Abstract
Background
Malaria can be eradicated from islands. To assess the prospects for eradication of malaria from the island of Príncipe in the Gulf of Guinea, we fitted a mathematical model to age-prevalence curves and thus obtained estimates of the vectorial capacity and of the basic reproductive number (R0) for malaria.
Methods
A cross-sectional malariological survey was carried out, in mid-1999, in six communities, comprising circa 17% of the total 6,000 population of the island. All houses in these communities were registered and their mode of construction recorded. Thick and thin blood films were prepared from all consenting individuals. Each individual was asked whether they possessed a mosquito net, whether they had slept under a mosquito net the previous night, whether they were allergic to chloroquine, and whether they had visited the main island of São Tomé since the beginning of the year. Outpatient records from March 1999 until the end of December 2000 were also examined and the age and place of residence of diagnosed cases noted.
Results
203 (19.8%) of the 1,026 individuals examined were found to be infected with Plasmodium falciparum. By fitting the mathematical model of the Garki project to the age-prevalence curve we estimate that the basic reproductive number, R0, on the island is approximately 1.6. Over a period of one year, a total of 1,792 P. falciparum cases reported to an outpatient facility at the island's hospital. Overall, 54% of the people interviewed slept under mosquito nets and were at reduced risk of infection. Conversely, people living in houses with openings between the top of the wall and the roof had higher risk of infection.
Conclusion
This high incidence suggests that most of the malaria cases on the island attend the hospital and that treatment of these cases is an important factor reducing the effective rate of transmission. Providing that clinical cases are effectively treated, endemic malaria can probably be eliminated from the island mainly by reducing exposure to the vector with simple measures such as insecticide-treated nets and mosquito-proofing of dwellings. In contrast to traditional malaria eradication strategies, this would avoid the risk of malaria epidemics because the reduction in R0 should be sustainable.
Background
If it is possible to eliminate malaria from anywhere in Sub-Saharan Africa, it should surely be on the island of Príncipe, the smaller partner island in the archipelago of São Tomé and Príncipe. Isolated in the Gulf of Guinea, Príncipe has a human population of only about 6,000 inhabitants (5,972 being recorded in the census of 2001, National Census Office, São Tomé). Plasmodium falciparum malaria is the most important vector-borne disease on the archipelago, being responsible for two thirds of outpatient diagnoses. It is the leading cause of death, especially in under five year olds and is transmitted by a population of the FOREST cytoform [1] of the M rna form of Anopheles gambiae, which appears to be isolated from that on the main island of São Tomé, some 160 km distant [2].
Prevalence rates have always been lower than on neighbouring São Tomé [3] and in 1997 a P. falciparum prevalence of 29% was recorded, (Plasmodium vivax 7.1%; Plasmodium malariae 2.4%) [1], higher than the 12.9% recorded in 1958 but still considerably lower than in most rural areas of central Africa.
Although the island has a total area of only 113 km2 there are large variations between different communities in their access to health services, which are largely limited to the hospital in the island's only town, Santo António. Exposure to malaria even within settlements is likely to vary considerably, due to house location relative to mosquito breeding sites, variations in house construction or in the use of mosquito nets.
We now report an analysis of risk factors for malaria infection on the island and of a large cross-sectional survey of malaria prevalence carried out in 1999. To assess the prospects for eradication of malaria from the island, we fitted the mathematical model of Dietz et al [4] to age-prevalence curves and thus obtained estimates of the vectorial capacity and of the basic reproductive number (R0) for malaria. Based on the results, we discuss the likely effects of different malaria control strategies and propose an approach using simple methods of vector control and personal protection.
Methods
Príncipe, (1°35'N, 7°25'E) is isolated in the Gulf of Guinea, about 160 km north-east of the companion island of São Tomé. It is volcanic in origin, mountainous, very wet and largely still covered in primary or secondary rainforest. For most of their history, the black population on the islands were slaves and so there was little or no interchange between most of the islands inhabitants. Following the abolition of slavery, indentured labourers, mainly from Cabo Verde, came to the islands. Portuguese is the island's official language but the islanders have their own language, Moncon.
Fishing villages usually with 100–300 inhabitants are located wherever there is a suitable beach and a supply of freshwater, especially in the north of the island. The nearest road to these villages can be several kilometres distant.
The majority of people of Cabo Verdian descent live in the presently dilapidated former coffee/cocoa estates, roças, where they practice subsistence agriculture and gain some cash from a declining cocoa industry. Although roças can be reached by road transport there are few vehicles. Their movement is generally limited to the islands paved roads. This also makes roças several hours distant from the hospital. Otherwise people tend to live in relative isolation, alongside the paved road, or in the island's only town, Santo António. People who live in the town (of circa 3, 000 inhabitants) have a wider variety of occupations among them the selling of salted fish in São Tomé.
When they have amassed sufficient fish, the fish traders take these to São Tomé by one of two boats that usually make a weekly 36-hour return journey between the islands. In addition to the boats, there is a thrice weekly plane service capable of transporting 20 people at a time between the islands. Hospital emergencies can be transported to São Tomé by the Portuguese Air Force.
It was not until the early 1980's that a sustained eradication campaign, using an indoor residual spraying of DDT and weekly chloroquine chemoprophylaxis, was undertaken. The breakdown of the campaign resulted in a serious epidemic with many deaths (especially in São Tomé) [5]. More recently, a pilot project using permethrin-treated bednets has been undertaken. However, although some people had nets, they were no longer available for sale during the studies reported here.
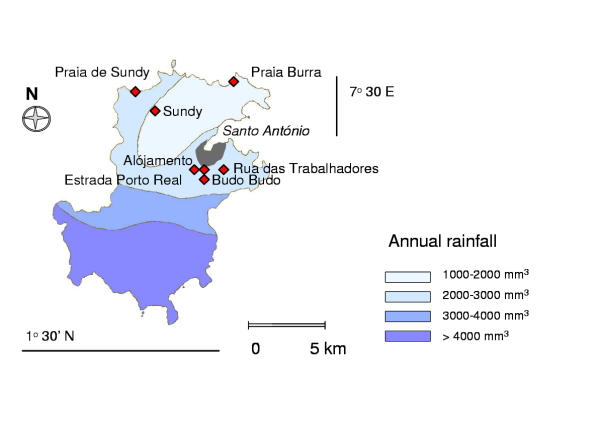
The present study was carried out in a subset of six communities, selected to form a transect across the northern part of the island (Figure 1). In mid-1999, all houses in these communities were registered and their mode of construction recorded (the material of the walls and roof, the number of stories of the building, and whether the building was raised on stilts). The presence of openings between the walls and the roof (eaves) was also registered, as were the number of windows, and the presence of intact gauze window screens. In addition, the availability of electricity and the fuel used for cooking were recorded as socio-economic indicators.
Figure 1.
Map of Príncipe showing collection sites
During May-August 1999 a malariological survey was conducted. Members of the six communities, comprising circa 30% of the total population of the island, were asked to sign a consent document, and thick and thin blood films were prepared from all consenting individuals. Each individual was asked whether they possessed a mosquito net, whether they had slept under a mosquito net the previous night, whether they were allergic to chloroquine, and whether they had visited the main island of São Tomé since the beginning of the year. A history of fever during the last 24 hours was recorded, and axillary temperatures were taken with an electronic thermometer. Febrile or pregnant individuals, and children (<15 years of age) who were found to be parasitaemic were treated with chloroquine (adult dose 600 mg base followed by 600 mg base at 24hr and 300 mg base at 48hr; paediatric dose 10 mg base/kg followed by 10 mg base/kg at 24hr and 5 mg base/kg at 48 hr) alone or, for those people who were allergic to chloroquine, in combination with an antihistamine. Treatment was followed by a physician and according to the Ministry of Health regulations.
Outpatient records from March 1999 until the end of December 2000 were examined and the age and place of residence of all diagnosed cases was noted. Area of residence was classified as being fishing village, roça, interior or town (Santo António). Self-treatment or treatment of malaria at the hospital was determined by questionnaire undertaken three months after the initial parasitological surveys.
Estimation of vectorial capacity and R0
The vectorial capacity [6] is the rate at which future inoculations arise from a currently infective host. It is a function is the product of the rate at which the host infects mosquitoes (mab1), the proportion of the vector population which has already survived through the sporogonic cycle (pT/(-ln p)), and the rate with which infective mosquitoes infect humans (ab2). i.e.
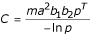 where m is the vector density, a the frequency with which vectors bite humans, b1- the susceptibility of the vector,b2 - the susceptibility of the humans,p - the daily survival of the vectors and T - the duration of the sporogonic cycle in days.
where m is the vector density, a the frequency with which vectors bite humans, b1- the susceptibility of the vector,b2 - the susceptibility of the humans,p - the daily survival of the vectors and T - the duration of the sporogonic cycle in days.
To estimate C, we used the mathematical model of the Garki project [4] which comprises a set of linked difference equations describing transitions among seven categories of host distinguished by their infection and immunological status. The model makes predictions of the age-specific prevalence of P. falciparum in humans as a function of C. For any given value of C, model predictions are made by simulating the transitions in an initially naive population until equilibrium is reached, and then simulating the experience of a birth cohort exposed to the equilibrium rate of inoculation.
To obtain a single estimate, 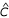 , for the vectorial capacity in Príncipe, we maximised the binomial likelihood for the observed age-prevalence curve by repeatedly evaluating the Garki model, using a golden section search routine[7] to locate
, for the vectorial capacity in Príncipe, we maximised the binomial likelihood for the observed age-prevalence curve by repeatedly evaluating the Garki model, using a golden section search routine[7] to locate 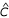 . For time-invariant C, eradication in the Garki model (i.e. R0 = 1) corresponds to C = 0.022 contacts per day. We have, therefore, estimated R0for Príncipe as the ratio
. For time-invariant C, eradication in the Garki model (i.e. R0 = 1) corresponds to C = 0.022 contacts per day. We have, therefore, estimated R0for Príncipe as the ratio 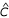 /0.022.
/0.022.
The above analysis does not allow for seasonal variation in C. Since the level of immunity is low it is reasonable to assume that the time series of parasite-positive clinical cases is roughly proportional to that of the force of infection in the population, and hence to use the time series of clinical cases to estimate the seasonality. We assumed the pattern of seasonal variation in inoculations h(t) to be proportional to the number of cases observed 15 days (N, the prepatent period) later, i.e.
I(t + N) = kh(t) (1)
where I(t) is the number of cases (incidence) during time interval t. We further assumed that this pattern recurs in an annual cycle and that the system is in equilibrium.
From the equations of the Garki model and equation 1:
![]()
Where y1(t) is the proportion of the human population infective to the vector (one of the model outputs) n is the duration of sporogony assumed in the Garki model, and g is a susceptibility parameter, to which, following Dietz et al [4], we assigned a value of 0.097.
We used (2) to predict the temporal pattern of C(t) from that of I(t-N-n). Starting from a guess C(0)() of the vector C, the Garki model is run until it reaches equilibrium, to make predictions y1(0)() and h(0)(). These predictions are used to generate a value k(0) for k from the means of the h(0)() and I() vectors, i.e.:
![]()
The elements of C(0)() are then updated using:
![]()
The Garki model is then run again using the updated vector C(1)() as input and the likelihood of the observed population survey data is then computed. The golden section search routine is then used to select the next k value. This iterative process is repeated until the maximum likelihood solution for k and hence for C(t) is found.
To obtain an estimate of R0, the vector C*(), at which the vectorial capacity is only just sufficient to sustain transmission, is estimated by running the model for different vectors C(0)().
As with any mathematical model, the results obtained in this way are subject to errors and approximations in the assumptions. Used in this way, the Garki model is expected to give reasonably good estimates of C(t), because it was originally developed by examining the fit of age-prevalence curves derived from slide data, to those predicted from field estimates of C(t). However the estimates of R0 are likely to be rather lower than the true values since the model ignores within-population heterogeneity in transmission [8].
Results
A total of 203 (19.8%) of the 1,026 individuals examined were found to be infected with P. falciparum in the cross-sectional survey. By microscopy, P. vivax was found on only two slides and P. malariae in a further two, corresponding to much lower prevalences of the latter two species than were found in 1997. Species identification in 1997 was carried out using polymerase chain reaction (PCR), which is more sensitive than microscopy. PCR analysis of a subset of the 1999 samples found 60/185 (32%) to be positive for P. falciparum, but the trend towards lower prevalence than in 1997 was confirmed for P. malariae (3.2%) and only 1/185 samples was positive for P. vivax even by PCR.
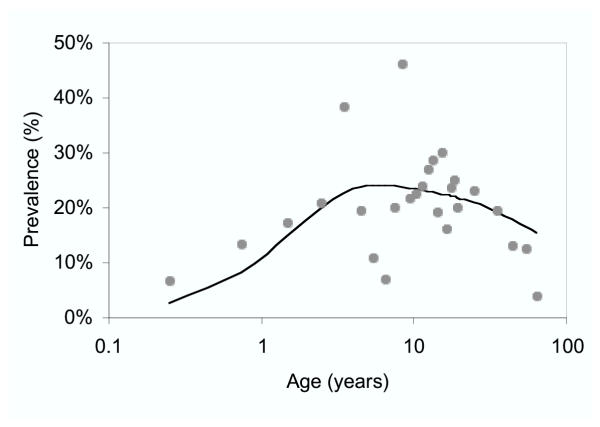
P. falciparum infections were fairly evenly distributed between age-groups. Maximum prevalence was observed in teenagers (Figure 2). There were substantial differences in the risk of infection between the different communities that were sampled (χ2= 29.04, d.f.=5, P<0.0001) (Figure 1, Table 1). Amongst those people who had a net, use was significantly higher among women than among men (χ2= 4.82, d.f.=1, P=0.028). Of 420 women who had a net, 337 (80.2%) used it. Of 316 men who had a net, only 231 (73.1%) made use of it. Overall 54% of the people interviewed slept under mosquito nets and were at reduced risk of infection (RR = 0.74, 95% CI; 0.58-0.94, P=0.016). Conversely, people living in houses with openings between the top of the wall and the roof had higher risk of infection (RR = 1.57, 95%CI; 1.22-2.02, P<0.001). Having openings in the eaves was significantly associated with having a wooden wall construction (χ2= 63.38, d.f.=1, P<0.0001). Houses with wooden walls were 2.3 times more likely to have openings between the roof and the wall. Effects of the other housing construction and socio-economic variables included in the questionnaire were not statistically significant. 198/1,002 (20%) of the people reported recent travel to the main island of São Tomé, but this too was unrelated to infection with P. falciparum. Nevertheless, 26 (12.6%) of the 206 people interviewed who had been to São Tomé since the start of the year had taken anti-malarials in the week prior to the surveys, whereas only 67 (7.9%) of the 846 who had not travelled had done so (χ2 = 4.54, d.f. = 1, P = 0.033).
Figure 2.
Prevalence of microscopy positive P. falciparum by age. Dots: observed prevalence, line: fitted line (using the Garki model, adjusted for seasonality)
Table 1.
Risk factors for malaria infection in Príncipe
| Risk factor | Level | Number infected/total slides (%) | Relative Risk (95% CI) | P-value$ |
| Community | Rua dos Trabalhadores | 94/349 (26.9) | <0.0001 | |
| Budu Budu | 17/167 (10.2) | |||
| Alójamento | 26/145 (17.9) | |||
| Estrada Porto Real | 8/31 (25.8) | |||
| Sundy | 55/313 (17.6) | |||
| Praia de Sundy | 8/18 (44.4) | |||
| Mosquito net use | Users | 97/556 (17.4) | 0.74 (0.58–0.94) | 0.016 |
| Non-users | 111/468 (23.7) | |||
| Openings between roof and wall | Openings present | 79/284 (27.8) | 1.57 (1.22–2.02) | 0.0008 |
| Openings absent | 109/614 (17.8) | |||
| Wire gauze covering windows | Intact | 4/36 (11.1) | 0.52 (0.21–1.33) | 0.2 |
| Absent or damaged | 186/875 (21.3) |
$Pearson χ2 tests
Migration rates also differed between zones, being highest in the Rua das Trabalhadores and lowest in the roça, Sundy. 234/694 (34%) of those people who were asked, indicated that they were allergic to chloroquine, with no difference in this proportion between men and women.
Mean vectorial capacity, estimated by fitting the Dietz et al [4] model to the age-prevalence curve but not allowing for temporal variation in transmission, was estimated to be C = 0.034 (95% Confidence Limits (CL): 0.033, 0.036).
During the whole period for which we analysed records of fever cases reporting to the hospital (from March 1999 until the end of December 2000), a total of 5,834 cases were recorded. 3,035 of these patients had positive blood films for P. falciparum, 82 for P. malariae and only five for P. vivax. 1,792 of these malaria cases reported during the 12 months from March 1999 onwards. Ninety-six residential areas were noted by hospital staff. These were classified into four categories: 'town', 'interior', 'roça' or 'beach' and the residential zone of 2,606 people with slides positive for P. falciparum and 77 with P. malariae determined (Table 2). People from roças or beach were more likely to have a grade 4 infection compared to those who had easier access to the hospital (comparison between Grade 4 and all other positive slides χ2 = 51.42, d.f. = 3, P < = 0.000). People from the town were also more likely to have a negative blood slide compared to those from other areas (χ2 = 49.51, d.f. = 3, P < = 0.000).
Table 2.
Number of cases and grade of malaria infection diagnosed at the hospital, Santo Antδnio, Príncipe, according to residence of the patient. (Grade 1 = 1–100 Grade 2 101–200; Grade 3 201–400; Grade 4 >400 parasites per 100 fields)
| P. falciparum Grade | Proportion by grade | P. malariae | ||||||||
| Locality | Neg | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Beach | 67 | 62 | 39 | 18 | 17 | 0.46 | 0.29 | 0.13 | 0.13 | 7 |
| Interior | 482 | 350 | 169 | 56 | 33 | 0.58 | 0.28 | 0.09 | 0.05 | 22 |
| Roça | 395 | 302 | 120 | 53 | 65 | 0.56 | 0.22 | 0.10 | 0.12 | 14 |
| Town | 1403 | 864 | 305 | 101 | 52 | 0.65 | 0.23 | 0.08 | 0.04 | 34 |
| Unknown | 334 | 243 | 108 | 46 | 32 | 0.57 | 0.25 | 0.11 | 0.07 | 21 |
There was no strong seasonal pattern in the outpatient attendances (Figure 3), and the estimate of vectorial capacity that allowed for seasonality was very similar to that which made no such allowance (C = 0.035; 95% CL: 0.032, 0.039). This gave an estimate of R0 of approximately 1.6, corresponding to an average annual inoculation rate of 1.36 infectious bites per person per annum. The ratio of the incidence of P. falciparum recorded at the hospital outpatient clinic to the estimated per capita force of infection k, was estimated to be approximately 13,300. Since the population of the island is only about 6,000, this implies that about 2.1 outpatient visits with P. falciparum occur for every new infection that contributes to the pool of asymptomatic carriers in the community.
Figure 3.
Seasonal pattern of fever incidence at the hospital, Santo Antδnio, Príncipe.
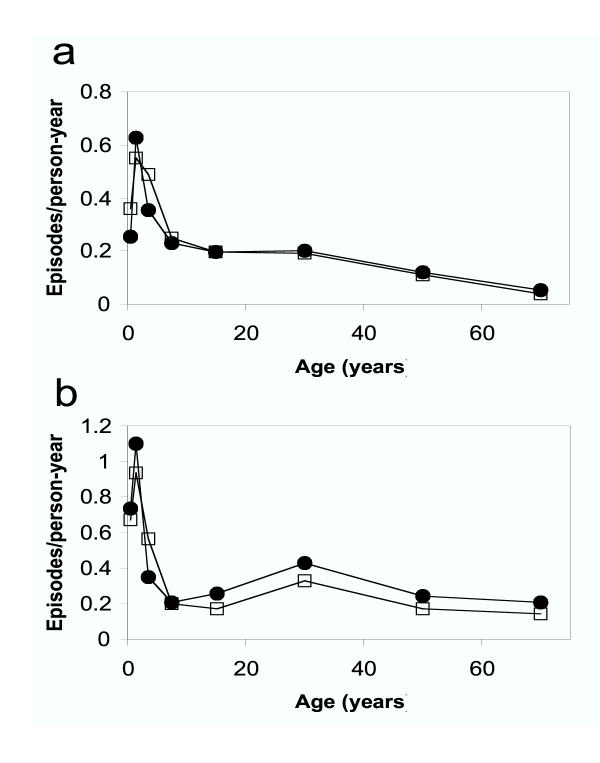
Attendance at the outpatient facility was highest on Mondays (31% of attendances compared with 20% expected) and showed a strong age trend (Figure 4) with the highest incidence in the second year of life. Young adult women had higher incidence than teenagers or adult men. This very likely represents the fact that mothers were often tested when they accompanied febrile children to the hospital.
Figure 4.
Age-sex pattern of incidence of fever with P. falciparum parasitaemia at the hospital, Santo Antδnio, Príncipe.
Discussion
In principle, malaria control and eradication are less difficult in island situations than they are in continental ones. Vectors may be absent, as is the case in the south and central Pacific, where Anophelines as a whole are completely absent [9] or their diversity is limited. On Príncipe, the presence of only a single vector which has an intrinsically low vectorial capacity due to its propensity to feed on domestic animals [10,11] does, to some extent, simplify vector control. However, the main factor that facilitates malaria control on islands is that there is a greater chance of limiting imported cases. Several important tropical islands, such as Réunion, St Helena, and the Seychelles have been kept free of malaria by keeping the parasite out, although potential vectors remain present [12].
Elimination of malaria from São Tomé and Príncipe was almost achieved during the early 1980s using indoor residual DDT spraying (IRS) and weekly prophylaxis with chloroquine [5]. This also illustrates the dangers of attempting malaria elimination when reduction of the basic reproductive number (R0) below 1 cannot be sustained. Disruption of the spraying program in 1982, together with introduction of chloroquine-resistant strains led to a severe epidemic [5]. Once IRS or mass drug treatment cease, the vectorial capacity returns quickly to its pre-control values, and the situation, of an uninfected human population facing a high vectorial capacity, is ripe for massive epidemics.
Our results suggest that it would be relatively easy in Príncipe to reduce R0 for P. falciparum below 1 in a sustainable way. This could be achieved without running the risk of epidemics, by intensifying the use of simple measures such as insecticide-treated nets or preventing mosquitoes entering through the eaves of houses. The promotion of permanently impregnated nets [13] would be especially helpful, since the existing nets are frequently washed and uptake of insecticide re-treatment is likely to be low even if it were available on demand. Novel interventions offering personal protection, such as insecticide-impregnated netting ceilings [14] may also be valuable. These measures all have substantial advantages over technologies such as vaccines and transgenic mosquitoes, namely they are available now and are known to work.
Measures offering personal protection against mosquitoes also have major advantages over the traditional eradication strategies of larviciding, IRS or mass treatment. Firstly, it should be possible to sustain them in the long term without major external inputs, since even if malaria transmission were reduced to very low levels or eradication achieved, nuisance biting by other mosquitoes would still provide an incentive for their use. Secondly, cessation of malaria control with these methods leads only to a very slow return of the vectorial capacity to pre-intervention levels, since the netting and gauze remain in place until the usual process of wear and tear take hold.
In the absence of mass treatment, personal protection measures take some time to reach their maximum effect on transmission, because equilibrium is approached only by a slow process that depends on the rate at which natural immunity clears pre-existing infections. In 1997, 67% of the population of Príncipe were using mosquito nets [1], many of them newly impregnated with insecticide. Two years later prevalence of all malaria species had declined, with P. malariae and P. vivax so infrequent that one doubts whether stable transmission of these species continues on the island. It may well be that these declines, which occurred in spite of an apparent decrease in mosquito net use to only 54% during the same period, represented the long-term effect of the nets introduced shortly before the 1997 study. Indeed, the excess incidence of clinical cases in children suggests that the older people have considerable clinical immunity, which could well reflect exposure to higher levels of infection in the past.
Given the low (and possibly declining) levels of infection in the community, the incidence of clinical malaria reported at the hospital is astonishingly high. Our estimate that, for every new infection contributing to the reservoir in the community, the hospital diagnoses 2.1 clinical cases, implies that this treatment plays a major role in controlling transmission on the island. Without this treatment, these cases would add to the estimates of R0 and of the vectorial capacity. It must be concluded that the hospital plays a crucial role in controlling malaria transmission as well as in morbidity control. This is despite the fact that many people are reluctant to take chloroquine because of allergies.
Chloroquine resistance has been reported from São Tomé [15-17]. Inadequate treatment of drug resistant parasites with chloroquine can enhance gametocyte production and hence transmission [18,19]. The spread of drug resistant P. falciparum would cancel out the effect of the case detection system on transmission, effectively leading to a substantial increase in vectorial capacity and hence, potentially, a major epidemic. For these reasons it is essential that the hospital should have adequate supplies of second and third-line drugs or that alternative first line treatment is considered. Moreover, if transmission is further reduced by protecting the population from mosquitoes, the role of the hospital will remain important in controlling R0, even if the number of cases also falls, and hence it is essential that the current passive case detection system is maintained or even improved. Possible improvements would be to introduce a daily service and to prioritize pregnant women and children for slide taking and reading. The introduction of sulfadoxine/pyrimethamine, as an alternative to chloroquine for treatment should be properly evaluated since it may enhance transmission, especially in a area of high asymptomatic carriers. Such drugs may jeopardize future control programs for the island.
At present, imported cases do not seem to be an important source of infection and therefore most transmission must be autochthonous. However, the prevalence of malaria is higher on São Tomé than it is in Príncipe [1], therefore São Tomé is likely to act as a net source of cases. If transmission in Príncipe is controlled, imported cases are likely to become a greater proportion of the total. Recent travel may then become a relevant factor in a patient's history. São Tomé is likely to be the source of drug-resistant parasites that very likely represent the main malariological threat to Príncipe, and so it will be increasingly crucial that the imported, potentially chloroquine resistant, cases should be effectively identified and treated. The sale of cheap repellents [20-22] would allow travellers to achieve some degree of protection whilst in São Tomé and so also reduce imported cases. A systematic survey at port of entry of potentially infected travellers from São Tomé should be included in the program.
The importance of efficient treatment of cases is reinforced by the higher prevalence of high grade infections in hospital patients from remote areas. The value of R0 that we have calculated is based on the assumption that transmission is homogeneous, and is thus an underestimate [23]. Unlike infectious diseases (such as measles) which induce life-long immunity, P. falciparum can persist even in very small communities if the same small group of people continually re-infects one another. Whether this would be a problem in Príncipe remains to be seen. An island-wide prevalence survey to determine if there were 'hotspots' of malaria on the island would enable adequate strategies to be determined. In practice this means that, if transmission declines, it would be logical to accompany reinforcement of personal protection in remote roças or fishing villages with treatment to eliminate pockets of asymptomatic infection. At the same time, villagers in these remote settlements, especially in any 'hotspots', could be trained to take temperatures, to use dipsticks for malaria diagnosis [24,25] and to provide fast acting, effective treatment. Availability of treatment in those areas would not only result in more rapid treatment among malaria sufferers but should help contain outbreaks of imported malaria in the future.
The reduction in malaria seen along the Thai-Myanmar border when artesunate based treatment was introduced was attributed to the treatments anti-gametocidal properties as much as its ability to cure the disease [26]. People who take artesunate based medicine feel better far more rapidly than with any other treatment. The introduction of combination therapy that includes artesunate or one of its derivatives would seem apposite. This would further reduce transmission as well as the number of severe, life-threatening infections that might occur.
Malaria can be eliminated from islands [27]. Schistosomiasis is endemic on São Tomé but absent in Príncipe [28]. Is it too much to hope that this could sometime be said for malaria on the island?
Abbreviations
CI Confidence interval, IRS Indoor Residual Spraying, PCR Polymerase Chain Reaction, MD Medical Doctor, CNE Centro Nacional de Endemias
Authors contributions
Hagmann, R helped design the study carried out the field work and analyzed data
Charlwood, JD Helped design the study, carried out the field work and wrote the manuscript
Gil V and Ferreira C Provided logistical support from São Tomé
do Rosário V Provided logistical support from Portugal
Smith T Helped design the study, helped with the fieldwork, analysed the data and wrote the manuscript.
Acknowledgments
Acknowledgements
This research was supported by Swiss National Science Foundation grant 31–52984.97, and INCO-DC project IC18CT960030. The surveys received approval from the São Tomé and Príncipe Ministry of Health. The authors would like to thank Izidro Bengala of the Centro Nacional de Endemias and the staff of the Hospital of Santo António for their assistance in the field. We would also like to thank Marcel Tanner, Director of the Swiss Tropical Institute, for his enthusiastic support of the project and Niels Ørnbjerg, Director, Danish Bilharziais Laboratory for his forbearance during manuscript preparation.
Contributor Information
Reto Hagmann, Email: Reto.Hagmann@bag.admin.ch.
J Derek Charlwood, Email: dc@bilharziasis.dk.
Vilfrido Gil, Email: mmalarst@cstome.net.
Conceição Ferreira, Email: malarst@cstome.net.
Virgíllo do Rosário, Email: cmdt@ihmt.unl.pt.
Tom A Smith, Email: Thomas-A.Smith@unibas.ch.
References
- Pinto J, Sousa CA, Gil V, Ferreira C, Gonçalves L, Lopes D, Petraraca V, Charlwood JD, do Rosário VE. Malaria in São Tomé and Príncipe: Parasite prevalences and vector densities. Acta Trop. 2000;76:185–193. doi: 10.1016/S0001-706X(00)00100-5. [DOI] [PubMed] [Google Scholar]
- Pinto J, Donnelly MJ, Sousa CA, Gil V, Ferreira C, Elissa N, do Rosario VE, Charlwood JD. Genetic structure of Anopheles gambiae (Diptera: Culicidae) in São Tomé and Príncipe (West Africa): implications for malaria control. Mol Ecol. 2002;11:2183–2187. doi: 10.1046/j.1365-294X.2002.01587.x. [DOI] [PubMed] [Google Scholar]
- Fraga de Azevedo J, Costa Mourão M, Castro Salazar JM, Tendeiro J, Almeida Franco LT. O paludismo na ilha do Príncipe (1958) Anais Inst Med Trop. 1960;17:955–966. [PubMed] [Google Scholar]
- Dietz K, Molineaux L, Thomas A. A malaria model tested in the African savannah. Bull World Health Organ. 1974;50:347–357. [PMC free article] [PubMed] [Google Scholar]
- Ceita JGV. Malaria in São Tomé and Príncipe. In: Buck AA, editor. In Proceedings of the Conference on Malaria in Africa. Washington DC, American Institute of Biological sciences; 1986. pp. 142–155. [Google Scholar]
- Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature. 1964;4964:1173–5. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes in C: the Art of Scientific Computing. Cambridge, Cambridge University Press. 1988.
- Dietz K. Mathematical models for transmission and control of malaria. In: Wernsdorfer WH, McGregor I, editor. Malaria, Principles and Practice of Malariology. Edinburgh, Churchill Livingstone; 1988. pp. 1091–133. [Google Scholar]
- Molineaux L. The epidemiology of human malaria as an explanation of its distribution, including some implications for its control. In: Wernsdorfer WH, McGregor I, editor. Malaria, Principles and Practice of Malariology. Edinburgh, Churchill Livingstone; 1988. pp. 913–998. [Google Scholar]
- Sousa CA, Pinto J, Almeida APG, Ferreira C, do Rosário VE, Charlwood JD. Dogs as a favoured host choice of Anopheles gambiae sensu stricto (Diptera:Culicidae) of São Tomé, West Africa. J Med Entomol. 2001;38:122–125. doi: 10.1603/0022-2585-38.1.122. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, Petrarca V, do Rosario VE. A mate or a meal' – Pre-gravid behaviour of female Anopheles gambiae from the islands of São Tomé and Príncipe, West Africa. Malaria J. 2:9. doi: 10.1186/1475-2875-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onori E, Beales PF, Gilles HM. From malaria eradication to malaria control: the past, the present, and the future. In: Gilles HM, Warrell DA, editor. Bruce-Chwatt's Essential Malariology. 3. London, Edward Arnold; 1993. pp. 267–282. [Google Scholar]
- Charlwood JD, Clarke SE, Bøgh C. Costing vaccines versus bednets. Trends Parasitol. 2001;17:121. doi: 10.1016/s1471-4922(01)01883-9. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Emerson PM, Charlwood JD. Reducing malaria by mosquito-proofing houses. Trends Parasitol. 2002;18:510–514. doi: 10.1016/S1471-4922(02)02382-6. [DOI] [PubMed] [Google Scholar]
- Martet G, Conceição S, Cordoliani G, Delolme H, Raphenon G, Hovette P, Doury JC, Baudon D, Touze JE, Lecamus JL. Le paludisme en République de São Tomé e Príncipe. Évaluation épidémiologique et chimiorésistance de P. falciparum. Bull Soc Path Exot. 1991;84:273–280. [PubMed] [Google Scholar]
- Loureiro LF, Cesário AM, Franco AS, Rosário VE, Benito A, Ferreira C, Eggelte TE. Malaria in São Tomé and Príncipe: prevalence and drug-susceptibility. Ann Trop Med Parasitol. 1994;90:223–224. doi: 10.1080/00034983.1996.11813048. [DOI] [PubMed] [Google Scholar]
- Baptista JL, Neves I, D'Alessandro U, Hendrix L, Wéry M. Plasmodium falciparum chloroquine and quinine sensitivity in asymptomatic and symptomatic children in São Tomé island. Trop Med Int Hlth. 1998;2:582–588. doi: 10.1046/j.1365-3156.1997.d01-325.x. [DOI] [PubMed] [Google Scholar]
- Osorio L, Ferro BE, Castillo CM. Effects of chloroquine and sulfadoxine/pyrimethamine on gametocytes in patients with uncomplicated Plasmodium falciparum malaria in Colombia. Mem Inst Oswaldo Cruz. 2002;97:1221–1223. doi: 10.1590/s0074-02762002000800026. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ, Alloueche A, Curtis J, Drakeley CJ, Ord R, Duraisingh M, Greenwood BM, Pinder M, Warhurst D, Targett GA. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg. 2002;67:578–585. doi: 10.4269/ajtmh.2002.67.578. [DOI] [PubMed] [Google Scholar]
- Charlwood JD. Repellent 'soap' for malaria vector control in Papua New Guinea. P N G Med J. 1987;30:99–103. [PubMed] [Google Scholar]
- Hoffmann EJ, Miller JR. Reduction of mosquito (Diptera: Culicidae) attacks on a human subject by combination of wind and vapor-phase DEET repellent. J Med Entomol. 2002;39:935–938. doi: 10.1603/0022-2585-39.6.935. [DOI] [PubMed] [Google Scholar]
- Peterson C. Insect repellents in urban settings. Biologist (London) 2003;501:39–43. [PubMed] [Google Scholar]
- Dye C, Hasibeder G. Population dynamics of mosquito-borne disease: effects of flies which bite some people more frequently than others. Trans R Soc Trop Med Hyg. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- Habluetzel A, Esposito F, Lombardi S. Immuno-techniques for epidemiology of malaria: appropriate tools for integration of primary health care with malaria reseach and control. Trans R Soc Trop Med Hyg. 1989;83:15–19. doi: 10.1016/0035-9203(89)90597-x. [DOI] [PubMed] [Google Scholar]
- Wongsrichanalai C. Rapid diagnostic techniques for malaria control. Trends Parasitol. 2001;17:307–309. doi: 10.1016/S1471-4922(01)01925-0. [DOI] [PubMed] [Google Scholar]
- van Vugt M, Leonardi E, Phaipun L, Slight T, Thway KL, McGready R, Brockman A, Villegas L, Looareesuwan S, White NJ, Nosten F. Treatment of uncomplicated multidrug-resistant falciparum malaria with artesunate-atovaquone-proguanil. Clin Infect Dis. 2002;35:1498–1504. doi: 10.1086/344901. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Taleob G, Kalkoab M, Yamarb S, Kobayakawaa T, Björkman A. Malaria eradication on islands. Lancet. 2000;356:1560–1564. doi: 10.1016/S0140-6736(00)03127-5. [DOI] [PubMed] [Google Scholar]
- Almeda J, Corachan M, Sousa A, Ascaso C, Carvalho JM, Rollinson D, Southgate VR. Schistosomiasis in the Republic of São Tomé and Príncipe: human studies. Trans R Soc Trop Med Hyg. 1994;88:406–409. doi: 10.1016/0035-9203(94)90402-2. [DOI] [PubMed] [Google Scholar]