Abstract
Explanations for left hemisphere language laterality have often focused on hemispheric structural asymmetry of the planum temporale. We examined the association between an index of language laterality and brain morphology in 99 normal adults whose degree of laterality was established using a functional MRI single-word comprehension task. The index of language laterality was derived from the difference in volume of activation between the left and right hemispheres. Planum temporale and brain volume measures were made using structural MRI scans, blind to the functional data. Although both planum temporale asymmetry (t(1,99) = 6.86, p < .001) and language laterality (t(1,99) = 15.26, p < .001) were significantly left hemisphere biased, there was not a significant association between these variables (r(99) = .01, ns). Brain volume, a control variable for the planum temporale analyses, was related to language laterality in a multiple regression (β = −.30, t = −2.25, p < .05). Individuals with small brains were more likely to demonstrate strong left hemisphere language laterality. These results suggest that language laterality is a multidimensional construct with complex neurological origins.
Keywords: Brain asymmetry, Cerebral asymmetry, Language laterality, Lateralization, Planum temporale
1. Introduction
More than a century of anatomical and neuropsychological research has led to a widely accepted model of language organization in which structural asymmetry of language-related brain areas provide the neurobiological substrate for left hemisphere language laterality (Broca, 1861; Foundas, Leonard, Gilmore, Fennell, & Heilman, 1994; Gazzaniga & Sperry, 1967; Geschwind & Levitsky, 1968; Wernicke, 1874). Much of this research has focused on leftward asymmetry of the posterior superior temporal plane, or planum temporale. Support for an association between brain asymmetry, planum temporale asymmetry in particular, and different measures of language laterality has been inconsistent, however (Binder, Frost, Hammeke, Rao, & Cox, 1996a, 1996b; Blonder, Pettigrew, & Smith, 1994; Chiarello, Kacinik, Manowitz, Otto, & Leonard, 2004; Dorsaint-Pierre et al., 2006; Foundas et al., 1994; Heiervang et al., 2000; Hellige, Taylor, Lesmes, & Peterson, 1998; Jancke & Steinmetz, 1993; Josse, Mazoyer, Crivello, & Tzourio-Mazoyer, 2003; Kertesz, Black, Polk, & Howell, 1986; Moffat, Hampson, & Lee, 1998; Tzourio, Nkanga-Ngila, & Mazoyer, 1998). These inconsistent findings may stem from the dimension of language examined, differences in how individuals perform laterality tasks, mathematical artifact (Mazoyer & Tzourio-Mazoyer, 2004) or methodological, and demographic factors.
The intracarotid amobarbital (Wada) test has been the gold standard for determining language laterality and for assessing whether a functional imaging task is a valid measure of language laterality (Kloppel & Buchel, 2005). For example, Binder et al. (1996b) demonstrated a highly significant correlation (r = .96) between laterality determined by the Wada test and fMRI BOLD response for an aurally presented single-word comprehension task. Fig. 1 shows that the single-word comprehension task engages left-lateralized frontal, lateral and ventral temporal, and angular gyrus regions, when contrasted with activation for a tone decision task. These findings suggest that engagement of the posterior superior temporal gyrus is not essential in the demonstration of language laterality as defined by the Wada test. In support of this notion, Lehericy et al. (2000) examined the association between regional activation for story listening and the Wada test. A left hemisphere bias for middle frontal gyrus activation, but not a left hemisphere bias for temporal lobe activation, was related to Wada language laterality.
Fig. 1.
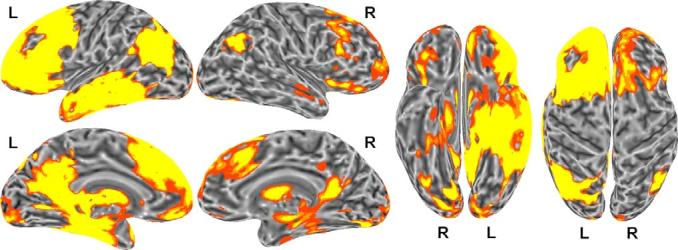
Brain activation associated with the semantic decision task (relative to the tone control task) in 100 healthy, right-handed adults. The group activation map was thresholded at a corrected p < .05 and mapped onto a representative "inflated" cortical surface. The data are shown in lateral, medial, ventral, and dorsal views. Activation is strongly left-lateralized and involves large regions of the prefrontal cortex, lateral and ventral temporal lobe (middle and inferior temporal gyri, anterior fusiform gyrus, parahippocampus, and hippocampus), posterior parietal lobe (angular gyrus), posterior cingulate region, and basal ganglia. Lateral temporal lobe activation extends upward into the superior temporal sulcus but not to the dorsal temporal plane.
Interestingly, there is evidence that a left hemisphere temporal lobe bias in story listening has a structural basis. Tzourio et al. (1998) and Josse et al. (2003) have both observed an association between left planum size and the amount of activation in the posterior superior temporal gyrus during story listening. These studies raise the possibility that functional laterality measured by the Wada test and story listening may have different structural associations.
In addition to the domain of language that is studied, associations between language laterality and brain structure may be influenced by factors such as hand preference and sex differences. People with a left hand preference or mixed handedness are more likely to exhibit reduced left hemisphere activation and increased right hemisphere activation during language tasks (Szaflarski et al., 2002). Non-right-handedness has been associated with greater anatomical variability (Foundas, Leonard, & Hanna-Pladdy, 2002), and people with a non-right-hand preference are more likely to exhibit symmetry and rightward structural asymmetry of the planum temporale than right-handed individuals (Foundas, Leonard, & Heilman, 1995; Steinmetz, Volkmann, Jancke, & Freund, 1991). Samples composed of only strongly right-handed individuals may fail to identify a linear relation between anatomical and laterality measures (Heiervang et al., 2000) because of the contribution of handedness to language and anatomical organization. A relation between planum temporale asymmetry and laterality has been reported with greater frequency when individuals with right hemisphere laterality for handedness and language are included in the study (Blonder et al., 1994; Foundas et al., 1994; Moffat et al., 1998). Some studies have failed to observe an association between handedness and planum morphology (Josse et al., 2003), however, suggesting that additional factors determine variation in handedness and planum morphology.
Sex differences have also been observed for measures of language laterality and anatomical asymmetry, albeit inconsistently. Increased right hemisphere activation has been observed in females compared to males performing language tasks (Baxter et al., 2003; Vikingstad, George, Johnson, & Cao, 2000). This finding is not always observed (Frost et al., 1999; Szaflarski et al., 2002), however, and may occur by chance (Sommer, Aleman, Bouma, & Kahn, 2004). Reduced planum temporale asymmetry has also been observed in females compared to males (Kulynych, Vladar, Jones, & Weinberger, 1994), but this finding is not always observed (Aboitiz, Scheibel, & Zaidel, 1992; Harasty, Double, Halliday, Kril, & McRitchie, 1997; Jancke, Schlaug, Huang, & Steinmetz, 1994), and planum temporale symmetry has been reported in males compared to females with leftward planum temporale asymmetry (Knaus, Bollich, Corey, Lemen, & Foundas, 2004). The reasons for these inconsistent findings are not clear and could be attributable to differences in planum temporale measurement methodology and/or sampling bias.
The Lehericy et al. (2000) study noted above suggests that there are different dimensions of language laterality, some of which relate to Wada language laterality and some that do not. This study examined the relations between measures of the planum temporale and language lateralization as assessed by lateral bias during a single-word comprehension task. Because of the potential influences of handedness and sex, we also examined the association between planum morphology and language laterality within handedness and sex groups. The results demonstrate that planum morphology does not relate to laterality of single-word comprehension processes and support the notion that language laterality is a multidimensional construct (Josse et al., 2003).
2. Methods
2.1. Participants
All participants gave written informed consent according to the Declaration of Helsinki and according to institutional guidelines for this Medical College of Wisconsin Human Research Review Committee approved project. Participants were screened using a medical history and demographics questionnaire. Participants were excluded if they were younger than 18 or older than 40; had a primary language other than English; had an estimated Full-Scale IQ < 90; were medically unable to undergo MRI scanning; had a history of brain injury or illness, substance abuse, psychiatric illness, auditory illness or symptoms, or cardiac disease; were taking psychoactive or vasoactive medications; or were found on screening examination to have neurologic or gross neuropsychologic abnormalities. Participants were also excluded if T1-weighted MRI scans revealed an intracranial structural abnormality.
One-hundred participants were recruited from Milwaukee, WI area universities and through advertisements in local newspapers. One participant was discarded because of poor image quality. There were 48 males with a mean age of 28.8 years (sd, 8.7) and 51 females with a mean age of 27.4 years (sd, 11.3). The sample included strongly right-handed, non-dominant and strongly left-handed participants. Handedness is defined below. These subjects represent a subset of those included in two prior fMRI studies of language dominance (Springer et al., 1999; Szaflarski et al., 2002). They were selected to provide a sample with maximal variation and range of language laterality and handedness, with the aim of optimizing the sensitivity for detecting any relationships between these variables and the morphological measures obtained in the present study.
2.2. Neuroimaging
2.2.1. Structural MRI acquisition
All MRI data were acquired on a 1.5 T GE Signa scanner (GE Medical Systems, Milwaukee, WI). Volumetric, gapless, 1.1–1.3 mm images were acquired using a spoiled gradient-recalled sequence (“SPGR”, matrix size = 256 × 128 and FOV = 24 cm). This structural imaging protocol produces gray-white contrast and spatial resolution suitable for the anatomical data collection. Statistical analyses were performed to control for potential influences of different section thickness of the images.
2.2.2. Image processing
Brain structure data analysis for this study was performed at the University of Florida McKnight Brain Institute (MBI) and Stanford University (SU). Structural MRIs obtained at MCW were sent digitally to MBI and SU, using a blind number to identify the MRI scan. No other identifying information was sent with the scan. The images were reformatted into 1 mm thick sections to correct for tip in the coronal, axial, and sagittal planes of section. Parameter files were created that store the distance between the anterior commissure and borders of the brain. Talairach coordinates were used to identify the same medial to lateral locations in each brain. The Talairach system standardizes positions by relating them to a brain atlas where the horizontal plane intersects the anterior and posterior commissure. The images were not warped or altered during the reformatting process.
2.3. Structural data analysis procedures
2.3.1. Planum temporale
The Sylvian fissure is surrounded by horizontal and vertical planes of cortical tissue. The planum temporale (PT) is defined by the horizontal bank that extends from Heschl's sulcus to the origin of the vertical bank or posterior ascending ramus. The posterior ascending ramus, also called the planum parietale (PP), rises from the termination of the PT into the parietal lobe. The surface area of the PT and PP were measured between 46 and 56 mm lateral to the midline in sagittal sections. PT asymmetry (PTA) is most dramatic in this lateral region of the planum temporale (Best & Demb, 1999). These coordinates were also chosen to replicate the methods of previous MRI studies showing cognitive associations with PT measures (Eckert, Lombardino, & Leonard, 2001; Foundas et al., 1994; Gauger, Lombardino, & Leonard, 1997; Leonard et al., 1996). Some authors have included the PP in their planum temporale asymmetry measure (Honeycutt, Musick, Barta, & Pearlson, 2000; Westbury, Zatorre, & Evans, 1999). An asymmetry measure of the combined PT and PP measurements was also collected (PTPPA). Asymmetry was defined according to the following formula: (surface area of the left planum – surface area of right planum)/average of the left and right planum. Inter-rater reliability for 40 planum temporale and 40 planum parietale measures was alpha = .97 and alpha = .94, respectively, for authors M.A.E. and C.M.L. Fig. 2 presents examples of the PT and PP measurements. Matlab 6.5 (Matworks, Natick, MA, USA) was used to randomly display and measure the planum from each hemisphere to avoid measurement bias that may occur from knowledge of which hemisphere is displayed. Our Matlab program did not warp the images into a normalized Talairach space. Instead, the medial and lateral boundaries of the measured surface were adjusted depending on the width of each individual hemisphere to maintain consistency of location.
Fig. 2.
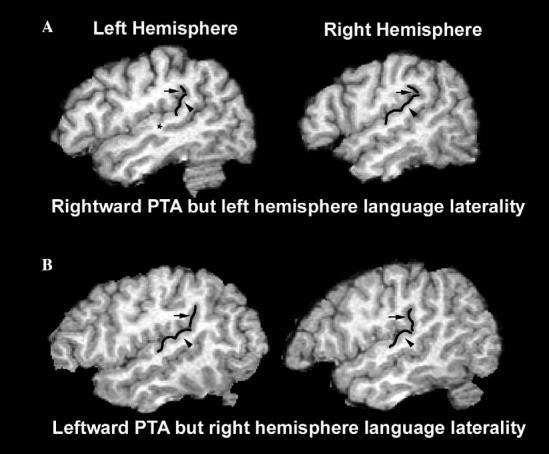
Left and right planum temporale of: (A) 44 years old non-right-handed female with left hemisphere language laterality (1.17) and rightward PTA (−.64); and (B) 20 years old right-handed male with bilateral language laterality (.13) and leftward PTA (.74). Arrowheads indicate planum temporale. Arrows indicate planum parietale. The star in the upper left image indicates the presence of a sulcus intermedius that divides Heschl's gyrus into two separate gyri.
2.3.2. Brain volume
Total brain volume was estimated by segmenting the structural images using SPM2 (Wellcome Department of Cognitive Neurology, University College, London). Segmentation of the images was based on the MNI prior probability gray, white, and CSF images that are packaged with SPM2. The number of gray, white, and CSF voxels in each image were calculated and summed to obtain an index of total brain volume.
2.4. fMRI methods
The fMRI methods used here have been described in detail elsewhere (Binder et al., 1997; Springer et al., 1999; Szaflarski et al., 2002). The difference in activation during a single-word comprehension task and a tone decision task was used to estimate language laterality. In the single-word comprehension task, participants heard spoken English nouns designating animals (e.g., “horse” and responded with a button press if they considered the animal to be both “found in the United States” and “commonly used by humans”. In the tone decision task, participants heard brief sequences of three to seven pure tones. All tones were either 500 Hz (“low”) or 750 Hz (“high”), and participants responded to sequences containing two “high” tones. Button presses for both tasks were made using the index finger of the non-dominant hand. The two tasks were matched for average stimulus intensity, average stimulus duration per trial (750 ms), average trial duration (3 s), and frequency of positive targets (1 target/8 s). The protocol used a block design with alternating periods of the single-word comprehension and tone tasks. The tone decision task was designed to produce activation of sensory, motor, short-term memory, and attention systems with minimal activation of phonological, lexical, or verbal semantic systems (Springer et al., 1999). The animal words varied widely in frequency (Kucera–Francis mean 7.84 per million, sd 26.2, range 0–203) and had generally high imageability ratings in the MRC Psycholinguistic Database (mean 594, sd 39.8, range 462–652, based on available ratings for 72 of the items).
2.4.1. fMRI acquisition
Functional T2*-weighted images were acquired in the sagittal plane using a blipped, gradient-echo echoplanar sequence. Imaging included the entire brain using the following parameters: TE = 40 ms, TR = 3000–4000 ms, flip angle = 90°, NEX = 1, slice thickness = 6–7 mm, FOV = 24 cm, matrix size = 64 × 64, and slice number = 19–21.
Functional image processing was performed at MCW using the MCW-AFNI software package (Cox, 1996). Event-related MRI signal changes were determined using a multiple regression approach on a voxel-by-voxel basis. The predicted change in fMRI signal was modeled by convolving a square wave representing the task alternation with an idealized (gamma function) hemodynamic response. Translation and rotation movement parameters were included in the model, as were linear trends and global MRI signal values for each imaging run. Individual subject activation maps were thresholded at an uncorrected p < .001.
Activation volumes were determined for each participant by counting the number of significantly activated voxels in each hemisphere. A laterality index was then calculated by determining the asymmetry in activation between the two hemispheres ((volume of left hemisphere – volume of the right hemisphere)/average volume of both hemispheres). This laterality index, computed using the same voxel threshold and voxel-counting method as used here, was shown previously to be highly correlated with language dominance as measured with the Wada test (Binder et al., 1996a, 1996b). This laterality index is also predictive of language outcome after left temporal lobe surgery for intractable epilepsy (Sabsevitz et al., 2003). Thus, this measure has been thoroughly validated as an index of language dominance. Fig. 1 demonstrates brain regions exhibiting significant activation during the semantic decision task relative to the tone control task.
2.5. Handedness
A modified version of the Edinburgh handedness inventory was used to provide a quantitative index of handedness (Oldfield, 1971). Participants were asked to indicate how frequently the left or right hand is used to perform tasks such as throwing a ball or brushing teeth. The quantitative score ranges from −100 to 100 for dominant left-handedness to dominant right-handedness. Forty-seven participants had handedness scores below 75 and were classified as non-right-handed (Eckert et al., 2001).
2.6. Statistics
Hierarchical regression analysis (Cohen & Cohen, 1983) was used to examine the amount of individual variability in language laterality that could be explained by planum temporale asymmetry, after controlling for the influences of sex, age, handedness, brain volume, and image section thickness. Pearson correlations were subsequently performed within sex groups because of previous studies suggesting sex influences on planum morphology and laterality (Knaus et al., 2004; McGlone, 1980).
3. Results
Tables 1 and 2 present descriptive statistics for the laterality and anatomical variables, respectively. The sample demonstrated significantly leftward PTA (t(1,98) = 6.86, p < .001), leftward PTPPA (t(1,98) = 2.13, p < .05), and leftward language laterality (t(1,98) = 15.26, p < .001). The language laterality index was significantly more leftward than PTA (t(1,98) = −6.24, p < .001). Table 3 shows that handedness, age, sex, and image section thickness did not contribute significantly to variance in planum asymmetry.
Table 1.
Descriptive statistics for the language laterality score, the amount of significantly activated voxels for each hemisphere in milliliters, and the measure of quantitative handedness for the entire sample and within male and female groups (sd)
| Language laterality | Left hemisphere activation | Right hemisphere activation | QH | |
|---|---|---|---|---|
| Males | .95 (.62) | 40.48 (22.94) | 14.88 (12.57) | 48 (62) |
| Females | .95 (.63) | 35.03 (16.01) | 13.71 (12.39) | 31 (75) |
| Males and females | .95 (.62) | 37.67 (19.76) | 14.28 (12.43) | 39 (70) |
QH, quantitative handedness.
Table 2.
Descriptive statistics for the left and right PT and PP surface area measures (cm2), the PT and PP asymmetry measures, and total brain volume (cm3) for the entire sample and within male and female groups (sd)
| Left PT | Right PT | PTA | Left PTPP | Right PTPP | PTPPA | Brain volume | |
|---|---|---|---|---|---|---|---|
| Males | 3.08 (.96) | 2.07 (1.06) | .44 (.63) | 4.40 (1.12) | 4.03 (.92) | .08 (.27) | 1506 (92) |
| Females | 2.59 (.97) | 1.81 (.98) | .37 (.61) | 3.91 (.79) | 3.72 (1.00) | .06 (.34) | 1325 (99) |
| Males and females | 2.83 (.99) | 1.94 (1.02) | .41 (.62) | 4.15 (.99) | 3.87 (97) | .07 (.30) | 1413 (131) |
PT, planum temporale; PTA, planum temporale asymmetry; PTPP, planum temporale + planum parietale; PTPPA, planum temporale + planum parietale asymmetry.
Table 3.
Correlations of the planum temporale measures with the language laterality, quantitative handedness, age, and sex variables
| Language laterality | Left hemisphere activation | Right hemisphere activation | QH | Age | Sex | Section thickness | |
|---|---|---|---|---|---|---|---|
| Left PT | −.07 | .00 | .06 | .10 | −.13 | −.23 | .08 |
| Right PT | −.04 | −.03 | .03 | .08 | .12 | −.13 | .15 |
| PTA | .01 | .00 | −.03 | .01 | −.19 | −.01 | −.10 |
| Left PTPP | −.08 | −.09 | .00 | .14 | −.11 | −.25 | .14 |
| Right PTPP | −.01 | −.07 | −.09 | .07 | .02 | −.16 | .13 |
| PTPPA | −.04 | .01 | .07 | .05 | −.14 | −.03 | .01 |
QH, edinburgh quantitative handedness; PT, planum temporale; PTA, planum temporale asymmetry; PTPP, planum temporale + planum parietale; PTPPA, planum temporale + planum parietale asymmetry.
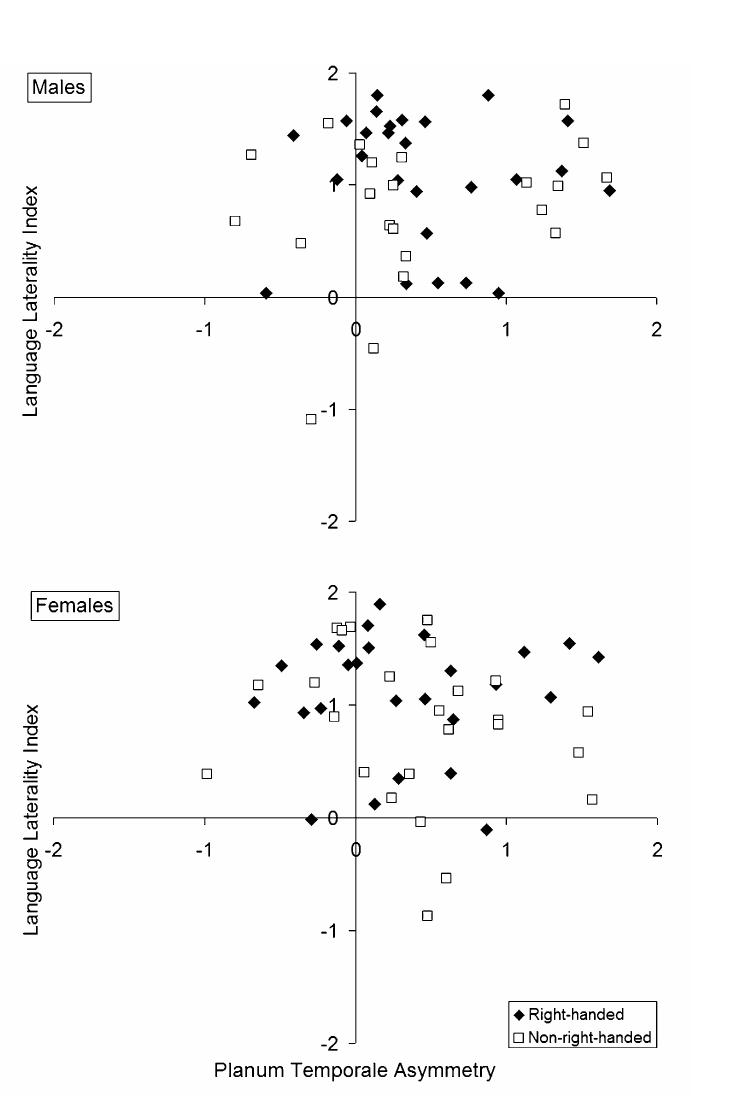
Hierarchical multiple regressions were performed to determine if PTA and PTPPA predicted the language laterality index, after controlling for potential effects of handedness, brain volume, age, sex, and image section thickness. Table 4 shows that neither PTA nor PTPPA significantly predicted the language laterality index. Fig. 2 presents examples of left and right planum temporale from participants whose direction of language laterality did not match the direction of their PTA. Fig. 3 presents the association between PTA and the language laterality index for male and female participants, by hand preference. In each regression, brain volume predicted unique variance in the language laterality index. Leftward language laterality was associated with the lowest brain volumes. There was more variation in the direction of language laterality for participants with the largest brain volumes. The strength of the brain volume and language laterality association was weak, however, and did not survive a post hoc correction for multiple comparisons.
Table 4.
Multiple regressions demonstrating the relation between language laterality and the planum temporale asymmetry measures (PTA and PTPPA, respectively) after controlling for handedness, brain volume, age, and image section thickness
| Standardized β | t | Sig. | |
|---|---|---|---|
| Level 1 (r = .01, F = 0.13, ns) | |||
| PTA | .04 | .36 | ns |
| Level 2 (r = .44, F = 3.66, p < .01) | |||
| PTA | .03 | .33 | ns |
| Handedness | .39 | 3.84 | *** |
| Brain volume | −.32 | −2.41 | * |
| Age | −.09 | −.98 | ns |
| Sex | −.15 | −1.17 | ns |
| Section thickness | −.02 | −.17 | ns |
| Level 1(r = .05, F = 0.20, ns) | |||
| PTPPA | −.05 | −.45 | ns |
| Level 2 (r = .44, F = 3.73, p < .01) | |||
| PTPPA | −.06 | −.66 | ns |
| Handedness | .40 | 3.88 | *** |
| Brain volume | −.31 | −2.35 | * |
| Age | −.11 | −1.14 | ns |
| Sex | −.15 | −1.16 | ns |
| Section thickness | −.02 | −.23 | ns |
ns, non-significant.
p < .05.
p < .001.
Fig. 3.
Planum temporale asymmetry (PTA) did not predict the language laterality index in males and females. The trend for greater leftward PTA and language laterality in non-right-handed males was due to the size of the right planum temporale. Post hoc analyses demonstrated that a right planum temporale was associated with greater right hemisphere representation for language in non-right-handed males only. Filled diamonds represent right-handers. Open squares represent non-right-handers.
We also categorized the planum temporale and language laterality measures to determine if there were qualitative associations between these measures that were not captured by the linear regressions above. The participants were categorized into three groups depending on whether their PTA, PTPPA, and the language laterality index fell within a distribution of scores that were within or beyond ±1 standard deviation from the mean of each measure. Chi-square analyses between the PTA () and PTPPA groups () with the language laterality groups were not significant.
Variation in the degree of PTA has been attributed to variation in the size of the right planum temporale (Galaburda, Rosen, & Sherman, 1990). In addition, a study by Foundas et al. (1994) suggests that PTA predicts right hemisphere language laterality in non-right-handed individuals. Post hoc analyses were performed in the non-right-handed sample to determine if the left and right hemisphere planum temporale measures predicted language laterality. Left hemisphere language laterality was less likely in males with a large right PT (r(20) = −.44, p < .05 uncorrected), but no such relationship with language laterality was observed for the right PTPP (r(20) = −.33, ns). A similar relation between language laterality and the right PT was not observed in females (r(23) = .33, ns). Language laterality was not associated with the left PT or left PTPP measures in either males or females.
4. Discussion
The goal of this study was to test the longstanding hypothesis that left hemisphere lateralization for language is associated with leftward asymmetry of the planum temporale. Surface area measures of the planum temporale and functional activation during a single-word comprehension task both demonstrated significant left hemisphere asymmetries, but were unrelated. These results are consistent with a recent study showing no association between volumetric and voxel based measures of planum temporale asymmetry and language dominance, as measured with the Wada test in epilepsy patients (Dorsaint-Pierre et al., 2006). The results from our study of normal individuals suggest either that planum morphology is unrelated to language laterality or that planum morphology predicts another processing dimension of language laterality not measured by the fMRI task used here.
Classic models of language organization, based on studies of patients with aphasia, implicate a network of perisylvian structures in expressive and receptive language. These models postulate a one-way projection from receptive language areas in the posterior superior temporal gyrus (including the planum temporale) and the inferior parietal lobule to expressive language areas in the inferior frontal gyrus (Heilman & Valenstein, 2003). Some support for such a projection is provided by a functional imaging connectivity study of language production that demonstrated that activation in all of the above brain regions was correlated during speech (Horwitz & Braun, 2004). However, the posterior superior temporal gyrus is rarely activated in child and adult fMRI studies of language when the experiment is designed to examine activation specific to language after controlling for auditory features of oral language stimuli (Ahmad, Balsamo, Sachs, Xu, & Gaillard, 2003; Balsamo et al., 2002; Binder et al., 2000; Binder et al., 1996a, 1996b; Scott, Blank, Rosen, & Wise, 2000).
The present study involved a level of semantic analysis that required accessing world knowledge after spoken word identification. Although this task requires phonological analysis of spoken lexical items, it does not require lexical phonological retrieval or production, which some authors have argued are processes in which the posterior superior temporal gyrus and planum temporale play a particular role (Hickok et al., 2000; Indefrey & Levelt, 2004). The single-word comprehension task used in this study elicits activation within the superior temporal sulcus and middle temporal gyrus rather than the superior temporal gyrus, in comparison to activation for a tone decision task (Binder et al., 1997; Binder et al., 1996a, 1996b). This pattern is consistent with the finding that electrical stimulation of the middle temporal gyrus in epilepsy patients produces auditory comprehension deficits but spares repetition and naming (Boatman et al., 2000), and with a large lesion-deficit correlation study in chronic aphasic patients, which showed a relationship between impaired sentence comprehension and middle temporal gyrus damage but not posterior superior temporal gyrus damage (Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004). While the posterior superior temporal gyrus appears to be important for processing frequency modulated auditory stimuli (Hart, Palmer, & Hall, 2004) and the fast-formant changes of human speech (Belin et al., 1998), it does not appear to be specifically involved in processing the semantics of language.
The non-significant association between planum temporale asymmetry and the language laterality index may not be surprising considering that the single-word comprehension task does not elicit superior temporal gyrus activation after controlling for activation related to low-level auditory processing. A significant association between left planum size and PET activation in the superior temporal gyrus has been observed for a story listening task that included a rest condition as the baseline contrast (Josse et al., 2003; Tzourio et al., 1998). The authors hypothesized that the association was due to the posterior superior temporal gyrus' role in perceptual representation of spoken language. It is possible that we would have observed a similar association using a resting condition contrast, but this approach would not have controlled for activation related to motor function, auditory processing, short-term memory, and attention. A strength of our task design was the isolation of linguistic representations from these other processes, an approach that produces lateralization results very similar to Wada testing (Binder et al., 1996b). Given the well documented leftward bias in planum morphology (which was further confirmed in this study), however, we predict that planum morphology correlates with other dimensions of language laterality not indexed by the single-word comprehension task.
Planum temporale morphology may predict laterality for lexical retrieval and production, processes that were not indexed in this study. Chiarello et al. (2004) examined the association between planum temporale asymmetry, using identical measurement techniques, and visual-half field measures of laterality. The planum temporale asymmetry of their sample composed only of male subjects (mean = .51, sd = .54) was similar to the planum temporale asymmetry of males in this study (mean = .44, sd = .63). Laterality was defined based on reaction times and accuracy for orthographic stimuli presented to the left versus right visual field for real word naming, non-word naming, and verb generation tasks. A left hemisphere advantage in reaction time and accuracy for the naming tasks was associated with leftward planum temporale asymmetry. Verb generation reaction time and accuracy, a semantically-loaded cognitive task more similar in nature to the one used in this study, was not associated with planum temporale morphology.
In addition to the dimension of language examined, hand preference may influence associations between planum morphology and language laterality. Post hoc analyses weakly suggested that right hemisphere language was related to a large right planum temporale in non-right-handed males. This relation did not survive post hoc correction for statistical significance, but the relation is consistent with an interpretation, based on previous studies (Blonder et al., 1994; Foundas et al., 1994; Moffat et al., 1998), that variation in right planum morphology reflects the degree of language laterality for individuals who have mixed laterality for language and handedness. A sample composed of greater number of individuals with mixed laterality for language and handedness may be necessary to observe this association. We did not observe strong associations between right planum size and the language laterality index across the combined sample of non-right-handed males and females, however.
The inconsistent associations reported between measures of language laterality, handedness, and planum morphology suggest that additional variable(s) influence the relation between these measures. Attention to space (auditory or visual) is a lateralized function that has rarely been examined in studies of the planum temporale. Laterality for attention and language are typically inversely associated and left-handed adults are more likely than right-handed adults to demonstrate mixed or atypical laterality for attention and language (Floel, Buyx, Breitenstein, Lohmann, & Knecht, 2005). Interestingly, functional imaging and lesion studies demonstrate the importance of the right posterior superior temporal gyrus for attention to auditory and visual space (Hillis et al., 2005; Krumbholz et al., 2005). An association between planum morphology and sensitivity and/or laterality for attention to auditory space could partly explain variation in the size of the right planum and influence associations between planum morphology, handedness, and laterality. In support of this premise, associations between laterality and planum morphology have been observed when the laterality task involved attention to left or right visual space (Chiarello et al., 2004; Hellige et al., 1998).
If planum temporale morphology is not a causal factor in left hemisphere language organization for single-word comprehension, then what neurobiological factors drive the development of laterality for semantic representation? The laterality task used in this study engages frontal, temporal, parietal, and cerebellar regions, supporting the notion that language laterality is a multidimensional construct (Josse et al., 2003). Variation in the development of each of the anatomical regions engaged in a laterality task, and the strength of their connectivity, could contribute to the degree of language laterality indexed by a particular task. For example, the degree of leftward white matter asymmetry for fiber bundles coursing near the superior temporal sulcus or middle frontal gyrus may relate to the degree of language laterality (Herve, Crivello, Perchey, Mazoyer, & Tzourio-Mazoyer, 2006).
4.1. Summary
The results of this study provide no support for the idea that the degree of planum temporale asymmetry influences language laterality, as defined by a single-word comprehension task. This does not preclude an association between planum morphology and other measures of behavioral asymmetry. Hemispheric asymmetries for perceptual representation of complex sounds, language production, or even attention to space may relate to planum temporale asymmetry. The findings do, however, question the widely held belief that planum temporale asymmetry is the foundation of a left hemisphere dominance for the production and reception of language.
Acknowledgments
The authors thank the participants in this study, the National Science Foundation (BCS 0224260), the National Institute for Neurological Disorders and Stroke (R01 NS/DC33576-06), and the National Center for Research Resources (C06 RR014516).
References
- Aboitiz F, Scheibel AB, Zaidel E. Morphometry of the sylvian fissure and the corpus callosum, with emphasis on sex differences. Brain. 1992;115(Pt 5):1521–1541. doi: 10.1093/brain/115.5.1521. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, et al. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Archives of Neurology. 2002;59(7):1168–1174. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- Baxter LC, Saykin AJ, Flashman LA, Johnson SC, Guerin SJ, Babcock DR, et al. Sex differences in semantic language processing: a functional MRI study. Brain and Language. 2003;84(2):264–272. doi: 10.1016/s0093-934x(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure MC, et al. Lateralization of speech and auditory temporal processing. Journal of Cognitive Neuroscience. 1998;10(4):536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- Best M, Demb JB. Normal planum temporale asymmetry in dyslexics with a magnocellular pathway deficit. Neuroreport. 1999;10(3):607–612. doi: 10.1097/00001756-199902250-00030. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, et al. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996a;119(4):1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996b;46(4):978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Pettigrew LC, Smith CD. Anomalous cerebral dominance: neurobehavioral studies and three-dimensional magnetic resonance imaging. Neuropsychiatry, Neuropsychology and Behavioral Neurology. 1994;7:41–50. [Google Scholar]
- Boatman D, Gordon B, Hart J, Selnes O, Miglioretti D, Lenz F. Transcortical sensory aphasia: revisited and revised. Brain. 2000;123(Pt 8):1634–1642. doi: 10.1093/brain/123.8.1634. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siege de la faulte du langage articule, suivies d une observation d aphemie (perte de la parole) Bulletins de la Societe Anatomique. 1861;36:330–357. [Google Scholar]
- Chiarello C, Kacinik N, Manowitz B, Otto R, Leonard C. Cerebral asymmetries for language: evidence for structural-behavioral correlations. Neuropsychology. 2004;18(2):219–231. doi: 10.1037/0894-4105.18.2.219. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Cox RW. Afni: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhune VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, et al. Asymmetries of the planum temporale and Heschl's gyrus: relationship to language lateralization. Brain. 2006;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr., Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lombardino LJ, Leonard CM. Planar asymmetry tips the phonological playground and environment raises the bar. Child Development. 2001;72(4):988–1002. doi: 10.1111/1467-8624.00330. [DOI] [PubMed] [Google Scholar]
- Floel A, Buyx A, Breitenstein C, Lohmann H, Knecht S. Hemispheric lateralization of spatial attention in right- and left-hemispheric language dominance. Behavioural Brain Research. 2005;158(2):269–275. doi: 10.1016/j.bbr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32(10):1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: do right- and left handers differ? Brain and Language. 2002;83(3):403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphologic cerebral asymmetries and handedness. The pars triangularis and planum temporale. Archives of Neurology. 1995;52(5):501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, et al. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122(Pt 2):199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28(6):529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1997;40(6):1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Sperry RW. Language after section of the cerebral commissures. Brain. 1967;90(1):131–148. doi: 10.1093/brain/90.1.131. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161(837):186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Archives of Neurology. 1997;54(2):171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- Hart HC, Palmer AR, Hall DA. Different areas of human non-primary auditory cortex are activated by sounds with spatial and nonspatial properties. Human Brain Mapping. 2004;21(3):178–190. doi: 10.1002/hbm.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiervang E, Hugdahl K, Steinmetz H, Inge Smievoll A, Stevenson J, Lund A, et al. Planum temporale, planum parietale and dichotic listening in dyslexia. Neuropsychologia. 2000;38(13):1704–1713. doi: 10.1016/s0028-3932(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Clinical neuropsychology. 4th ed. Oxford University Press; Oxford: 2003. [Google Scholar]
- Hellige JB, Taylor KB, Lesmes L, Peterson S. Relationships between brain morphology and behavioral measures of hemispheric asymmetry and interhemispheric interaction. Brain and Cognition. 1998;36(2):158–192. doi: 10.1006/brcg.1997.0951. [DOI] [PubMed] [Google Scholar]
- Herve PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. 2006;29(4):1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hickok G, Erhard P, Kassubek J, Helms-Tillery AK, Naeve-Velguth S, Strupp JP, et al. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: implications for the explanation of conduction aphasia. Neuroscience Letters. 2000;287(2):156–160. doi: 10.1016/s0304-3940(00)01143-5. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience. 2005;25(12):3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt NA, Musick A, Barta PE, Pearlson GD. Measurement of the planum temporale (PT) on magnetic resonance imaging scans: temporal PT alone and with parietal extension. Psychiatry Research. 2000;98(2):103–116. doi: 10.1016/s0925-4927(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Braun AR. Brain network interactions in auditory, visual and linguistic processing. Brain and Language. 2004;89(2):377–384. doi: 10.1016/S0093-934X(03)00349-3. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jancke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. Neuroreport. 1994;5(9):1161–1163. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- Jancke L, Steinmetz H. Auditory lateralization and planum temporale asymmetry. Neuroreport. 1993;5(2):169–172. doi: 10.1097/00001756-199311180-00019. [DOI] [PubMed] [Google Scholar]
- Josse G, Mazoyer B, Crivello F, Tzourio-Mazoyer N. Left planum temporale: an anatomical marker of left hemispheric specialization for language comprehension. Brain Research Cognitive Brain Research. 2003;18(1):1–14. doi: 10.1016/j.cogbrainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Black SE, Polk M, Howell J. Cerebral asymmetries on magnetic resonance imaging. Cortex. 1986;22(1):117–127. doi: 10.1016/s0010-9452(86)80036-3. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Buchel C. Alternatives to the Wada test: a critical view of functional magnetic resonance imaging in preoperative use. Current Opinion in Neurology. 2005;18(4):418–423. doi: 10.1097/01.wco.0000170242.63948.17. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Sex-linked differences in the anatomy of the perisylvian language cortex: a volumetric MRI study of gray matter volumes. Neuropsychology. 2004;18(4):738–747. doi: 10.1037/0894-4105.18.4.738. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Schonwiesner M, von Cramon DY, Rubsamen R, Shah NJ, Zilles K, et al. Representation of interaural temporal information from left and right auditory space in the human planum temporale and inferior parietal lobe. Cerebral Cortex. 2005;15(3):317–324. doi: 10.1093/cercor/bhh133. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl's gyrus and the planum temporale. Cerebral Cortex. 1994;4(2):107–118. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the wada test. Neurology. 2000;54(8):1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Lombardino LJ, Mercado LR, Browd SR, Breier JI, Agee OF. Cerebral asymmetry and cognitive development in children: a magnetic resonance imaging study. Psychological Science. 1996;7:79–85. [Google Scholar]
- Mazoyer BM, Tzourio-Mazoyer NG. Planum temporale asymmetry and models of dominance for language: a reappraisal. Neuroreport. 2004;15(6):1057–1059. doi: 10.1097/00001756-200404290-00025. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in human brain organisation: a critical survey. Behavioral and Brain Sciences. 1980;3:215–227. [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right hemisphere speech representation. Brain. 1998;121(Pt 12):2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, 3rd, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127(Pt 8):1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Volkmann J, Jancke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Annals of Neurology. 1991;29(3):315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Nkanga-Ngila B, Mazoyer B. Left planum temporale surface correlates with functional dominance during story listening. Neuroreport. 1998;9(5):829–833. doi: 10.1097/00001756-199803300-00012. [DOI] [PubMed] [Google Scholar]
- Vikingstad EM, George KP, Johnson AF, Cao Y. Cortical language lateralization in right handed normal subjects using functional magnetic resonance imaging. Journal of Neurological Sciences. 2000;175(1):17–27. doi: 10.1016/s0022-510x(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Des aphasiche symptomenkomplex. Cohn and Weigart; Breslau: 1874. [Google Scholar]
- Westbury CF, Zatorre RJ, Evans AC. Quantifying variability in the planum temporale: a probability map. Cerebral Cortex. 1999;9(4):392–405. doi: 10.1093/cercor/9.4.392. [DOI] [PubMed] [Google Scholar]