Prostate cancer is the most commonly diagnosed cancer in United Kingdom men.w1 In 2002 it was diagnosed in 32 000 men, and more than 10 000 deaths were attributed to it. Its incidence increased with the introduction of the prostate specific antigen (PSA) blood test, and disease specific mortality has declined. This review provides evidence about risk factors, prevention, detection, natural course, and treatment, with a focus on clinically localised disease, to guide primary care doctors.
Summary points
Prostate cancer is a common and potentially serious disease
Risk factors include increasing age, family history of prostate cancer, and black race. There are few established prevention strategies
Early detection and treatment may prevent future cancer-related illness, extend life, and provide peace of mind
However, prostate cancer testing, by digital rectal examination and prostate specific antigen testing, can have false positive and false negative results and detects many cancers that would never cause symptoms.
Lower urinary symptoms such as frequency, hesitancy, night time urination, and slow stream do not increase prostate cancer risk but are associated with higher PSA values
The probability that further evaluation with a prostate biopsy will be required as a result of testing is relatively high. Biopsy can cause adverse effects including pain, bleeding, and urinary infection
Treatment options include observation, surgery, radiation, and early hormonal deprivation. Aggressive treatment is needed to realise any benefit from discovery of a tumour, but such treatment may not be necessary or effective
Treatment is associated with a small risk of death and a higher risk of side effects, particularly regarding sexual, urinary, and bowel function
Men with a life expectancy of <10-15 years (due to advanced age or a serious coexisting condition) are unlikely to benefit from routine testing
Data sources and selection criteria
We searched Medline and the Cochrane Library during August 2006 for randomised trials, systematic reviews, and recent evidence based guidelines from the US Preventive Services Task Force (USPSTF), the American Urological Association (AUA), and the National Institute for Health and Clinical Excellence (NICE)
We searched personal archives of references and the reference lists of relevant articles, including the chapter on prostate cancer (non-metastatic) from Clinical Evidence updated in 2003
What factors increase prostate cancer risk?
Advanced age is the primary risk factor for prostate cancer. About 80% of cases, and 90% of deaths, occur in men over the age of 65.1 The prevalence of subclinical prostate cancer is high at all ages and greatly exceeds the lifetime risk of dying of prostate cancer (3%). Geographical variation is probably due to racial, dietary, and environmental factors as well as differences in the intensity of cancer detection efforts. Prostate cancer is more common in black men and those with a first degree relative who has had prostate cancer. Mortality may be associated with obesity.2 High intake of dairy products and calcium as well as red meat have been claimed to increase risk, though any effect is small.3 Testosterone replacement to treat erectile dysfunction and enhance libido, muscle strength, and wellbeing is popular despite little information about potential risks of prostate cancer or benign prostate problems.w2
Can prostate cancer be prevented?
Few proved strategies exist to prevent prostate cancer. Diets high in soya, selenium, vitamin E, fruits, and lycopenes (tomato based products) may be associated with lower risk of prostate cancer. However, in patients with cancer or preinvasive lesions no association was found between nutritional interventions and cancer mortality or disease-free survival.w3 One randomised trial showed that finasteride, a 5α reductase inhibitor, reduced the risk of incident prostate cancer at seven years by about six men in 100.4 Detected cancers considered high grade, and thus potentially of greater risk for causing morbidity and mortality, were more common in the finasteride than the placebo group.
What signs or symptoms are due to prostate cancer?
Prostate cancer can cause haematuria or urinary obstruction due to local progression. Cancer that spreads outside the gland may result in lower extremity oedema from regional lymphatic obstruction or pain from bone metastasis. However, the vast majority of men with prostate cancer have no symptoms, and their tumours are detected by routine testing. Bothersome lower urinary tract symptoms due to benign prostatic obstruction are common in elderly men, result in increased concentrations of prostate specific antigen but do not increase prostate cancer risk.5 PSA testing based on the presence of these urinary symptoms is not supported by the evidence.
What detection strategies exist and do they reduce morbidity and mortality?
Digital rectal examination
Digital rectal examination has not been proved to improve morbidity or mortality, and its accuracy is user dependent, with poor reproducibility even among trained clinicians.6 About 3-6% of examinations can be expected to find abnormal results that raise suspicions for cancer (induration or nodularity). The examination's predictive value varies substantially with the patient's age, family history of prostate cancer, and PSA concentration. A challenge with testing by digital rectal examination alone is that more than half of the men with prostate cancer detected by the technique will have disease outside the gland at diagnosis.w4 Among men with low PSA values (<4.0 ng/ml), the positive predictive value of an abnormal result from digital rectal examination is low, resulting in unnecessary prostate biopsies.7 Many of the tumours detected in these men are small, found serendipitously, and of questionable clinical significance.
PSA blood test
Prostate specific antigen is a protein found in the blood of all men. Raised concentrations are associated with prostate cancer, but also with enlargement, infection, or inflammation of the prostate. PSA testing detects more cancers at earlier stages and of smaller size than does digital rectal examination. From 1990 to 2002, when PSA testing increased, the annual, age adjusted incidence of prostate cancer in the UK increased nearly twofold.w1
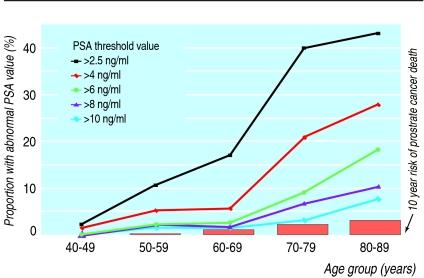
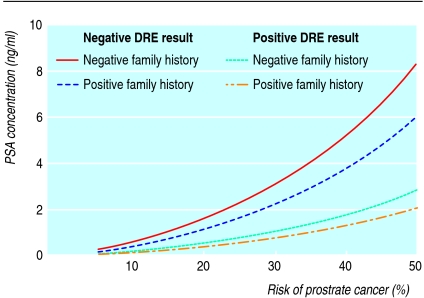
A cut-off value of 4.0 ng/ml for normal PSA levels has been proposed as the optimal balance between detecting cancer and avoiding unnecessary extra evaluations. However, lower levels have been detected with many cancers, including high grade, aggressive tumours.7 Categorising PSA results as normal or abnormal does not adequately assess prostate cancer risk. PSA values, results from digital rectal examination, family history, age, ethnic origin, and results of prior prostate biopsies are all predictive of prostate cancer risk and risk of high grade disease.8 Reducing the PSA threshold that prompts a biopsy detects more cancers and might improve morbidity and mortality from prostate cancer. However, lowering the threshold from 4.0 ng/ml to 2.5 ng/ml would more than double the number of men referred for possible prostate biopsy (fig 1). An individual's risk can be calculated (see www.compass.fhcrc.org/edrnnci/bin/calculator/main.asp) and may be useful for decision making (fig 2).8
Fig 1 Proportion of US men eligible for prostate specific antigen (PSA) testing who would have abnormal results for different PSA cut-off values. (Adapted from Welch et al9)
Fig 2 Risk of prostate cancer associated with prostate specific antigen (PSA) concentrations by digital rectal examination result and family history. (Adapted from Thompson et al8)
Effects of testing on morbidity and mortality
A Cochrane review identified two randomised trials of screening.10 Both had methodological flaws, but the pooled results found no difference in prostate cancer mortality between men randomised to screening with digital rectal examination and PSA testing and the unscreened controls. Case-control studies have generally not found reduced mortality with testing.11 High quality randomised controlled trials in the United States and Europe are currently evaluating whether screening reduces prostate cancer mortality.w5 w6
Testing for prostate cancer is associated with adverse effects including anxiety related to abnormal results; pain, infection, and bleeding from diagnostic prostate biopsies; and detection and treatment of prostate cancers unlikely to cause health problems.12 13 Although mortality from prostate cancer has been declining in several countries and some age groups, it is not clear if this is due to increased PSA testing. In the US, where PSA testing is common, the incidence of prostate cancer is about seven times higher than in England and Wales, where testing rates are much lower.
The US Preventive Services Task Force and the UK National Institute for Health and Clinical Excellence and UK Prostate Cancer Risk Management Programme Scientific Reference Group do not recommend routine testing. Men interested in having a test should be informed of the potential but unproved benefits and associated harms.
How is prostate cancer diagnosed and progression risk predicted?
Men with an abnormal test result should be referred for further evaluation and possible prostate biopsy to determine if cancer is present. Multiple tissue cores are removed under transrectal ultrasound guidance. The natural course of a cancer can be estimated from tumour volume, aggressiveness, and disease extent. Volume measures include local stage, number of biopsy cores with cancer, and extent of cancer in the affected cores. The primary measure of aggressiveness is the Gleason histological score: tumours scored 8-10 are considered the most aggressive, while those with scores ≤6 are potentially indolent. One classification predicts disease progression without intervention as well as recurrence after treatment:
Low risk—PSA value ≤10 ng/ml, Gleason score ≤6, and clinical stage T1c or T2a
Intermediate risk—PSA >10-20 ng/ml, Gleason score 7, or clinical stage T2b
High risk—PSA >20 ng/ml, Gleason score 8-10, or clinical stage T2c.14
How is prostate cancer treated and what are the risks and benefits?
The commonest treatments include watchful waiting, surgery (radical prostatectomy), external beam and interstitial (brachytherapy) radiotherapy, and early androgen deprivation (see box). Because of a lack of randomised trials, the optimal treatment is not known, and preferred choices and recommendations vary widely. The cancer's natural course, the patient's life expectancy based on age and comorbidities, treatment complications, and patient priorities are important considerations. Factors incorporated into the patient decision process include cancer eradication, adverse effects, doctors' recommendations, convenience, and costs.15
Treatment options for clinically localised prostate cancer
Watchful waiting (active surveillance, expectant management)
Active plan to postpone intervention. May involve monitoring with digital rectal examination or PSA blood test and repeat prostate biopsy, with further treatment (curative or palliative) based on patient preference, symptoms, and clinical findings
Potential benefits—No immediate side effects; low initial cost; most men (especially of low to intermediate risk) do not need treatment and survive at least 10 years
Potential risks—Cancer not removed, so could advance, become incurable, and cause death; patients' quality of life could be painfully restricted; other treatments may be necessary, not effective, and have side effects; patients may be too anxious or worried to monitor cancer without treatment
Radical prostatectomy (retropubic or perineal)
Complete surgical removal of prostate gland with seminal vesicles, ampulla of vas, and sometimes pelvic lymph nodes. Sometimes done laparoscopically or with robotic assistance and attempt to preserve nerves for erectile function
Potential benefits—May eliminate cancer; generally well tolerated. One randomised controlled trial showed reduced mortality from prostate cancer and metastasis compared with watchful waiting
Potential risks—Hospitalisation for major surgery; operative related death and perioperative cardiovascular complications and bleeding; may not eradicate cancer; long term urinary incontinence, urethral stricture, bladder neck contracture, and bowel and erectile dysfunction
External beam radiation therapy (EBRT)
Multiple doses of radiation from an external source applied over several weeks. Dose and physical characteristics of beam may vary. Conformal radiotherapy uses three dimensional planning systems to maximise dose to prostate cancer and to spare normal tissue
Potential benefits—May eliminate cancer; generally well tolerated; avoids operative risk
Potential risks—Does not remove prostate gland and may not eradicate cancer; 5-8 weeks of outpatient therapy; treatment related death, incontinence, proctitis, diarrhoea, cystitis, erectile dysfunction, urethral stricture, bladder neck contracture, and bleeding. Contraindicated in men with inflammatory bowel disease because of risk of bowel injury. One small, older randomised controlled trial showed greater risk of disease progression compared with prostatectomy
Brachytherapy
Radioactive implants placed under anaesthesia using radiological guidance. Lower dose, permanent implants typically used. External beam “boost” radiotherapy or androgen deprivation sometimes recommended
Potential benefits—May eliminate cancer; generally well tolerated; avoids operative risk; single outpatient session
Potential risks—Does not remove prostate gland and may not eradicate cancer; may not be effective for larger prostate glands or more aggressive tumours; urinary retention, incontinence, impotence, cystitis or urethritis, and proctitis; long term outcomes from representative national sample not reported. Contraindicated in patients with prior transurethral resection of the prostate
Androgen deprivation therapy
Oral or injected drugs or surgical removal of testicles to lower or block circulating androgens
Potential benefits—Avoids risks of prostatectomy and radiotherapy; usually lowers PSA levels and may slow cancer progression
Potential risks—Does not remove prostate cancer and may not eradicate cancer; gynaecomastia, impotence, diarrhoea, osteoporosis, lost libido, hot flushes, and “androgen deprivation syndrome” (depression, memory difficulties, fatigue)
Cryoablation
Destruction of cells through rapid freezing and thawing using transrectal guided placement of probes and injection of freezing and thawing gases
Potential benefits—May eliminate cancer; generally well tolerated; avoids operative risk; single outpatient session
Potential risks—Does not remove prostate gland and may not eradicate cancer; impotence, incontinence, scrotal oedema, pelvic pain, sloughed urethral tissue, prostatic abscess, urethrorectal fistula; no long term outcomes from national sample
Options are associated with short and long term risks including urinary, bowel, and sexual dysfunction, a small risk of treatment related death, as well as cancer spread and death. Treatments vary in the frequency and severity of complications, but all carry some risk. In general, surgery is more likely to cause urinary and sexual dysfunction than radiotherapy, whereas bowel and rectal injury are more common with radiotherapy. Patient priorities are often the primary factor in treatment selection. Patients particularly averse to the risk of cancer may prefer more aggressive treatments at the potential expense of urinary, bowel, or sexual dysfunction. Patients who particularly value quality of life may prefer watchful waiting or less invasive options. Functional issues should be considered. For example, patients with inflammatory bowel disease may be poor candidates for radiotherapy, as bowel symptoms could be exacerbated. Poor bladder function (such as obstructive symptoms or unstable detrusor activity) may be ameliorated by some treatments but exacerbated by others.
Watchful waiting
One report assessed 20 year outcomes from watchful waiting with delayed palliative interventions for cancer detected before the use of PSA testing. Men with low grade disease (Gleason score 2-4) had a minimal risk of dying from prostate cancer in 20 years of follow-up (6 deaths/1000 person years).16 Men with high grade disease (Gleason score 8-10) had a high probability of dying from prostate cancer within 10 years of diagnosis (121 deaths/1000 person years). Because PSA testing allows detection of cancers some 5-15 years earlier than digital rectal examination, men with PSA detected tumours treated by watchful waiting will probably have better than 20 year disease specific survival.
Radical prostatectomy
Two randomised trials compared surgery with watchful waiting.w5 Few of the men had tumours detected by PSA testing. One small trial found no difference in survival after 23 years. A more recent and larger study found that surgery improved both disease specific and overall survival by 5% after 10 years: 9% of men assigned to surgery and 14% of those assigned watchful waiting died of prostate cancer (fig 3).17 Surgery reduced the risk of metastatic and local disease spread but was associated with greater risk of urinary dysfunction (49% v 21%) and sexual dysfunction (80% v 45%). Bowel function, anxiety, and general quality of life were similar. Mortality reduction was limited to men less than 65 years old. Ongoing trials in the US and UK are evaluating surgery, watchful waiting, and radiation therapy in men with primarily PSA screen detected cancers.w7 w8
Fig 3 Cumulative mortality from prostate cancer for treatment by radical prostatectomy versus watchful waiting. (Adapted from Bill-Axelson A et al17)
External beam radiation therapy
This has been compared with surgery in only one small randomised trial. Despite the findings that disease progression (measured by bone scan and acid phosphatase) was greater in patients treated with radiation therapy, the study is generally considered not applicable to current practice.18 Recent modifications include high dose conformal radiation therapy, which uses three dimensional planning systems and methods to match radiation treatment to prostate and tumour volumes. Compared with conventional radiotherapy, high dose conformal therapy decreased the rate of recurrence or progression of abnormal PSA values (but not mortality) without increasing acute or late serious urinary or rectal complications.19 w9
Brachytherapy
This delivers radiation through the implantation of needles containing small radioactive pellets into the prostate gland under general or spinal anaesthesia. The pellets can be left in place permanently, or temporarily for high dose pellets, and emit a low dose of radiation over several weeks or months. Brachytherapy is used as a primary therapy, in combination with external beam radiation therapy or with androgen deprivation therapy. Brachytherapy has become increasingly used for selected men with low to moderate risk prostate cancers despite no survival data from randomised trials. A review of case series concluded that efficacy and some long term adverse effects were similar to those with surgery or external beam radiation therapy in well selected patients.20 Disease specific quality of life may be lower in patients receiving brachytherapy than in those receiving external beam radiation therapy. Preliminary results of trials comparing different isotopes or adjuvant therapies and other underpowered studies have been published,w10-w13 but no conclusions can yet be drawn regarding the relative efficacy versus other treatments as well as optimal forms of brachytherapy.
Androgen deprivation therapy
Use of continuous or intermittent long term androgen deprivation therapy as primary treatment has increased. This approach has not been shown to improve survival, although it lowers PSA concentrations. Among patients with intermediate and high risk localised or locally advanced prostate cancer, addition of adjuvant or neoadjuvant androgen deprivation therapy to external beam radiation therapy, but not to radical prostatectomy, improves survival.21 w14-w16 In addition to treatment costs, adverse effects of androgen deprivation therapy include erectile dysfunction, loss of libido, breast tenderness, hot flushes, depression and mood changes, memory difficulties, fatigue, muscle and bone loss, and fractures.22
Improving the quality of prevention, testing, and treatment
Given the evidence that finasteride reduces the risk of prostate cancer, doctors should consider providing this information to men over the age of 50. Decisions whether to start prophylactic treatment should take account of patient life expectancy and the long term adverse effects and costs of finasteride. Men interested in testing for prostate cancer should first consult their doctor. Testing can lead to a cascade of unanticipated events if patients do not understand the potential but unproved impact on survival, treatment effectiveness, side effects, and lifelong changes associated with being a “cancer survivor.” Rather than recommending for or against prostate cancer testing, doctors should encourage informed decision making among men who inquire about testing, are at least 50 years old, and have a life expectancy of at least 10-15 years. Tools are available to assist in presenting balanced information about the potential benefits and established harms of testing. For men with shorter life expectancy due to age or comorbidities, recommending against testing is likely to lead to superior health outcomes.23
A review of treatments should include their risks and benefits, incorporating results from any randomised trials and cancer outcomes (see box of treatment options). A multidisciplinary approach to deciding about treatment should be encouraged because recommendations are based in part on doctors' specialties.24 25 Decisions can be based on patients' expectations and priorities for outcomes (such as cancer control, anxiety, urinary or sexual function), as well as their life expectancy. Structure and process measures of care reflect attributes of the healthcare setting (such as type of hospital or surgeon's work volume) or describe details of the interactions between provider or patient (such as discussion of alternative treatment risks and benefits). These are modifiable and can influence treatment effectiveness, adverse effects, and patient satisfaction. Initiatives are under way to measure and improve the quality of care for patients with prostate cancer.26
Ongoing research priorities
Conduct large scale, randomised controlled trials of prostate cancer prevention, detection, and treatment
Identify biomarkers that can provide reliable identification of cancers requiring treatment
Standardise reporting of prostate cancer and quality of life outcomes
Identify measures that improve quality of prostate cancer care, including informed decision making
Unanswered questions
Do dietary or drug interventions prevent prostate cancer?
Does screening with PSA blood test and digital rectal examination improve prostate cancer and overall survival?
What is the comparative effectiveness and adverse effects of different treatments for localised prostate cancer (especially those detected by PSA testing)? Do these vary according to patient, tumour, or provider characteristics?
Can biomarkers be identified to predict the relative effectiveness of interventions in order to maximise treatment effectiveness while minimising excess detection and treatment?
What structural and process measures of care are most closely linked to quality outcomes and how can these be improved?
Additional educational resources
Watson E, Jenkins L, Bukach C,Austoker J. The PSA test and prostate cancer: information for primary care. Sheffield: NHS Cancer Screening Programmes, 2002
Aus G, Abbou CC, Bolla M, Heidenreich A, van Poppel H, Schmid H-P, et al. Guidelines on prostate cancer. European Association of Urology, 2006. (www.uroweb.nl/files/uploaded_files/guidelines/07%20Prostate%20Cancer.pdf)
American Urological Association. Guidelines for the management of clinically localized prostate cancer. (Update in press)
Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-29
National Institute for Health and Clinical Excellence (www.nice.org.uk). Prostate cancer: diagnosis and treatment. (Scheduled publication 2007)
NHS Cancer Screening Programmes. Prostate cancer risk management questions and answers. www.cancerscreening.nhs.uk/prostate/faqs.html
Agency for Healthcare Research and Quality. Comparison of therapies for clinically localized prostate cancer. (Release date 2007) (http://effectivehealthcare.ahrq.gov/synthesize/cers.cfm?topic=41)
Foundation for Informed Medical Decision Making. www.fimdm.org
Health Dialog. www.healthdialog.com
The Cochrane Library. www.cochrane.org
References
- 1.Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-64. [DOI] [PubMed] [Google Scholar]
- 2.Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review). Int J Oncol 2006;28:737-45. [PubMed] [Google Scholar]
- 3.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 2005;97:1768-77. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215-24. [DOI] [PubMed] [Google Scholar]
- 5.Young JM, Muscatello DJ, Ward JE. Are men with lower urinary tract symptomas at increased risk of prostate cancer? A systematic review and critique of the available evidence. BJU Int 2000;85:1037-48. [DOI] [PubMed] [Google Scholar]
- 6.Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Int Med 2002;137:917-29. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level of ≤4.0 ng per milliliter. N Engl J Med 2004;350:2239-46. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Feng Z, et al. Assessing prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98:529-34. [DOI] [PubMed] [Google Scholar]
- 9.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United Sates: implications of various definitions for abnormal. J Natl Cancer Inst 2005;97:1132-7. [DOI] [PubMed] [Google Scholar]
- 10.Ilic D, O'Connor D, Green S, Wilt TJ. Screening for prostate cancer. Cochrane Database Syst Rev 2006;3:CD004720. [DOI] [PubMed] [Google Scholar]
- 11.Barry MJ. The PSA conundrum. Arch Intern Med 2006;166:7-8. [DOI] [PubMed]
- 12.McNaughton-Collins M, Fowler FJ Jr, Caubet JF, Bates DW, Lee JM, Hauser A, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med 2004;117:719-25. [DOI] [PubMed] [Google Scholar]
- 13.Ransohoff D. Why is prostate cancer screening so common when the evidence is so uncertain? A system without negative feedback. Am J Med 2002;113:663-7. [DOI] [PubMed] [Google Scholar]
- 14.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 2001;58:843-8. [DOI] [PubMed] [Google Scholar]
- 15.Zeliadt SB, Ramsey SD, Penson DF, Hall IJ, Ekwueme DU, Stroud L, et al. Why do men choose one treatment over another? A review of patient decision making for localized prostate cancer. Cancer 2006;106:1865-74. [DOI] [PubMed] [Google Scholar]
- 16.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-101. [DOI] [PubMed] [Google Scholar]
- 17.Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84. [DOI] [PubMed] [Google Scholar]
- 18.Paulson DF, Lin GH, Hinshaw W, Stephani S. Radical surgery versus radiotherapy for adenocarcinoma of the prostate. J Urol 1982;128:502-4. [DOI] [PubMed] [Google Scholar]
- 19.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ Jr, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233-9. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Clinical Excellence. Low dose rate brachytherapy for localised prostate cancer. www.nice.org.uk/IPG132
- 21.D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004;292: 821-7. [DOI] [PubMed]
- 22.Shahinian VB, Fuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med 2006;166:465-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilt TJ, Partin MR. Reducing PSAnxiety: The importance of noninvasive chronic disease management in prostate cancer detection and treatment. Am J Med 2004;117:796-8. [DOI] [PubMed] [Google Scholar]
- 24.Fowler FJ Jr, McNaughton Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA 2000;283:3217-22. [DOI] [PubMed] [Google Scholar]
- 25.Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-3. [DOI] [PubMed] [Google Scholar]
- 26.Spencer BA, Steinberg M, Malin J, Adams J, Litwin MS. Quality-of-care indicators for early-stage prostate cancer. J Clin Oncol 2003;21:1928-36. [DOI] [PubMed] [Google Scholar]