/TRAIL-induced apoptosis (Frese et al., 2003), However, it is still unclear how this tumor-specific killing occurs. Further investigation is needed to clarify the mechanism behind this interesting phenomenon.
2. Enlarged efficacy of herbal remedies in combination with routine chemical reagents
Some studies have assessed the use of herbs in combination with routine chemical reagents. Asiaticoside could enhance the anti-tumor activity of vincristine in several cancer cell lines including KB, KBv200, MCF-7, and MCF-7/ADM. The apoptosis rates and the Bcl-2 phosphorylation levels were much higher in asiaticoside plus vincristine groups than in vincristine or asiaticoside groups. Asiaticoside plus vincristine enhanced S-G (2)/M arrest, up-regulated Cyclin B1 protein expression, and down-regulated P34 (cdc2) protein expression in KB cells (Huang et al., 2004). PC-SPES enhanced the antiproliferative and prodifferentiative effects of ATRA (all-trans retinoic acid) on leukemia cells. PC-SPES (0.125 μl/ml) alone, ATRA (10−8 mol/L) alone or the combination of the two inhibited growth of HL-60 cells after 4 d of culture, by approximately 40%, 30% and 80%, respectively. Measured by expression of CD11b antigen, the induced differentiation rate was also much higher in the combination group (60%) than in PC-SPES group (27%) or ATRA group (18%) alone (Ikezoe et al., 2003a). An extract of the common dietary herb rosemary (Rosemarinus officinalis Labiatae) increased the intracellular accumulation of commonly used chemotherapeutic agents, including doxorubicin (DOX) and vinblastine (VIN), in drug-resistant MCF-7 human breast cancer cells (Plouzek et al., 1999).
Current status of clinical trials
Simultaneously, clinical trials of different phases have been conducted. The critical data from the seven clinical trials, searched from PubMed, are listed in Table 2. These studies were carried out in the USA (Wheeler et al., 2003; Oh et al., 2004; Soignet et al., 1998), Egypt (Mabed et al., 2004), China (Shen et al., 2004; Piao et al., 2004), Russia, Bulgaria and Ukraine (Semiglasov et al., 2004) from 1998 to 2004. Various kinds of herbs were used, including Semen coicis, Arsenic trioxide, Viscum fraxini-2, standardized mistletoe extracts, PC-SPES. Various types of cancer have been investigated, including acute promyelocytic leukemia, thyroid cancer, nasopharyngeal carcinoma, esophagus cancer, mesothelioma, NSCLC, hepatocellular carcinoma, pancreatic cancer, colon cancer, prostate cancer, breast cancer, ovarian cancer, carcinoid, and liposarcoma.
Table 2.
Anticancer effects of CHM (clinical trials)
| Patients |
Herb | Herb dosage | Treatments | Effect | Reference | |
| Type of cancer | No. | |||||
| Lung | 3 | Kanglaite injection, an extract of the coix seed | 10000 mg/d up to 50000 mg/d, for 21 consecutive days every 4 weeks (increased by 10000 mg/d in each of four subsequent levels) | Herb | No dose limiting toxicities were observed in the first cycle up to the maximum dose of 50000 mg/d. A maximum tolerated dose (MTD) could not be defined. | Wheeler et al., 2003 |
| Esophagus | 3 | |||||
| Prostate | 2 | |||||
| Mesothelioma | 2 | |||||
| Colon | 2 | |||||
| Carcinoid | 1 | |||||
| Pancreas | 1 | |||||
| Thyroid | 1 | |||||
| Liposarcoma | 1 | |||||
| HCC | 23 | Viscum fraxini-2 | 2 ampoules (10000 ng/ml), subcutaneously, once weekly | Herb | CR: 13.1%; PR: 8.1%; 39.1%: progressive; 39.1%: no evaluation of response due to early death | Mabed et al., 2004 |
| Prostate cancer | 90 | PC-SPES | 960 mg orally, t.i.d., (DES 3 mg orally q.d. as control) | Herb | PSA declines ≥50% were noted in 40% with PC-SPES and 24% with DES | Oh et al., 2004 |
| APL | 12 | Arsenic trioxide | 0.06 to 0.2 mg/(kg·d), cumulative doses, 160~515 mg | Herb | Complete remission: 92% | Soignet et al., 1998 |
| APL | 61 | Arsenic trioxide | ATRA: 25 mg/m2 per day; Arsenic trioxide: 0.16 mg/kg per day | Arsenic trioxide+ATRA (appropriate chemotherapy) | The time to achieve complete rates was the shortest one in the combination group | Shen et al., 2004 |
| Breast cancer | 272 | sME | 0.5 ml (10, 30, 70 ng/ml) twice weekly subcutaneously for 15 consecutive weeks | Herb+CMF chemotherapy | QoL was significantly improved (P<0.01) with the medium dose (30 ng/ml) | Semiglasov et al., 2004 |
| Breast cancer | 68 | sME | 1 mg up to 200 mg, subcutaneously, 3 times per week | Herb+chemotherapy (Breast cancer: CAP, CAF; Ovarian cancer: CP, IcP; NSCLC: VP, MViP) | QoL was significantly (P<0.05) improved | Piao et al., 2004 |
| Ovarian cancer | 71 | |||||
| NSCLC | 94 | |||||
Note: Kanglaite injection, produced by the Chinese company Zhejiang Kanglaite pharmaceutical (ZKP, Hangzhou). APL: Acute promyelocytic leukemia; HCC: Hepatocellular carcinoma; CR: Complete response; PR: Partial response; DES: Diethylstilbestrol; PSA: Prostate-specific antigen; ATRA: All-trans retinoic acid; IA: Idarubicin, Ara-C; sME: Standardized mistletoe extracts; CMF: Cyclophosphamide, methotrexate, fluorouracil; QoL: Quality of life; NSCLC: Non-small cell lung cancer; CAP: Cyclophosphamide, adriamycin, cisplatin; CAF: Cyclophosphamide, adriamycin, 5-fluorouracil; CP: Cyclophosphamide, cisplatin; IcP: Ifosfamide, carboplatin; VP: Vinorelbine, cisplatin; MViP: mitomycine, vindesine, cisplatin
Sample sizes in the clinical trials ranged from 12 to 272. Dropouts and withdrawals were mentioned in (Wheeler et al., 2003; Oh et al., 2004; Soignet et al., 1998; Shen et al., 2004; Semiglasov et al., 2004; Piao et al., 2004). Adverse effects of CHM were reported in all of the clinical trials. In these trials, patients received herbal medicine alone (Wheeler et al., 2003; Mabed et al., 2004; Oh et al., 2004; Soignet et al., 1998) or along with standard tumor destructive treatment (Shen et al., 2004; Semiglasov et al., 2004; Piao et al., 2004). The phase I trial (Wheeler et al., 2003) focusing on Semen coicis demonstrated the safety of the drug. The six phase II trials (Mabed et al., 2004; Oh et al., 2004; Soignet et al., 1998; Shen et al., 2004; Semiglasov et al., 2004; Piao et al., 2004) showed that these herbs were helpful against cancer, alone or in combination with chemotherapy. It is demonstrated that herbs could improve survival (Mabed et al., 2004; Shen et al., 2004), increase tumor response (Mabed et al., 2004; Oh et al., 2004; Soignet et al., 1998; Shen et al., 2004), improve quality of life or reduce chemotherapy toxicity (Semiglasov et al., 2004; Piao et al., 2004).
DISCUSSION
Benefits and concerns
Science has long acknowledged the value of healing substances found in nature, such as digitalis, aspirin, penicillin, insulin, steroids, etc. There has been a resurgence of interest, both scientifically and popularly, in the utilization of natural approaches.
Experiments in test tubes and in animals demonstrated that CHM played its anticancer role by inducing apoptosis and differentiation, enhancing the immune system, inhibiting angiogenesis, reversing multidrug resistance (MDR), etc. However, the mechanism of the anticancer role of CHM has not yet been fully elucidated. Further research is needed to explore the molecular mechanism of CHM. The pharmacological effects of CHM are still not clear although it is proved to be effective against disease, which is also the major reason preventing CHM from being accepted worldwide. Although the clinical trials showed that herbs were helpful against cancer, these outcomes require further confirmation with rigorously controlled trials. In China, many clinical trials focusing on the anticancer effects of herbal formulas have been conducted. Though many of them demonstrated that herbs are helpful against cancer, especially useful in improving survival and quality of life in patients suffering from advanced cancer, the lack of controls and reporting bias have been severe flaws. Researchers must pay attention to the scientific rigor of studies of CHM in the future to improve the status.
Some Chinese herbs may be harmful to the human body if they are used improperly. Some herbs may cause serious toxicity when taken excessively or under inappropriate circumstances (Chan et al., 2005). Herbal remedies should be prescribed carefully by qualified practitioners according to the herb character and the whole body condition of the patient. Also, potential herb-drug interactions should be taken into consideration if multiple drugs are prescribed simultaneously (Cheng et al., 2003b).
Herbal therapies have already led to the development of a number of anticancer drugs. The key to choosing the anticancer CHM is to optimize an effective formula. With the development of modern science and medicine, CHM modernization is on the way. Using state-of-the-art technology in CHM extraction, purification and drug production can efficiently improve the CHM industry. Traditionally, herbs have been made into pills, powders and astopical plasters. Today, herbal companies have developed other forms like capsules, tablets and injections which are more convenient and palatable. Kanglaite injection is an extract produced from coix seed (Semen coicis). The anticancer mechanisms of Kanglaite injection have been widely explored in China. It has been proven to play its anticancer role through inhibition of the mitosis of tumor cells during G2/M phases, induction of apoptosis and inhibition of the formation of newly generated blood vessels (Li, 2001). This botanical drug, which is approved in China for treatment of lung cancer, hepatic cancer and gastric cancer, has been extensively used in more than 2000 hospitals around the country. After one year of clinical experiments which proved its beneficial effects in the treatment of lung cancer and gastric cancer, it has been recently permitted by the Russian health authorities for clinical application for cancer treatment. A phase I trial for Kanglaite in Salt Lake City, USA, has been completed, verifying the safety of the drug (Wheeler et al., 2003). The phase II trial, approved by the United States Food and Drug Administration (FDA), will pair Kanglaite with another chemotherapy to treat NSCLC. The global market of CHM for anticancer therapy will probably be prosperous from this new start.
However, caution should be used. Due to contamination with undeclared prescription drugs, such as DES, indomethacin and warfarin, PC-SPES was withdrawn from the market in 2002. The withdrawal of PC-SPES can be viewed as due to inadequate quality control in the production of the herb remedies. The product should be fully characterized and standardized, properly labelled for its contents. It is unclear whether these prescription drugs account for the anticancer effect of the herb remedies. Future research is required to determine which ingredients are effective. However, PC-SPES did have anticancer effects against prostate cancer. If PC-SPES free of contaminating drugs is not as effective as the drug-containing PC-SPES, the original mixture (combining herbs and drugs) may provide valuable clues for researching and developing anticancer drugs in the future.
Combination of CHM and chemotherapy
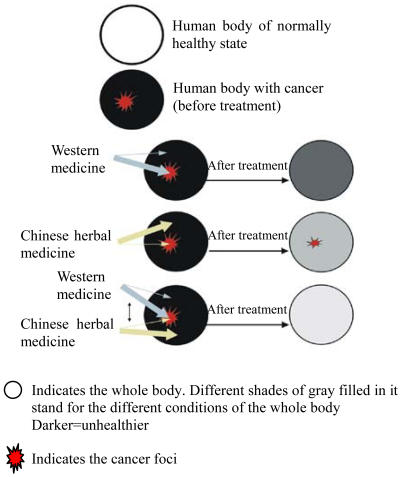
Although much remains to be determined regarding the objective effects of CHM in vitro and in human in the context of clinical trials, Arsenic trioxide has confirmed efficacy against APL. Arsenic trioxide has proven to be very effective in obtaining high clinically complete remission rates in APL patients (Soignet et al., 1998). This drug has been widely used on APL patients around the world. Recent study showed that the ATRA/Arsenic trioxide combination for remission/maintenance therapy of APL lead to much better results than either of the two drugs alone in terms of the quality of complete remission and the status of the disease-free survival (Shen et al., 2004). The authors also conducted experiments to explore the possible underlying molecular mechanisms. As a result, ATRA and Arsenic trioxide were shown to have synergistic effect on apoptosis and degradation of PML-PARα oncoprotein. This successful research, together with other studies demonstrating the enlarged efficacy and lowered toxicity of the combination therapy in vitro (Huang et al., 2004; Ikezoe et al., 2003a; Plouzek et al., 1999) and in clinical trials (Shen et al., 2004; Semiglasov et al., 2004; Piao et al., 2004) holds the promise of better treatment of cancer. However, the possibility of detrimental effects from the combination cannot be ruled out. Further studies, including basic experiments conducted in different systems and rigorously controlled clinical trials, are required to better define the objective effects of the combination therapy. Western therapies, including surgery, chemotherapy, and radiotherapy are tumor destructive. From the Chinese practitioners’ viewpoint, the whole unhealthy condition should be treated successfully to cure the patients suffering from cancer. The two different systems of medicine target “cancer” from different perspective. This is due to difference in the way of thinking. Westerner usually use the direct thinking way. When they want to target “A”, they usually try their best to target “A” itself, with that requisite, they will impair the factors related to “A”-B, C, D. Chinese usually use the indirect way of thinking. When they want to target “A”, they are apt to first target the factors related to “A”-B, C, D, to change the environment and conditions of “A”, which ultimately leads to change in “A”. It is difficult to draw a conclusion about which one is superior to the other. They are two ways from different perspective to solve the problem. One might provide a complementary remedy to the other. Chinese practitioners hold the belief that western therapy strongly targets the cancer foci and CHM strongly targets the unhealthy condition of the whole body. Combination therapy, bringing together the advantages of each type of medicine for an overall enhancement of cancer treatment, is regarded as one of the most important principles for the treatment of cancer in China (Fig.1). However, due to the culture difference, the philosophy accepted in China needs further research to verify its scientific basis from the perspective accepted all around the world, which needs the effort from Chinese practitioners and oncologists worldwide.
Fig. 1.
Cancer treatment with western medicine and Chinese herbal medicine
Acknowledgments
We thank Mari Swift (University of Washington, USA), Janice Lee (Harvard University, USA) for the serious proofreading work, Bingjian Lv, Weiqun Xu, Xiuxu Chen, Jing Deng and Lina Shao for helpful discussion in revising the article, Richard T. Penson (Massachusetts General Hospital, USA), Mohammed Mabed (Mansoura University, Egypt) for critical comments, Peng Fang for help with figure preparation.
Footnotes
Project (No. 011107607) supported by the Science and Technology Foundation Grant of Zhejiang Province, China
References
- 1.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 2.Cha YY, Lee EO, Lee HJ, Park YD, Ko SG, Kim DH, Kim HM, Kang IC, Kim SH. Methylene chloride fraction of Scutellaria barbata induces apoptosis in human U937 leukemia cells via the mitochondrial signaling pathway. Clin Chim Acta. 2004;348(1-2):41–48. doi: 10.1016/j.cccn.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Chan TY, Tam HP, Lai CK, Chan AY. A multidisciplinary approach to the toxicologic problems associated with the use of herbal medicines. Ther Drug Monit. 2005;27(1):53–57. doi: 10.1097/00007691-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88(3):1052–1061. [PubMed] [Google Scholar]
- 5.Chen RC, Su JH, Ouyang GL, Cai KX, Li JQ, Xie XG. Induction of differentiation in human hepatocarcinoma cells by isoverbascoside. Planta Med. 2002;68(4):370–372. doi: 10.1055/s-2002-26759. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Peng W, Qi S, Xu A. Apoptosis of human highly metastatic lung cancer cell line 95-D induced by acutiaporberine, a novel bisalkaloid derived from Thalictrum acutifolium. Planta Med. 2002;68(6):550–553. doi: 10.1055/s-2002-32546. [DOI] [PubMed] [Google Scholar]
- 7.Cheng YL, Chang WL, Lee SC, Liu YG, Lin HC, Chen CJ, Yen CY, Yu DS, Lin SZ, Harn HJ. Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. 2003;73(18):2383–2394. doi: 10.1016/S0024-3205(03)00648-9. [DOI] [PubMed] [Google Scholar]
- 8.Cheng KF, Leung KS, Leung PC. Interactions between modern and Chinese medicinal drugs: a general review. Am J Chin Med. 2003;31(2):163–169. doi: 10.1142/S0192415X0300093X. [DOI] [PubMed] [Google Scholar]
- 9.Efferth T, Davey M, Olbrich A, Rucker G, Gebhart E, Davey R. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol Dis. 2002;28(2):160–168. doi: 10.1006/bcmd.2002.0492. [DOI] [PubMed] [Google Scholar]
- 10.Frese S, Pirnia F, Miescher D, Krajewski S, Borner MM, Reed JC, Schmid RA. PG490-mediated sensitization of lung cancer cells to Apo2L/TRAIL-induced apoptosis requires activation of ERK2. Oncogene. 2003;22(35):5427–5435. doi: 10.1038/sj.onc.1206842. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi I, Ohotsuki M, Suzuki I, Watanabe T. Effects of oral administration of Echinacea purpurea (American herb) on incidence of spontaneous leukemia caused by recombinant leukemia viruses in AKR/J mice. Nihon Rinsho Meneki Gakkai Kaishi. 2001;24(1):10–20. doi: 10.2177/jsci.24.10. [DOI] [PubMed] [Google Scholar]
- 12.Hong-Fen L, Waisman T, Maimon Y, Shakhar K, Rosenne E, Ben-Eliyahu S. The effects of a Chinese herb formula, anti-cancer number one (ACNO), on NK cell activity and tumor metastasis in rats. Int Immunopharmacol. 2001;1(11):1947–1956. doi: 10.1016/S1567-5769(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh TC, Lu X, Guo J, Xiong W, Kunicki J, Darzynkiewicz Z, Wu JM. Effects of herbal preparation Equiguard on hormone-responsive and hormone-refractory prostate carcinoma cells: mechanistic studies. Int J Oncol. 2002;20(4):681–689. [PubMed] [Google Scholar]
- 14.Hsieh CC, Lin WC, Lee MR, Hsu SL, Liu HS, Kao ST, Hsieh MT. Dang-Gui-Bu-Xai-Tang modulated the immunity of tumor bearing mice. Immunopharmacol Immunotoxicol. 2003;25(2):259–271. doi: 10.1081/IPH-120020474. [DOI] [PubMed] [Google Scholar]
- 15.Huang YH, Zhang SH, Zhen RX, Xu XD, Zhen YS. Asiaticoside inducing apoptosis of tumor cells and enhancing anti-tumor activity of vincristine. Ai Zheng. 2004;23(12):1599–1604. (in Chinese) [PubMed] [Google Scholar]
- 16.Huh JE, Kang KS, Ahn KS, Kim DH, Saiki I, Kim SH. Mylabris phalerlata induces apoptosis by caspase activation following cytochrome c release and Bid cleavage. Life Sci. 2003;73(17):2249–2262. doi: 10.1016/S0024-3205(03)00568-X. [DOI] [PubMed] [Google Scholar]
- 17.Iizuka N, Hazama S, Yoshimura K, Yoshino S, Tangoku A, Miyamoto K, Okita K, Oka M. Anticachectic effects of the natural herb Coptidis rhizoma and berberine on mice bearing colon 26/clone 20 adenocarcinoma. Int J Cancer. 2002;99(2):286–291. doi: 10.1002/ijc.10338. [DOI] [PubMed] [Google Scholar]
- 18.Ikezoe T, Chen S, Saito T, Asou H, Kyo T, Tanosaki S, Heber D, Taguchi H, Koeffler HP. PC-SPES decreases proliferation and induces differentiation and apoptosis of human acute myeloid leukemia cells. Int J Oncol. 2003;23(4):1203–1211. [PubMed] [Google Scholar]
- 19.Ikezoe T, Chen SS, Yang Y, Heber D, Taguchi H, Koeffler HP. PC-SPES: molecular mechanism to induce apoptosis and down-regulate expression of PSA in LNCaP human prostate cancer cells. Int J Oncol. 2003;23(5):1461–1470. [PubMed] [Google Scholar]
- 20.Li DP. Progresses in basic studies of anticancer mechanisms of Kanglaite injection (KLT) Zhong Yao Xin Yao Yu Lin Chuang Yao Li. 2001;12(2):122–124. (in Chinese) [Google Scholar]
- 21.Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH, Lee WS. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem. 2002;84(3):532–544. doi: 10.1002/jcb.10059. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Dong HF, Oppenheim JJ, Howard OM. Effects of astragali radix on the growth of different cancer cell lines. World J Gastroenterol. 2003;9(4):670–673. doi: 10.3748/wjg.v9.i4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, Fu NY, Pang DB, Wu WY, Xu AL. Apoptosis induced by isoliquiritigenin in human gastric cancer MGC-803 cells. Planta Med. 2001;67(8):754–757. doi: 10.1055/s-2001-18361. [DOI] [PubMed] [Google Scholar]
- 24.Mabed M, El-Helw L, Shamaa S. Phase II study of viscum fraxini-2 in patients with advanced hepatocellular carcinoma. Br J Cancer. 2004;90(1):65–69. doi: 10.1038/sj.bjc.6601463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh WK, Kantoff PW, Weinberg V, Jones G, Rini BI, Derynck MK, Bok R, Smith MR, Bubley GJ, Rosen RT, et al. Prospective, multicenter, randomized phase II trial of the herbal supplement, PC-SPES, and diethylstilbestrol in patients with androgen-independent prostate cancer. J Clin Oncol. 2004;22(18):3705–3712. doi: 10.1200/JCO.2004.10.195. [DOI] [PubMed] [Google Scholar]
- 26.Piao BK, Wang YX, Xie GR, Mansmann U, Matthes H, Beuth J, Lin HS. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004;24(1):303–309. [PubMed] [Google Scholar]
- 27.Plouzek CA, Ciolino HP, Clarke R, Yeh GC. Inhibition of P-glycoprotein activity and reversal of multidrug resistance in vitro by rosemary extract. Eur J Cancer. 1999;35(10):1541–1545. doi: 10.1016/S0959-8049(99)00180-X. [DOI] [PubMed] [Google Scholar]
- 28.Powell CB, Fung P, Jackson J, Dall′Era J, Lewkowicz D, Cohen I, Smith-McCune K. Aqueous extract of herba Scutellaria barbatae, a chinese herb used for ovarian cancer, induces apoptosis of ovarian cancer cell lines. Gynecol Oncol. 2003;91(2):332–340. doi: 10.1016/j.ygyno.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Semiglasov VF, Stepula VV, Dudov A, Lehmacher W, Mengs U. The standardised mistletoe extract PS76A2 improves QoL in patients with breast cancer receiving adjuvant CMF chemotherapy: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2004;24(2C):1293–1302. [PubMed] [Google Scholar]
- 30.Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, Shi JY, Zheng PZ, Yan H, Liu YF, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2004;101(15):5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 32.Takeda Y, Togashi H, Matsuo T, Shinzawa H, Takeda Y, Takahashi T. Growth inhibition and apoptosis of gastric cancer cell lines by Anemarrhena asphodeloides Bunge. J Gastroenterol. 2001;36(2):79–90. doi: 10.1007/s005350170135. [DOI] [PubMed] [Google Scholar]
- 33.Tsai YJ, Chen IL, Horng LY, Wu RT. Induction of differentiation in rat C6 glioma cells with Saikosaponins. Phytother Res. 2002;16(2):117–121. doi: 10.1002/ptr.752. [DOI] [PubMed] [Google Scholar]
- 34.Wartenberg M, Budde P, de Marees M, Grunheck F, Tsang SY, Huang Y, Chen ZY, Hescheler J, Sauer H. Inhibition of tumor-induced angiogenesis and matrix-metalloproteinase expression in confrontation cultures of embryoid bodies and tumor spheroids by plant ingredients used in traditional chinese medicine. Lab Invest. 2003;83(1):87–98. doi: 10.1097/01.LAB.0000049348.51663.2F. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler RH, Busby L, Samlowski W, et al. Phase I Study of Kanglaite (KLT) a Botanical Product Based on Traditional Chinese Medicine; 2003. Presented at American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL. [Google Scholar]
- 36.Xue T, Roy R. Studying traditional Chinese medicine. Science. 2003;300(5620):740–741. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]
- 37.Yang LL, Lee CY, Yen KY. Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells. Cancer Lett. 2000;157(1):65–75. doi: 10.1016/S0304-3835(00)00477-8. [DOI] [PubMed] [Google Scholar]
- 38.Yeh CW, Chen WJ, Chiang CT, Lin-Shiau SY, Lin JK. Suppression of fatty acid synthase in MCF-7 breast cancer cells by tea and tea polyphenols: a possible mechanism for their hypolipidemic effects. Pharmacogenomics J. 2003;3(5):267–276. doi: 10.1038/sj.tpj.6500192. [DOI] [PubMed] [Google Scholar]
- 39.Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi J, Zhang W, Wei H, Sung M, Wang W, et al. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 2003;63(14):4037–4043. [PubMed] [Google Scholar]
- 40.Zhong L, Chen F, Han J, Shao N, Ouyang R. Effects of red orpiment on cell morphology and expression of PML mRNA and protein in NB4 and HL-60 cells. Chin Med J. 2003;116(1):148–150. [PubMed] [Google Scholar]