Abstract
To investigate the possible role of nitric oxide (NO) produced locally or intramurally in the quiescence of the pregnant myometrium, nitric oxide synthase (NOS) activity was measured in samples from first trimester (villous, and non villous-trophoblast), term placenta and pregnant myometrium. Trophoblast tissue was obtained from psychosocial termination of pregnancy (9 – 12 weeks' gestation) whereas placenta and myometrium, from the same patient, at deliveries by Caesarean section. NOS activity was measured in both cytosolic and particulate fractions by the formation of 14C-citrulline from 14C-arginine. Western immunoblotting was used to identify the endothelial NOS (eNOS) and neuronal (nNOS) isoforms. The activity of NOS in particulate fractions from all preparations was considerably higher than the cytosolic fractions. Activity in all fractions except the myometrium was highly Ca-dependent. More than 50% of particulate NOS from the myometrium was Ca-independent. NOS activity was highest in the villous trophoblast and there was a significant difference between the villous and non-villous trophoblast. In placenta and myometrium, NOS was 2–4 fold and 20–28-fold lower than the villous trophoblast, respectively. Western blot analysis showed clearly eNOS in the particulate fraction and a weak eNOS band in the cytosolic fractions, whereas nNOS was not detectable in any of the fractions. In view of the marginal activity of NOS in the myometrium, NO produced by the trophoblast and placenta could play a significant role in maintaining uterine quiescence by paracrine effect.
Background
Nitric oxide (NO) is a multifunctional signal and important modulator of cellular responses in a variety of tissues including those involved in human reproduction. NO is generated from L-arginine by the catalytic action of the enzyme, nitric oxide synthase (NOS). So far three different isoform of NOS have been identified, cloned and characterised. While two of the three (type I or neuronal and III or endothelial) are constitutively expressed in a variety of tissues, the expression of the third isoform (type II or inducible) can be induced by cytokines and some other agents [1-3].
NO is known to have a powerful vasodilatory effect in resistance vessels throughout the body. This action is, however, mediated by locally produced NO and in most instances by the subsequently generated second messenger guanosine 3–5 cyclic monophosphate (cGMP).
There is considerable evidence that local production of NO contributes to the maintenance of low vascular resistance in the fetoplacental circulation [4.6]. Since umbilical cord and chorionic plate vessels are unlikely to contribute greatly to the regulation of fetoplacental blood flow because of their large calibre, stem villous arterioles of the placenta are thought to be the major site of resistance. The biosynthesis of NO and cGMP is increased during pregnancy in rat and sheep [7,9] and NO was reported to decrease in rats at parturition [10,11], but the evidence in human is inconclusive.
The histological unit of the placenta consists of villous trophoblast and non-villous trophoblast, remaining trophoblast not used for villous formation, found in all other parts of the placenta: cell columns, cell islands, chorionic plate, membranes, septa, basal plate and spiral arteries [12].
The villous cytotrophoblast is the inner layer of the villous surface trophoblast whereas the villous syncytiotrophoblast is the superficial layer facing the intervillous space. Formation of NO by placental NOS, released in the intervillous space, may prevent platelet adhesion and aggregation and importantly contribute to dilatation of villous smooth muscle [12,13]. The trophoblast is considered to be important in implantation, placental development and adhesion of the placenta to the uterine wall, which are mediated by invasion of extravillous trophoblast into the endometrium [12,13]. The villous trophoblast is considered to be largely responsible for the regulation of blood perfusion in the fetus.
NOS has been identified in human placenta at term as well as primordial placenta [14,15]. The NOS activity in these tissues was found to be highly Ca-dependent suggesting constitutive NOS. The activity was largely found in the microsomal (particulate) fraction. Our recent studies in rat and rabbit reproductive tract tissue have shown that NOS activity was predominantly found in the cytosolic fraction [17,18]. Ramsay et. al presented data on NOS activity in the villous trophoblast in the three trimesters of pregnancy and found the highest activity in the first trimester [19]. It has been suggested that production of NO by placenta would be an important factor acting in a paracrine manner to contribute to uterine quiescence [19,20]. From our recent studies showing important differences between NOS activity in human and rat or rabbit myometrium, it was concluded that placental and fetal NOS could be of considerable importance in maintaining quiescence of the myometrium in human pregnancy [21].
In order to gain further insight into the functional role of NOS in trophoblast, we have in the present study obtained data on the NOS activity in cytosolic and particulate fractions prepared from villous and non-villous trophoblast. For both comparison with trophoblast and elucidation of any paracrine effect, NOS activity was also measured in term placenta and pregnant myometrium.
Methods
The study was approved by the local Ethics committee (University Hospital, Lund, Sweden) and informed constant was obtained from all patients.
Samples from primordial human placentae were obtained from psychosocial termination of pregnancy between 9 – 12 weeks gestation (n = 17). Pregnancies were dated by measuring crown rump length using transvaginal ultrasound approximately one week prior to the termination. Samples were obtained by suction and curettage. Term placenta and myometria were obtained from deliveries by elective Caesarean section (n = 15).
After washing the first trimester samples 3 times in ice-cold saline solution, villous tissue was separated by careful scraping and both villous and non-villous trophoblast tissue were collected. A sample approximately 2–3 g of tissue was excised from the term placenta near the insertion of umbilical cord. Myometrial tissue (approximately 3 cm × 0.5 cm) was obtained from the upper margin of the transverse section in the lower uterine segment. All tissues were rinsed with ice-cold saline and frozen by immersion in liquid nitrogen and stored at -80°C until used (within 3 weeks) for the experiment.
In addition, five samples were collected from each tissue for histological tissue control. These samples were fixed in formalin, paraffin embedded and stained with haematoxylin-eosin.
Preparation of subcellular fractions
The frozen tissues were thawed and then homogenised in approximately 10 vol. buffer containing 320 mM sucrose, 10 mM HEPES buffer (pH 7.5), 0.1 mM EGTA, 1 mM DTT, 10 μg/ml trypsin inhibitor, 10 μg/ml leupeptin and 2 μg/ml aprotinin with a Polytron homogenizer for four periods of 15 s (per gram tissue) with intermittent cooling pauses of 20 s. The homogenate was centrifuged at 1000 g for 10 minutes and the pellet was discarded. The supernatant was filtered through one layer of gauze and centrifuged 40.000 g for 45 minutes and the pellet and supernatant were saved as cytosolic and particulate fractions, respectively. In some experiments the above pellet was suspended in 1 M KCl and centrifuged to remove any bound cytosolic NOS. There was no significant difference in the specific NOS activity between the unwashed pellet and pellets washed with 1 M KCl.
Measurement of NOS activity
NOS activity was determined by measuring the formation of 14C-citrulline from 14C-arginine essentially according to the procedure described by Salter et al.18 Incubation mixture contained 20 μL of cytosolic or particulate fractions (40 – 60 μg protein) added to 100 μL of phosphate buffer containing 50 mM K-phosphate (pH 7.5), 1.2 mM MgCl2, 0.24 mM CaCl2, 50 mM valine, 0.1 mM β-nicotinamide adenine dinucleotide phosphate (NADPH), 1 mM citrulline, 2-μM flavin adenine dinucleotide (FAD), 2 μM flavin mononucleotide (FMN), 10 μM tetrahydro-L-biopterin (BH4), 50 IU/mL calmodulin, 20 μM arginine and 0.6 – 0.8 μM [-14C] arginine (sp. Act 0.32 Ci/mM). Samples were incubated for 20 minutes at 28°C. Preliminary experiments showed that the reaction was linear for at least 30 minutes. Incubation was terminated by removal of substrate and dilution by addition of 1.5 mL of 1:1 (v/v) pre-washed resin. AG50W-X8, in ice-cold water, and measured for quantification of [-14C] citrulline by liquid scintillation spectrometry.
Protein concentrations in subcellular fractions were determined by method of Peterson using bovine serum albumin as standard [23].
Presentation of data
Specific NOS activity (in pmol/mg protein/min) is reported as the difference between the activity (total) and that in the presence of the inhibitor 1 mM L-NAME and 1 mM EGTA (non-specific). Calcium independent activity was the activity measured in the incubation mixture lacking CaCl2 and containing 1 mM EGTA.
Student's t-test was used for statistical analysis of the observed differences.
Western blots
Aliquots of 50 μl of supernatant or particulate fraction were diluted 1:2 in electrophoresis sample buffer (62.5 nM Tris-HCL, pH 6.8, 25% glycerol, 2% SDS, 0.01% bromophenol blue and 5% β-mercaptoethanol) to yield a protein amount of 10 μl/cell and 30 μl/cell for endothelial NOS (eNOS) and neuronal NOS (nNOS) analysis, respectively. The samples were reduced by boiling for 5 min. Human placenta and rat cerebral cortex were used as a positive control for eNOS and nNOS, respectively. Sodium dodecyl sulphate-polyacryl-amide gel electrophoresis (SDS-PAGE) was carried out on a 7% polyacrylamide gel at 200 V for 30 min.
After electrophoretic transfer to a PVDF membrane at 10–15 V for 30 min, blocking with dry milk diluted in TBS-Tween was carried out over night at 4°C. The blots were then incubated with primary polyclonal antibodies diluted in TBS-Tween dry milk (eNOS 1:2000 and nNOS 1:1000) for 1 h at room temperature. The blots were rinsed in TBS-Tween for 1 × 15 min followed by 3 × 5 min and incubated with secondary anti-rabbit IgG-HRP diluted in TBS-Tween (1:10 000) for 1 h at room temperature. The blots were rinsed as above and then subjected to enhanced chemiluminescence (ECL) using the Western blot detection system. Autoradiographic film was applied to the blot until satisfactory exposure was obtained.
Results
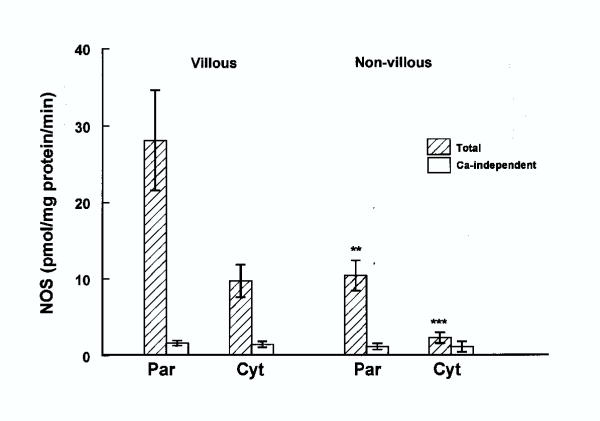
Figure 1 shows NOS activity in both particulate and cytosolic fractions prepared from villous and non-villous trophoblast, both total and Ca-independent activities are shown. The activity of NOS in particulate fractions from both preparations was considerably higher than the cytosolic fractions.
Figure 1.
NOS activity in particulate (par) and cytosolic (cyt) fractions isolated from early placenta (first trimester) villous (n = 17) and non-villous (n = 17) trophoblasts. Significance of differences between villous and extravillous are shown by ** (p < 0.01) and *** (p < 0.005).
In villous trophoblast the total activity in the particulate fraction was 3-fold higher than the cytosolic fraction. The Ca-independent activities in both fractions were similar and very low, compared with the total.
In non-villous trophoblast NOS activity was also higher in particulate than in the cytosolic fraction, but Ca-independent activity in the two fractions was nearly the same.
The particulate NOS in the villous was 2–3-fold higher than that in the non-villous trophoblast and the difference was statistically significant (P < 0.01), but Ca-independent NOS in particulate fraction from villous and non-villous trophoblast was similar. In villous trophoblast, the total cytosolic NOS was nearly 3-fold higher than that in the non-villous trophoblast and the difference between the two was highly significant (P < 0.005), but the Ca-independent NOS was similar.
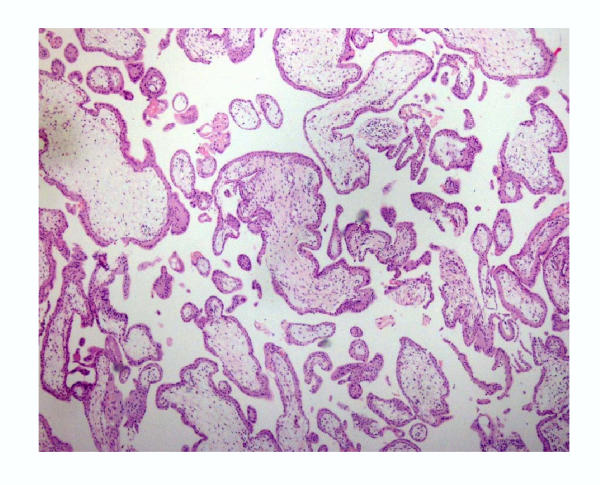
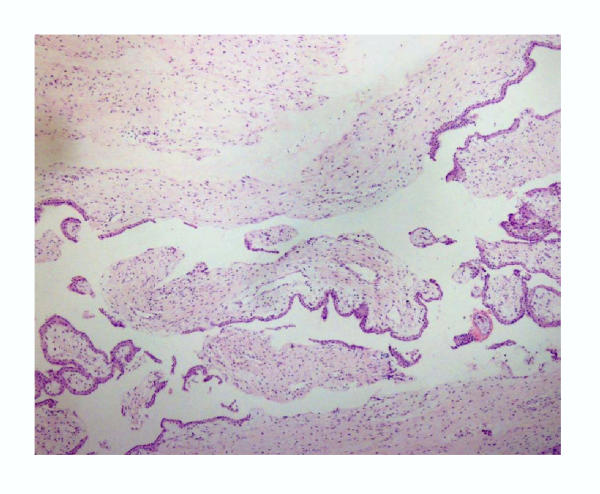
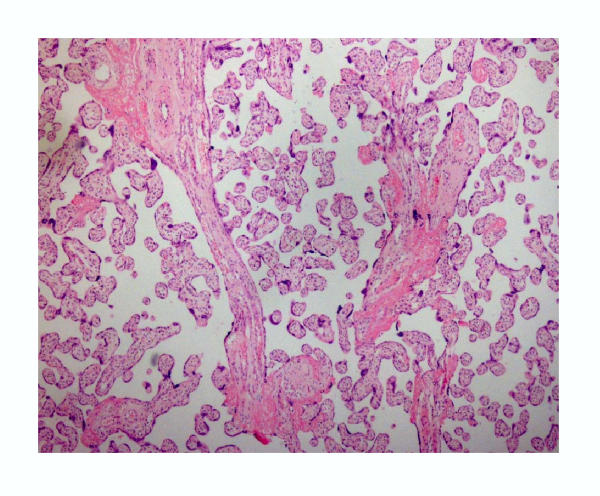
Histological examination of villous samples showed that they consisted predominantly of villi covered by villous trophoblasts (Fig. 2). Samples from non-villous trophoblast showed a predominance of connective tissue (stroma), limited amount of non-villous trophoblast and few villi (Fig. 3).
Figure 2.
General view of the villous sample from first trimester showing a predominance of villous-trophoblast. (Hematoxylin-eosin, 60×)
Figure 3.
General view of the non-villous sample from first trimester showing a predominance of stromal connective tissue and limited amounts of non-villous trophoblast. (Hematoxylin-eosin, 60×)
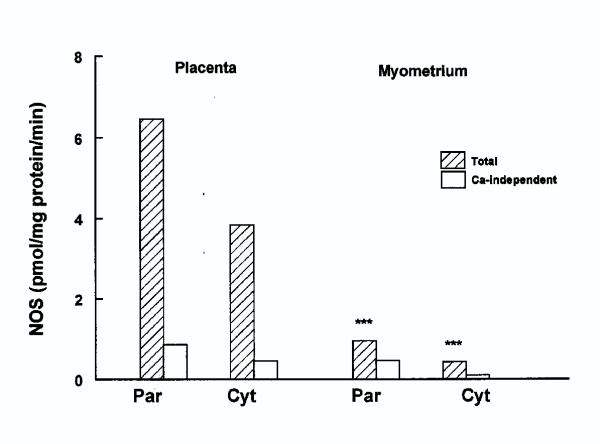
Figure 4 shows both total and Ca-independent NOS activities in the placenta and myometrium obtained from the same patients. In these tissues the NOS activity was markedly low compared to the villous and extravillous trophoblast, by about 3–5-fold.
Figure 4.
NOS activity in particulate (par) and cytosolic (cyt) fractions isolated from placenta (n = 15) and pregnant myometrium (n = 14). Significance of difference, between placenta and myometrium is shown by *** (p < 0.005).
Although, NOS in the placenta was predominantly particulate bound, the cytosolic fraction contained considerable amount of NOS, being nearly 60% of that in the particulate fraction. The Ca-independent activity was very low being less than 15% of the total. The NOS activity in placenta was considerably lower than the villous trophoblast, being more than 4-fold and 2-fold lower in particulate and cytosolic fraction, respectively. Although placental particulate NOS was lower and the cytosolic NOS higher than the corresponding fractions in non-villous trophoblast, the difference was not statistically significant.
In the myometrium the concentration of NOS was very low in both the particulate and cytosolic fractions being 6 – 9-fold lower than the corresponding fractions from placenta. A considerable portion of the total activity in the particulate fraction, which was the predominant form was Ca-independent, as it was more than 50% of the total activity. There was no correlation between myometrial and placental activities, in the individual patients (data not shown).
Histological examination of term placenta samples showed an even mixture of villi covered by villous trophoblast and connective tissue (stroma) from villi and septae (Fig. 5).
Figure 5.
Microscopic appearance of the term placenta sample showing a mixture of villous-trophoblast (villi) and stromal connective tissue from villi and septae. (Hematoxylin-eosin, 60×)
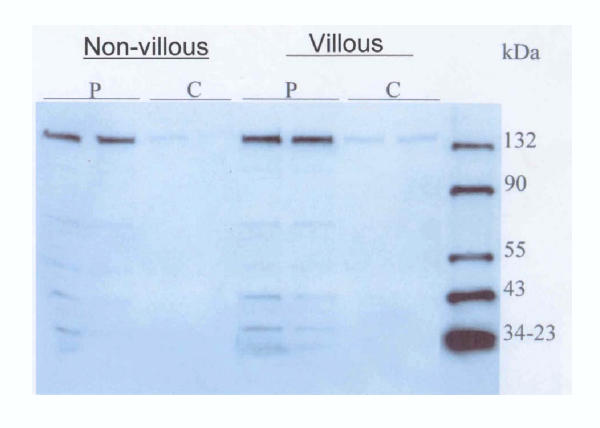
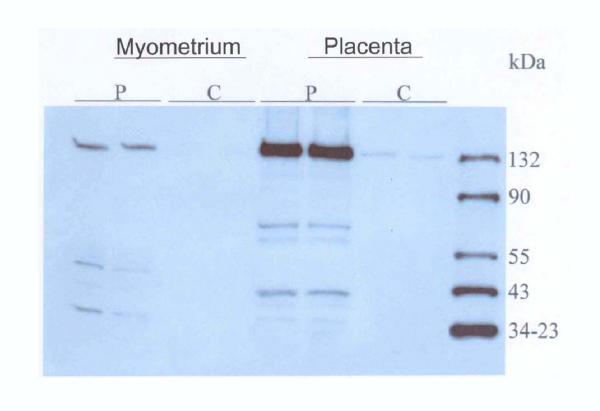
Data on Western blot analysis performed on particulate and cytosolic fractions of non-villous and villous trophoblast is shown in Figure 6. The eNOS antibody reacted with a 133 kDa protein corresponding to the size of eNOS. In both villous and non-villous trophoblast dense bands corresponding to eNOS can be seen. In addition a very faint band could also been seen in the cytosolic fraction from the villous trophoblast. The fractions from human myometrium showed a specific band of 133 kDa, corresponding to eNOS, which was detected in particulate but not cytosolic fractions (Fig. 7). In the fractions from placenta a band corresponding to eNOS was clearly seen in the particulate fraction and again a faint band in the cytosolic fraction.
Figure 6.
Western blots of particulate (P) and cytosolic (C) fractions isolated from non-villous and villous trophoblasts (first trimester) using an antibody directed against eNOS. The putative bands are seen at 135 kDa corresponding to eNOS protein.
Figure 7.
Western blots of particulate (P) and cytosolic (C) fractions isolated from myometrium and term placenta using an antibody directed against eNOS. The bands are seen at 135 kDa corresponding to eNOS protein.
The nNOS antibody showed a clear and intense reactivity with a 161 kDa protein from rat cortex (positive control), which corresponds to the expected size of nNOS. However, with this antibody we were unable to see any reaction in cytosolic or the particulate fraction from any of the tissues.
Discussion
In the present study we have measured NOS activities in human trophoblast from early placenta (first trimester), term placental and myometrium. A recent immunohistochemical study on trophoblastic NOS has demonstrated the presence of NOS in microvilli as well as in extravillous trophoblastic cells at the implantation site [13]. It was, therefore, of interest to quantify NOS activities in villi and non-villous cells of the trophoblast both in early placenta (first trimester) and term placenta and further investigate whether there were any differences in their subcellular distribution and Ca-dependence.
In agreement with the above immunohistochemical study [13], we have found considerable NOS activity in particularly the villous trophoblast. Ramsay et al. have recently presented data on villous trophoblast in each of the three trimesters during pregnancy, but only cytosolic (supernatant) activity was analysed in their samples [19]. The mean values for NOS activity in trophoblast from the first trimester, in their data, are generally comparable with the present data on cytosolic fractions. We have, however, found that NOS is much more concentrated in the particulate fraction than the cytosolic fraction, but the former was not analysed in the study by Ramsay et al [19]. In view of the fact that in the present study, an expression of nNOS was not seen in Western blots, it is likely that the activity measured in cytosolic fraction is by and large a result of contamination of this fraction with particles originally associated with the particulate fraction. This contamination most probably occurred during the isolation of the fractions. This is supported by the present data showing cross-reactivity in the cytosolic fraction particularly from placenta and villi with a protein corresponding to eNOS, which is predominantly associated with the particulate fraction. A contamination of the cytosolic fraction with fragments from the particulate fraction as suggested by the present findings would apparently vary depending on the method of preparation, which should be importantly considered when comparing the subcellular distribution of NOS reported in various studies, in addition to consideration of differences in organs and species (24).
Intimate relationship exists between maternal blood and trophoblastic cells in the implantation site. It is conceivable that relaxation of the vascular wall of muscles at the implantation site by NO may be produced by extravillous trophoblastic cells. This would be consistent with the present finding of a considerable NOS activity in non-villous trophoblast. However, significantly higher activity was found in the villous trohpoblast suggesting a relation between number of trophoblastic cells and NOS activity.
Due to the highly proliferative and invasive nature of trophoblastic cells in early pregnancy as they penetrate the endometrium, one might expect an inducible Ca-independent NO (Type II), particularly in the extravillous trophoblast. In the present data we found that in contrast to the villous, the non-villous trophoblast had a considerable Ca-independent NOS as it was approximately half of the total activity in the cytosol. Corresponding figure for the villous trophoblast was only 15%. Ramsay et al. have also reported that only a very small portion of total NOS in the cytosol of villous trophoblast was Ca-independent [19].
The NOS activity in the placenta at term was considerably lower than in samples from early placenta (first trimester), which is in agreement with the recent immunohistochemical study [13]. The histological examination of the samples showed less villous trophoblast when compared with samples from early placenta (first trimester). Our data on Ca-dependent and Ca-independent activities in placenta are in agreement with the recently published data [14,15]. A higher activity in trophoblast in early pregnancy than in term placenta might be expected due to a heavy demand placed on the utero-placental circulation which is achieved by decreased resistance to blood flow. It is, however, possible that NOS activity in trophoblastic cells surrounding the spiral arteries as well as in the spiral arteries themselves at term may be of greater significance than the activity in the rest of the placenta [25,26].
The present data showing very low NOS activity in the human pregnant myometrium would suggest that it is unlikely that a local production of NO in the myometrium plays an important role in the maintenance of uterine quiescence. On the contrary, it is probable that trophoblastic NOS activity, which in relatively high both in villous and non-villous cells may have a paracrine effect during early pregnancy on the myometrium and thereby contribute to maintenance of uterine quiescence [27]. From the data of our recent study it was suggested that increasing levels of estrogen and progesterone could contribute to a downregulation of NOS activity in myometrium during pregnancy (24). This suggestion is consistent with present data showing higher NOS activity in early placenta (first trimester) than in term placenta. Similar paracrine effect of placental NOS activity, which was 2–4-fold higher than the myometrium, could be envisioned during late pregnancy [26]. It should be emphasized, however, that the villous and non-villous trophoblast represents a major cellular component related to both the maternal (intervillous space) and fetal (villi) circulation suggesting also a major role in the haemodynamic control of these circulations.
Studies in animals not only provide a strong support for a paracrine role of placental NO but also led to the suggestion that fetal-placental unit increases the availability of NO generated by NO donors [27-29]. In a recent study it was suggested that NO synthesised in fetal membranes may act either directly to inhibit myometrial contractility or indirectly through an interaction with cyclooxygenase [31]. This would also apply to NO produced by trophoblasts and placenta, which appear to be the major sources of NO production during pregnancy.
Data from the current study demonstrate considerable amounts of NOS activity in both trophoblastic villi and extravillous cells and suggest a physiologic role of trophoblastic NO in early stages of pregnancy. Appreciable concentration of NOS was found in term placenta and very low in the myometrium but there was no correlation between the myometrial and placental activities. Further work is needed to establish the role of NO produced at specific sites in the uteroplacental circulation and paracrine effect of trophoblastic NOS in maintenance of uterine quiescence during pregnancy.
Conclusion
In view of the marginal activity of NOS in the pregnant myometrium, NO produced by the trophoblast and placenta could play a significant role in maintaining uterine quiescence by paracrine effect.
Contributor Information
J Al-Hijji, Email: alhiji@hotmail.com.
Ellika Andolf, Email: Ellika.Andolf@kvk.ds.sll.se.
Ricardo Laurini, Email: Ricardo.Laurini@skane.se.
Satish Batra, Email: satish.batra@gyn.lu.se.
References
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Ignarro LJ. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-F. [DOI] [PubMed] [Google Scholar]
- Sessa VC. The nitric oxide synthase family of protein. J Vasc Res. 1994;31:131–143. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- Gude NM, King RG, Brennecke SP. Role of endothelium-derived nitric oxide in maintenance of low fetal vascular resistance in placenta. Lancet. 1990;336:1589–90. doi: 10.1016/0140-6736(90)93374-X. [DOI] [PubMed] [Google Scholar]
- Gude NM, Xie CY, King RG, et al. Effects of eicosanoid and endothelial cell derived relaxing factor inhibition of fetal vascular tone and responsiveness in the human prefused placenta. Trophoblast Res. 1993;7:133–145. [Google Scholar]
- Wieczorek KM, Brewer AS, Myatt L. Shear stress may stimulate release and action of nitric oxide in the human fetal-placental vasculature. Am J Obstet Gynecol. 1995;173:708–713. doi: 10.1016/0002-9378(95)90327-5. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Joffe GM, Kruszyna H, et al. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993;7:566–571. [PubMed] [Google Scholar]
- Yang D, Lang U, Greenberg SG, et al. Elevation of nitrate levels in pregnant ewes and their fetuses. Am J Obstet Gynecol. 1996;174:573–577. doi: 10.1016/s0002-9378(96)70430-1. [DOI] [PubMed] [Google Scholar]
- Yllampalli C, Dong Y-L. Estradiol-17β in hibits oxide synthase (NOS)-II and stimulates NOS-III gene expression in the rat uterus. Biol Reprod. 2000;63:34–41. doi: 10.1095/biolreprod63.1.34. [DOI] [PubMed] [Google Scholar]
- Natuzzi ES, PC Ursell, Harrison M, Buscher C, Riemer RK. Nitric oxide synthase activity in the pregnant uterus decreases at parturition. Biochem Biophys Res Comm. 1993;194:1–8. doi: 10.1006/bbrc.1993.1776. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;41:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Benirschke KK, Kaufmann P. Pathology of the human Placenta. New York: Springer-Verlag. 1995. pp. 182–189.
- Buttery LDK, McCarthy A, Springall DR, et al. Endothelial nitric oxide synthase in the human placenta: Regional distribution and proposed regulatory role at the fetomaternal interface. Placenta. 1994;15:257–265. doi: 10.1016/0143-4004(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Kohnen G, et al. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod. 1997;12:2546–2552. doi: 10.1093/humrep/12.11.2546. [DOI] [PubMed] [Google Scholar]
- Sahin-Tóth M, Kukor Z, Tóth M. Tetrahydrobiopterin preferentially stimulates activity and promotes subunit aggregation of membrane-bound calcium-dependent nitric oxide synthase in human placenta. Mol Hum Reprod. 1997;3:293–298. doi: 10.1093/molehr/3.4.293. [DOI] [PubMed] [Google Scholar]
- Otsubo Y, Hori H, Nishino T, et al. Comparisons of nitric oxide synthases in normal human placenta from 37 to 41 weeks gestation: qualitative and quantitaative analyses. J Matern Fetal Invest. 1998;8:139–144. [PubMed] [Google Scholar]
- Al-Hijji J, Larsson B, Batra S. Nitric oxide synthase in the rabbit uterus and vagina: Hormonal regulation and functional significance. Biol Reprod. 2000;62:1387–1392. doi: 10.1095/biolreprod62.5.1387. [DOI] [PubMed] [Google Scholar]
- Al-Hijji J, Larsson I, Batra S. Effect of ovarian steroids on nitric oxide synthase in the rat uterus, cervix and vagina. Life Sci. [DOI] [PubMed]
- Ramsay B, Sooranna SR, Johnson MR. Nitric oxide synthase activities in human myometrium and villous trophoblast throughout pregnancy. Obstet Gynecol. 1996;87:249–253. doi: 10.1016/0029-7844(95)00391-6. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Florio P, Nappi C, et al. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17:156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- Batra S, Iosif C, Al-Hijji J, Larsson I. Important differences in nitric oxide synthase activity and predominant isoform in reproductive tissues from human and rat. RB & E. 2002. [DOI] [PMC free article] [PubMed]
- Salter M, Knowles RG, Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca 2+-dependent and Ca 2+-independent nitric oxide synthases. FEBS Lett. 1991;291:145–149. doi: 10.1016/0014-5793(91)81123-P. [DOI] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al., which is more generally acceptable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Batra S, Iosif C, Larsson I. Important differences in nitric oxide synthase activity a predominant isoform in reproductive tissues from human rat. Reprod Biol Endocrinol. 2003;1:10. doi: 10.1186/1477-7827-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel I, Hochberg A, Shochina M. Endothelial nitric oxide synthase immunoreactivity in early gestation and in trophoblastic disease. J Clin Phathol. 1998;51:427–431. doi: 10.1136/jcp.51.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalisz K, Buhimschi I, Garfield GE. Role of nitric oxide in obstetrics. Neonatal Med. 1996;14:293–328. [Google Scholar]
- Petraglia F, Florio P, Nappi C, Genazzani AR. Peptide signaling in human placenta and membranes: Autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17:156–185. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- Purcell TL, Buhimschi IA, Given R, Chwalisz K, Garfield RE. Inducible nitric oxide synthase is present in the rat placenta at the fetal-maternal interface and decreases prior to labour. Mol Hum Reprod. 1997;3:485–491. doi: 10.1093/molehr/3.6.485. [DOI] [PubMed] [Google Scholar]
- Syal A, Okawa T, Vedernikow Y, Chwalisz K, Saade GR, Garfield RE. Effect of placental tissue on inhibition of uterine contraction by nitric oxide donors. Am J Obstet Gynecol. 1999;181:415–418. doi: 10.1016/s0002-9378(99)70571-5. [DOI] [PubMed] [Google Scholar]
- Buhimschi C, Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Contrasting effects of diethylenetriamine-nitric oxide, a spontaneously releasing nitric oxide donor, on pregnant rat uterine contractility in vitro versus in vivo. Am J Obstet Gynecol. 1997;177:690–701. doi: 10.1016/s0002-9378(97)70166-2. [DOI] [PubMed] [Google Scholar]
- Dennes WJB, Slater DM, Bennett PR. Nitric oxide synthase mRNA expression in human fetal membranes: a possible role in parturition. Biochem Biophys Res Commun. 1997;233:276–278. doi: 10.1006/bbrc.1997.6439. [DOI] [PubMed] [Google Scholar]