Abstract
Background
FcɛRI expression by monocytes can affect monocyte function via multiple mechanisms, thereby potentially influencing the generation of allergic inflammation. Previous studies on the in vivo regulation of monocyte FcɛRI expression by ambient IgE have yielded conflicting results.
Objective
We hypothesized that monocyte FcɛRI expression is limited to a specific monocyte subset and that within that subset FcɛRI surface expression is correlated to serum IgE.
Methods
Study 1: Blood was obtained from nonallergic subjects (n = 14) and subjects with allergic asthma (n = 18), hypereosinophilic syndrome (n = 2), hyper-IgE syndrome (n = 6), and helminth infection (n = 4). Study 2: Blood was obtained from allergic subjects in a clinical trial of omalizumab before and during study drug treatment. Monocyte surface FcɛRI expression was measured using flow cytometry.
Results
FcɛRI expression was significantly greater in the CD2high vs. CD2low monocyte subsets (31% vs. 1.9% median FcɛRI+, respectively). In asthmatic and non-atopic healthy control subjects, CD2low monocytes expressed little or undetectable FcɛRI. In study 1, FcɛRI expression was highly correlated to serum IgE in the CD2high, but not in the CD2low monocyte subpopulation (R values of 0.67 and 0.41, respectively). In study 2, omalizumab but not placebo, caused a significant and sustained drop in FcɛRI expression within the CD2high monocyte subset.
Conclusions
CD2 defines a monocyte subset with high FcɛRI expression, the magnitude of which is highly correlated to serum IgE. As such, this new description of CD2high monocytes as FcɛRI bearing cells suggests that they may be potential targets of anti-IgE immunomodulatory therapies.
Keywords: Monocyte, FcɛRI, IgE, antigen presenting cell, CD2, allergy, omalizumab, allergy, asthma, flow cytometry
Abbreviations: APC: Antigen presenting cell, DC: Dendritic cell, FcɛRI: High affinity IgE receptor, MEPE: Molecules of equivalent phycoerythrin
Introduction
The high-affinity IgE receptor (FcɛRI) and allergen specific IgE play an essential role in mast cell and basophil activation, leading to immediate-type hypersensitivity. Antigen presenting cells (APCs), such as monocytes and dendritic cells (DCs), also express FcɛRI[1–6]. FcɛRI expression by APCs can increase the efficiency of allergen presentation to T cells up to 1000-fold, through an antigen focusing mechanism in which FcɛRI-bound IgE on APCs selectively captures allergen[7]. In this manner, FcɛRIα expression by APCs may augment the activation and differentiation of allergen-specific Th2 cells, thereby increasing allergic inflammation[8]. Additionally, FcɛRI cross-linking on monocytes causes NF-κB activation[9], prevention of apoptosis[10], and induction of IL-10 expression, which then inhibits monocyte differentiation into DC[11]. Lastly, monocytes are the precursors of both DCs and macrophages, and thus FcɛRI expression by monocytes may have the potential to influence the generation of allergic inflammation through multiple mechanisms.
IL-4 and IL-13 increase monocyte FcɛRIα expression in vitro[12] and may play a major role in the increased expression found on monocytes obtained from atopic patients. In vitro studies of FcɛRI transfected cell lines have shown that IgE binding to FcɛRIα stabilizes the complex resulting in greater FcɛRI surface expression[13]. This phenomenon is borne out in human disease in which there is a strong correlation between serum IgE and FcɛRIα surface expression by basophils and DCs[14]. This suggests that the increased monocyte FcɛRI expression found in allergic disease is at least in part a result of the increased serum IgE that is characteristic of these disorders[3, 15, 16]. However, a study that carefully examined monocyte FcɛRIα expression in non-allergic patients with highly elevated serum IgE concentrations found no correlation between monocyte FcɛRI expression and serum IgE[17]. This latter study suggests that monocyte surface FcɛRIα expression is independent of ambient IgE concentrations and thus regulated in a fundamentally different manner from that of other FcɛRI expressing cells.
Monocytes are defined by their characteristic morphology and cell surface phenotype. Recently it has been appreciated that monocytes, although morphologically uniform, are composed of subsets with different capacities for lineage differentiation, tissue homing and contribution to tissue inflammation[18, 19]. These subsets have largely been phenotypically defined using cell surface markers including CD2, CD14, CD16, CD64 and chemokine receptor expression. In specific, CD16+, CX3CR1+ monocytes differentiate into tissue homing resident macrophages found in uninflammed spleen, liver and lungs. In contrast, CD16−, CCR2+ monocytes are the precursors of macrophage and DC populations found in inflammatory sites[18, 20]. Recently, it has been reported that the CD16+, CD64− monocyte subpopulation is increased in the blood of patients with atopic dermatitis[21].
However, previous studies of monocyte FcɛRI expression have examined the total monocyte population without subset analysis and were technically limited by the use of unconjugated low signal:noise FcɛRIα mAbs, that in some cases recognized an FcɛRI epitope that competed with IgE binding, and required acid stripping to remove bound IgE. The conflicting results in the literature thus led us to carefully reexamine the issue of monocyte FcɛRI expression using multiparameter flow cytometry and a directly labeled high signal:noise non-competitive anti-FcɛRIα mAb[22] to examine FcɛRI expression. We used two complementary clinical approaches to examine the relationship between serum IgE and monocyte FcɛRI : in study 1, we investigated patient populations with a 1000-fold range of serum IgE concentrations and in study 2, we employed a clinical trial of omalizumab, in which the serum IgE concentration was reduced 10–30 fold. In this work, we localize FcɛRIα expression to a subpopulation of CD2high human monocytes, and using the above clinical approaches, we demonstrate a high correlation between serum IgE and FcɛRI expression within the CD2high monocyte subpopulation. These findings explain the conflicting results from previous studies and demonstrate that surface FcɛRI expression by monocytes is, in fact, regulated in a similar manner to other hematopoietic cells, such as basophils, mast cells and DC.
Methods
Antibodies
Anti-FcɛRIα (clone AER-37, originally described as CRA1 [22]) PE, APC, biotin was obtained form eBiosciences, San Diego, CA. This mAb does not compete with IgE for FcɛRI binding and thus is an accurate means to assess surface FcɛRIα expression. Anti-IgE phycoerythrin (PE) (clone G7-26); CD123/anti–IL-3R phycoerythrin (PE), PE/cyanin-5 (PE/Cy5), biotin; mouse IgG1 unlabeled, biotin, PE; mouse IgG2b unlabeled, PE, PE/Cy5; mouse IgG2a PE; streptavidin-PE; CD64 (clone 10.1) biotin, PE; HLA-DR PE, PE/Cy5; CCR3 (clone 1C6) PE; CCR4 (clone 1G1) PE, CCR5 (clone 2D7/CCR5) PE; CCR6 (clone 11A9) PE were obtained form BD-PharMingen Corporation, San Diego, CA. Anti-CD14 (clone RM052) FITC, APC; CD2 unlabeled (clone T11) (Immunotech, Marseille, France); CCR2 (clone 48607) PE; CCR7 (clone 150503) PE (R&D Systems Inc, Minneapolis, MN.); CD1c/BDCA-1 biotin, PE (clone AD5-8E7); BDCA-2 PE (clone AC144); BDCA-3 (clone AD5-14H12) PE (Militenyi Biotec, Auburn, CA); Goat-anti-Mouse IgG1 Alexa 647; streptavidin-APC (Molecular Probes, Eugene, OR); anti-CD16 (clone 3G8) FITC (BioSource, Camarillo, CA) were obtained commercially. The basophil granule-specific mAb 2D7[23] was a gift from Dr. Lawrence Schwartz (Virginia Commonwealth University, VA).
Reagents
Paraformaldehyde, dimethyl sulfoxide, histopaque-1077 and -1088 (Sigma Chemicals, St. Louis, MO) and Sphero Rainbow Calibration Particles (BD-PharMingen) were obtained commercially.
Study subjects and cells
Study 1
Eighteen allergic asthmatic subjects (Figs. 1–5) met American Thoracic Society criteria for the diagnosis of asthma[24], had either moderate or severe asthma as defined by the National Asthma Education Prevention Program, had 3 or more positive skin test responses (≥3 mm induration over glycerol negative control) out of a panel of 10 aeroallergens, and were not experiencing an exacerbation at the time of the study. Thirteen of these asthmatic subjects were using inhaled corticosteroid therapy; none were receiving systemic corticosteroid therapy. Fourteen healthy control subjects had no history of allergic disease or asthma and had negative skin tests (≤2 mm induration) to the panel of aeroallergens. The helminth infected patients had intestinal helminths (n=1), onchocerciasis (n=2) and loiasis (n=1). The hyper IgE recurrent infection (Job’s syndrome) patients had a history of multiple characteristic infections and elevated IgE. The 2 hypereosinophilic syndrome patients met established criteria[25].
Figure 1. Identification of CD14+, FcɛRIα+ cells as monocytes.
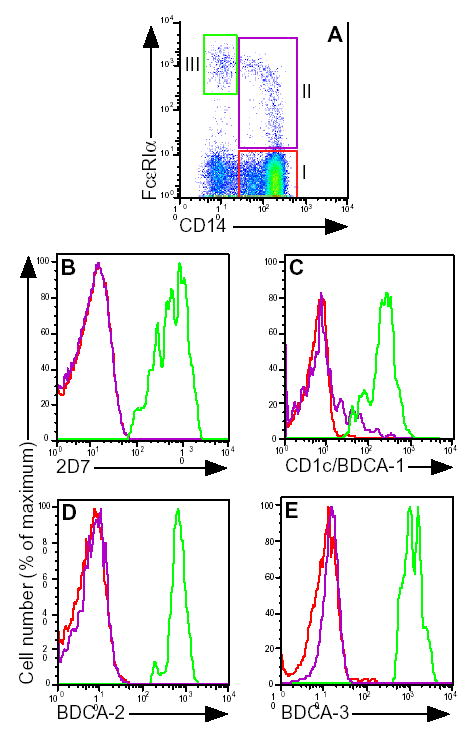
A, after gating on typical monocyte scatter, a CD14 vs. FcɛRI dot plot was generated. Gates for FcɛRI− (I) and FcɛRI+ monocytes (II) and basophils/DC (III) are shown. Expression of B, 2D7; C, CD1c/BDCA-1; D, BDCA-2; and E, BDCA-3, after gating on the FcɛRI− (red histograms) or FcɛRI+ (purple histograms) monocyte, basophil (green histogram in B) or DC (green histograms in C–E) populations. Results shown are representative of 3 non-atopic control and 3 allergic asthmatic subjects.
Figure 5. Monocyte FcɛRIα expression as a function of disease category.
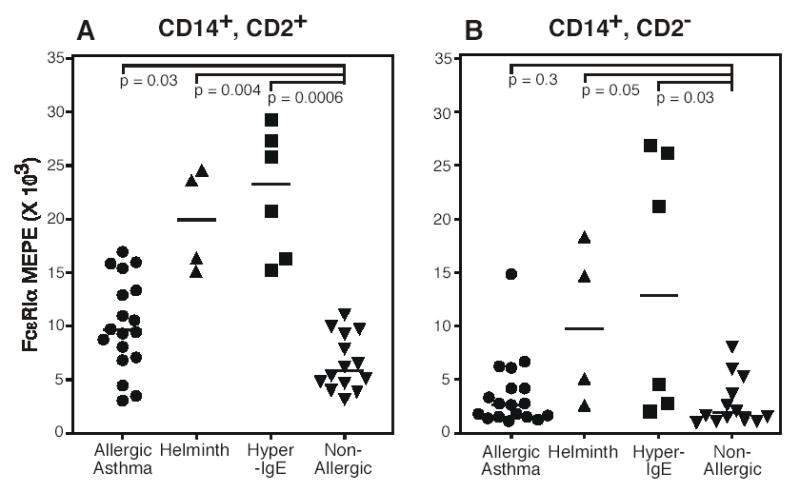
Surface FcɛRIα expression by CD2high (A) and CD2low (B) monocytes was determined and plotted against diseases category. Subject information is detailed in table I. Each symbol represents a unique subject. Horizontal bars denote median values. Statistical significance was determined using the Mann-Whitney U test.
Study 2
The omalizumab clinical study (Fig. 6) was a randomized, double blind, placebo-controlled clinical trial of 24 subjects (16 omalizumab and 8 placebo) with seasonal allergic rhinitis, as reported[5, 26]. Subjects with a history of ragweed-induced seasonal allergic rhinitis, a positive ragweed skin test, and a serum IgE concentration of less than 700 IU/mL were enrolled. Subjects were randomly assigned to receive either omalizumab (0.016 mg·kg−1·IU−1·mL−1 of IgE) or placebo on days 0 and 28. At the completion of the trial, the data were analyzed in a blinded fashion. Monocyte FcɛRIα expression was determined by flow cytometry on day 0 (baseline) and on days 7, 14, 28, and 42.
Figure 6. Omalizumab downregulates FcɛRIα expression selectively in CD2high monocytes.
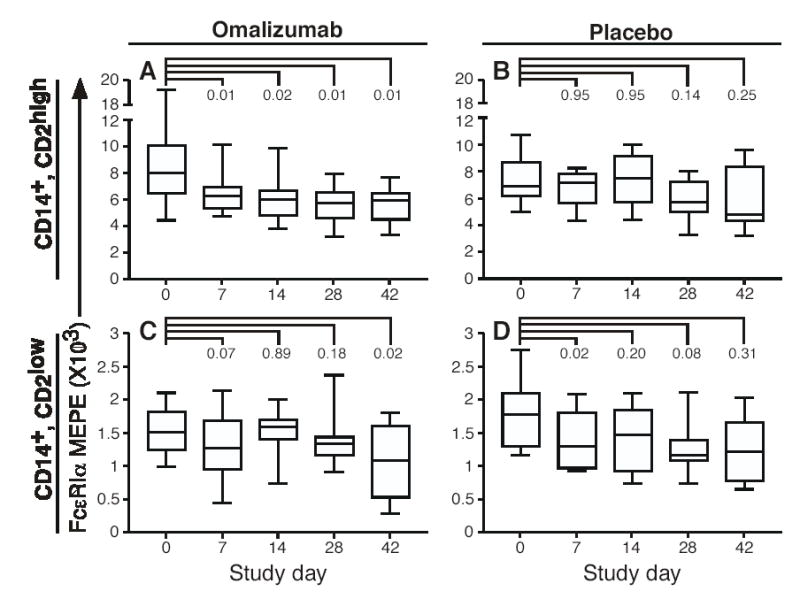
A–D, Box-and-whisker plots showing FcɛRIα expression in CD2high (top) and CD2low monocytes plotted for each study visit. The horizontal middle line represents the median, the top and bottom lines represent the quartile values, and the T bars represent the maximum and minimal values. Statistical significance was determined by using the Wilcoxon signed-rank test.
The clinical protocols for studies 1 and 2 were approved by the National Institute of Allergy and Infectious Diseases and the Creighton University Institutional Review Boards, respectively. All subjects gave informed consent.
PBMC were isolated from EDTA anticoagulated blood by means of density gradient separation with either Histopaque-1077 (study 1, Figs 1–5) or Histopaque-1088 (study 2, Fig 6), fixed in 4% paraformaldehyde for 5 minutes at 37°C, and cryopreserved in 10% dimethyl sulfoxide/PBS at −80°C, according to published methods[27]. Serum IgE determinations (Fig 4, Table I) were performed by the National Institutes of Health Clinical Center Department of Laboratory Medicine using a chemiluminescence immunoassay.
Figure 4. CD2high monocyte FcɛRIα expression correlates with serum IgE.
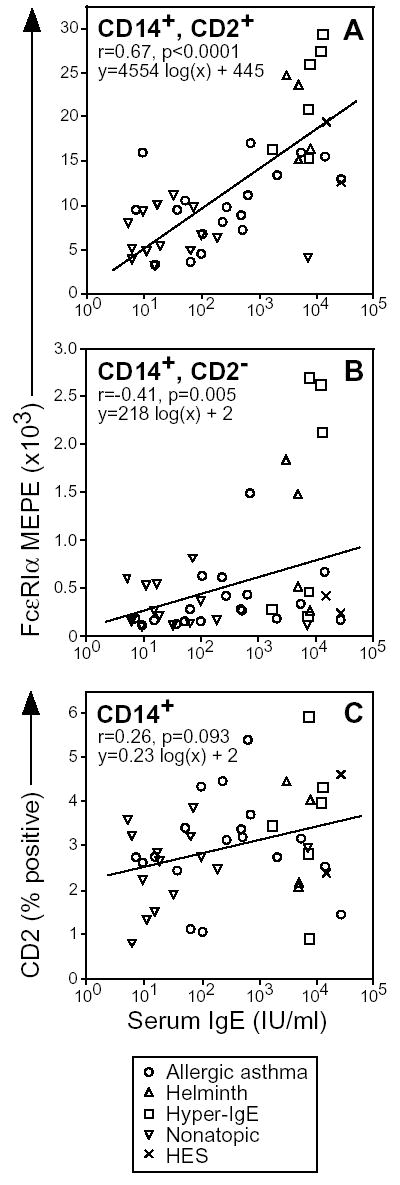
Surface FcɛRIα expression by CD2high (A) and CD2low (B) monocytes was plotted vs. serum IgE concentration. In (C) the percentage of monocytes staining CD2high, FcɛRI+ was plotted vs. serum IgE. Subject information is detailed in table I. Each symbol represents a unique subject. Correlation of FcɛRI and serum IgE was determined using the Spearman rank correlation test. The line fitting these results was determined using linear regression analysis.
Table I.
Subject characteristics
| Diagnosis | Number of subjects | Age (years) | Sex (M/F) | Serum IgE (IU/ml) |
|---|---|---|---|---|
| Allergic Asthma | 18 | 36 | 10/8 | 361 |
| Non-atopic Healthy Control | 14 | 46 | 9/5 | 16 |
| Hyper-IgE/Job’s | 6 | 26 | 2/4 | 7188 |
| Helminth | 4 | 58 | 1/3 | 4780 |
| Hypereosinophilic | 2 | 29 | 1/1 | 20,212 |
Values for age and serum IgE are median values for each subject group.
Antibody staining
Multiple combinations of antibodies were used in the experiments, employing a nonpermeabilizing adaptation of described procedures[4, 27]. The following is an example of the staining for CD2, CD14, FcɛRIα and BDCA-1 that was used in figures 3–6. Cryopreserved fixed cells were thawed, washed once in PBS with 0.1% BSA (PBS/BSA), and then blocked in PBS/BSA/5% nonfat dry milk (PBS/BSA/milk) for 1 hour on ice. Cells were incubated with CD14 FITC, FcɛRIα or IgG2a PE, BDCA-1 biotin, and CD2 or IgG1 control in PBS/BSA/milk for 30 minutes at 4°C, washed twice in PBS/BSA, and incubated with Goat-anti-Mouse IgG1 Alexa 647 and streptavidin conjugated PE/Cy5.5 in PBS/BSA for 30 minutes, washed, and analyzed by flow cytometry. The CD2 mAb was an IgG1 isotype, the CD14, FcɛRI and BDCA-1 mAbs were Ig2a and were thus not recognized by the Goat-anti-Mouse IgG1 secondary antibody in pilot experiments (data not shown).
Figure 3. CD2high monocytes express high levels of surface FcɛRIα.
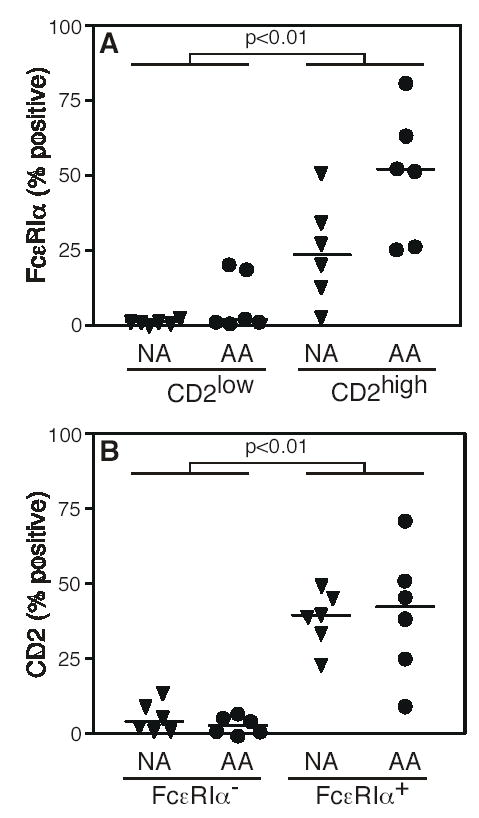
A, CD2low and CD2high monocytes from 6 allergic asthmatic (AA) and 6 healthy non-atopic healthy control (NA) subjects were analyzed for FcɛRI expression. B, FcɛRI− and FcɛRI+ monocytes from allergic asthmatic and healthy non-atopic control subjects were analyzed for CD2 expression. Each symbol represents a unique subject. Horizontal bars denote median values. Statistical significance was determined using the Mann-Whitney U test.
Flow cytometry
Data were acquired with a 2-laser, 4-parameter FACSCalibur flow cytometer (Becton-Dickinson Biosciences) and analyzed on Cellquest (Becton-Dickinson Biosciences) or FlowJo software (Tree Star, San Carlos, CA). Typically, 200,000 total events were acquired to obtain adequate numbers of FcɛRIα+ monocytes. FcɛRIα expression was quantitated as molecules of equivalent PE (MEPE) using Sphero Rainbow Calibration particles, as per the manufacturer’s instructions.
The CD14+, CD2high and CD14+, CD2low subsets were identified by first gating on CD14+ cells, then back gating on cells of the corresponding scatter, which yielded two distinct populations of cells differentially expressing CD2 and FcɛRI (Fig. 2E, F). The CD14+, CD2high population, although enriched for FcɛRI+ cells, contained a minority population of CD2bright, FcɛRI− cells (bold arrows in Fig. 2E, F), which in additional experiments were found to be largely CD3+ T cells (mean 84% CD3+, n=6 donors, 3 allergic asthma, 3 non-atopic control). Because these cells were not monocytes, in the additional experiments performed in figures 4–6, this population was excluded from the analysis of CD2high monocytes.
Figure 2. FcɛRIα expression by monocyte subpopulations.
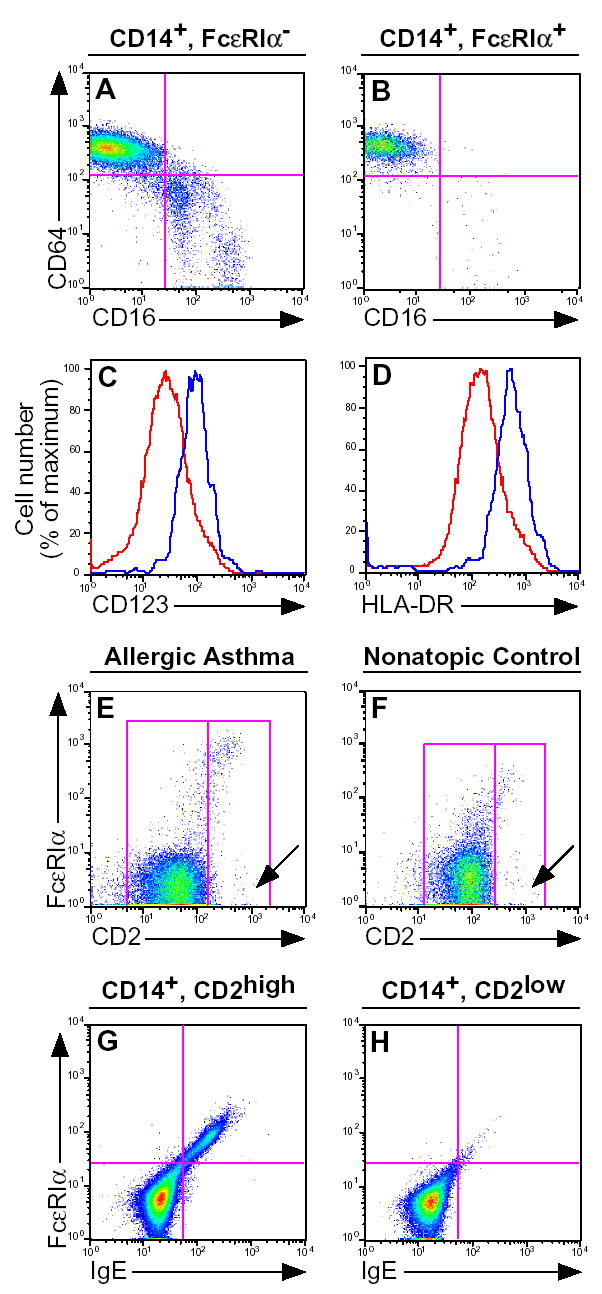
After gating on FcɛRI− (A) or FcɛRI+ (B) monocyte populations, a CD16 vs. CD64 dot plot was generated. FcɛRI− (red histogram) or FcɛRI+ monocytes (blue histogram) expression of CD123 (C) and HLA-DR (D). After gating on CD14+ monocytes from allergic asthmatic (E) and non-allergic (F) donors, a CD2 vs. FcɛRI dot plot was generated. After gating on (G) CD14+, CD2+ or (H) CD14+, CD2− monocytes from an allergic asthmatic donor, an FcɛRI vs. IgE dot plot was generated. Results shown are representative of 6 (A, B), 7 (C, D), 18 asthmatic and 14 non-atopic control (E, F) and 6 (G, H) subjects, respectively.
Statistical analysis
The Mann-Whitney U test was used to compare FcɛRIα expression between different subject groups (Figs. 2 and 5). The Spearman rank test was used to evaluate correlative data in figure 4. Paired data in figure 6 were analyzed using the Wilcoxon signed rank test. A P value of less than 0.05 was considered significant. Statistical calculations and linear regression analysis were performed with Prism software (GraphPad Software, San Diego, CA).
Results
To explore monocyte heterogeneity, we first examined FcɛRIα expression in monocytes as a function of CD14. As shown in figure 1A, the majority of monocytes stained negative for FcɛRIα (gate I) and a minority subpopulation (gate II) extended as a characteristic curved FcɛRI+ tail. A third population of CD14−, FcɛRIbright cells was noted (gate III), which upon further analysis consisted of basophils and DCs (data not shown). In subsequent experiments in Figs. 1–3, we define FcɛRI− and FcɛRI+ monocytes as the populations defined by gates I and II, respectively. These results demonstrate that monocytes consist of two populations: a majority or “mainstream” population with little or no FcɛRI expression and a second population consisting of a tail of FcɛRI+ cells extending 1–2 logs above the mainstream cluster.
Because basophils and DCs express FcɛRIα, we next sought to demonstrate that the FcɛRI+ monocyte population was not composed of either of basophils or DCs. Both the FcɛRI+ and FcɛRI− monocyte populations stained negatively for the basophil granule protein 2D7 (Fig. 1B) as well as for the BDCA-2 and BDCA-3 dendritic cell markers (Fig. 1D, E)[28]. Most of the FcɛRI+ monocytes were negative for the CD1c/BDCA-1 marker, however there was a shoulder of CD1c intermediate cells noted (Fig. 1C). These results demonstrate that the FcɛRI+ monocyte subpopulation is not contaminated by substantial numbers of other known FcɛRI bearing cell populations.
We next sought to determine if any previously reported monocyte subset markers could be used to better define the FcɛRI+ monocyte subset. The best accepted monocyte subsets consist of reciprocal CD16−, CD64+ and CD16+, CD64− subpopulations [18], both of which were found in the mainstream FcɛRI− monocyte population (Fig. 2A). In contrast, the FcɛRI+ monocyte subpopulation consisted almost entirely of cells expressing the CD16−, CD64+ phenotype (mean 92% of FcɛRI+ monocytes were CD16−, CD64+). However, because most monocytes are CD16−, CD64+, these markers did not further discriminate the FcɛRI+ monocyte subpopulation. We next examined HLA-DR and CD123, which identify different DC subsets and maturation stages[29] and found they were expressed at 2.7 and 2.0 greater levels, respectively, in the FcɛRI+ vs. FcɛRI− monocyte subsets (Fig. 2C and D), but did not sufficiently discriminate the FcɛRI+ monocyte population (data not shown). We further examined CCR2, CCR3, CCR4, CCR5, CCR6 and CCR7 and found none of these chemokine receptors were differentially expressed in the FcɛRI+ vs. FcɛRI− monocyte populations (data not shown).
CD2 is expressed at high levels by a subset of monocytes, which are capable of rapid differentiation into functional dendritic cells and may represent DC precursors[30–32]. We thus examined CD2 expression and found that FcɛRI was highly expressed on the CD2high monocyte subset (Figs. 2E and F). FcɛRI staining by CD2high monocytes was verified by demonstrating that FcɛRI+ cells stained concordantly for IgE (Fig. 2G), whereas no staining was found in CD2low monocytes (Fig. 2H).
We next analyzed the relative expression of FcɛRI in the CD2high and CD2low monocyte subsets to determine if CD2 could be used to better discriminate the FcɛRI+ subset. The CD2high monocyte subset contained a significantly greater fraction of FcɛRI+ cells than were present in the CD2low monocyte subset (39% vs. 1.9%, median) and this relationship was found in monocytes from both allergic asthmatic and non-atopic healthy control subjects (Fig. 3A). CD2high monocytes from allergic asthmatic donors contained greater numbers of FcɛRI+ cells relative to healthy non-atopic donors, and approached statistical significance (p = 0.065). Conversely, the FcɛRI+ monocyte subset contained a significantly greater fraction of CD2high cells, relative to the FcɛRI− subset (Fig. 3B). In sum, these data demonstrate that the CD2high monocyte subset is highly enriched for FcɛRI expressing cells and suggests that CD2 may be used as a marker to identify FcɛRI expressing monocytes.
Previous studies have shown that monocytes are capable of expressing FcɛRI, but have yielded conflicting results regarding the relationship between monocyte FcɛRI expression and serum IgE [16, 17]. We hypothesized that this discrepancy between studies could be due to the inability to accurately measure the small fraction of FcɛRI+ monocytes within the much larger pool of mainstream FcɛRI− monocytes. To address this, we next examined the relationship between serum IgE and FcɛRI expression within the CD2high and CD2low monocyte subsets.
We first used a similar study design to Saini et al[17], and studied subjects with serum IgE concentrations encompassing a 10,000-fold range. Five patient groups were studied: healthy non-atopic controls, allergic asthma, helminth infection, hyper-IgE (Job’s) syndrome and hypereosinophilic syndrome (Table I). All 4 disease cohorts had elevated serum IgE levels, but the latter three did not have allergic disease. Within the CD2high monocyte subset, FcɛRI expression correlated well with serum IgE (r= 0.67, Fig. 4A). In contrast, a lower correlation was found in the CD2low subset (r= 0.41, Fig. 4B). A subgroup analysis using only the allergic asthmatic and non-atopic control subjects yielded a significant correlation for the CD2high (r= 0.39, p= 0.028), but not the CD2low (r= 0.30, p= 0.99) monocyte subsets. We additionally examined the percentage of CD2high, FcɛRI+ monocytes (Fig. 4C), but did not find a significant correlation (r= 0.26, p=0.09). Repeat analyses of the CD2high monocyte FcɛRI MEPE from 6 asthmatic and 6 non-atopic control subjects acquired at separate time points over a year apart demonstrated a high degree of correlation (r= 0.71), demonstrating that this is a stable characteristic. These data demonstrate that FcɛRI expression and serum IgE are highly correlated in the CD2high but not the CD2low monocyte subset.
We then examined surface FcɛRIα expression as a function of disease status. In the CD2high monocyte subset, FcɛRI was expressed at significantly higher levels in all 3 disease categories relative to the non-atopic subjects (Fig. 5). In contrast, no such relationship to disease status was found for CD2low monocytes when comparing allergic asthma to non-atopic healthy control subjects. Interestingly, CD2low monocytes from both the helminth infected and hyper-IgE syndrome subjects had higher FcɛRI expression than those from the non-atopic control subjects, although this finding was less significant than that for the CD2high monocytes.
We next hypothesized that the correlation between monocyte FcɛRI and serum IgE found in figures 4 and 5 would be reiterated when serum IgE was therapeutically decreased 10–30-fold during a clinical trial of omalizumab[26]. Omalizumab caused a significant drop in surface FcɛRI expression in the CD2high monocyte subpopulation that was noted at all study time points (Fig. 6A). In contrast, the change in FcɛRI expression within the CD2low population was not significant at any of the first 3 times points measured (days 7, 14 and 28), but did reach statistical significance at the single day 42 time point (Fig. 6C). The drop in FcɛRI expression during omalizumab treatment was 22% and 11% for the CD2high and CD2low monocyte subsets, respectively. These data demonstrate that therapeutic reduction in serum IgE causes a reduction in FcɛRIα surface expression in the CD2high monocyte subset.
Discussion
Monocytes express low levels of FcɛRI, however the relative magnitude of this expression and its relationship to serum IgE concentration has been disputed. In this report, we demonstrate for the first time that FcɛRIα surface expression is limited to the CD2high monocyte subset, and that FcɛRI expression by this monocyte subset is highly correlated to serum IgE concentration. These results demonstrate that FcɛRI surface expression by this subset of CD2high monocytes responds to ambient IgE levels similarly to that shown previously in other FcɛRI bearing cells.
In basophils,[17] dendritic cells,[5] and mast cells[33] in vivo, as well as in in vitro studies of transfected cell lines[13] and monocyte derived DC[34], surface FcɛRIα expression is highly dependent on ambient IgE concentration. These studies have led to the general understanding that surface FcɛRI is stabilized by IgE occupancy of the receptor, resulting in greater levels of surface FcɛRI expression. Sirha et al [16] found a high correlation between monocyte FcɛRI and serum IgE. However, a carefully performed study of monocytes found no correlation between serum IgE and monocyte FcɛRI, suggesting that the regulation of monocyte surface FcɛRI expression was singularly different from that of other cell lineages previously studied [17]. Our results in Figs. 1–3, demonstrating that FcɛRI surface expression is largely limited to a subset of CD2high monocytes, help explain the seeming contradiction of these previous reports. We found that surface FcɛRI expression by the majority CD2low monocyte subset was generally below the level of detection of our high signal:noise assay, and in that subset, FcɛRI expression was not responsive to ambient IgE levels. In contrast, as shown in Figs. 4–6, the CD2high monocyte subset expresses substantial amounts of surface FcɛRI, the expression of which is highly responsive to ambient IgE concentrations. These data support the concept that surface FcɛRI expression regulation by CD2high monocytes is regulated in a similar manner to other cell lineages studied.
We used two complementary clinical approaches, both of which demonstrate the IgE dependence of monocyte FcɛRI surface expression. First, in a similar manner to Saini et al[17], we utilized a cohort of subjects with a 4 log range of serum IgE concentrations (Figs. 4 and 5). Second, we utilized a clinical trial of omalizumab to decrease serum IgE (Fig. 6). The magnitude of reduction in FcɛRI expression induced by omalizumab was less than that seen for basophils and DCs analyzed from the same clinical trial [5, 26]. Further work is needed to determine if this finding is a technical artifact or represents actual differences in the biology of FcɛRI surface expression.
To more clearly define the FcɛRI+ monocyte population, we examined a number of different cell surface markers. In these experiments, CD2 was uniquely capable of identifying the FcɛRI+ monocyte subpopulation. However, despite our identification of the CD2high monocyte as being the major FcɛRI bearing monocyte population, some FcɛRI expressing monocytes were found in the CD2low population. This spillover may explain the higher levels of FcɛRI in CD2low monocytes from subjects with hyper-IgE syndrome or helminth infections. This finding may also explain the drop in FcɛRI expression in CD2low monocytes in the omalizumab treated group on study day 42. Future advances in understanding FcɛRI expression by monocyte subsets will require additional markers to more precisely identify this subpopulation.
CD2high monocytes rapidly acquire DC activity in vitro and may represent the immediate precursors to DC [30–32]. Given the capacity for CD2high monocytes to acquire DC activity, it may be argued that CD2high monocytes are simply DC themselves and thus, express FcɛRI in a similar manner to DC. However, FcɛRI+ monocytes did not express the DC specific BDCA-2 and BDCA-3 markers, supporting the conclusion that these FcɛRI+ monocytes are not simply CD14+ DC. A fraction of FcɛRI+ monocytes expressed intermediate levels of CD1c/BDCA-1, which although lower expression than that found on DCs, could indicate that these cells are in transition from the monocyte to DC lineages. Further investigation is needed to better define the lineage relationship between the FcɛRI+ monocyte subset, the mainstream FcɛRI− monocyte population, and DCs; as well as the role of the CD2high monocyte subset in allergic disease pathogenesis and as a target for immunomodulatory therapies. These results confirm and extend our previous findings on DCs[5] and suggest that surface FcɛRI is upregulated prior to monocyte differentiation into DCs.
FcɛRI expression by APCs increases the efficiency of antigen presentation of allergen to T cells via an antigen focusing mechanism by as much as 1000-fold[8]. Given that we have shown that the CD2high, FcɛRI+ monocyte is the dominant FcɛRI expressing monocyte subset, it is possible that these cells are responsible for IgE mediated antigen focusing by monocytes. Alternatively, crosslinking of FcɛRI on antigen presenting cells may induce expression of proinflammatory mediators and chemokines[8].
In conclusion, we have demonstrated high level FcɛRI expression by CD2high monocytes, and furthermore, that this FcɛRI expression is limited to the CD2high monocyte subset, and is highly correlated to serum IgE. These findings help explain the conflicting results of previous studies on the relationship of monocyte FcɛRI to serum IgE[3, 16, 17], and suggest that CD2high monocytes may be a potential target of anti-IgE immunomodulatory therapy [35].
Acknowledgments
The authors thank Leigh Bernardino, Linda Scott, Mary Huber, Victoria Anderson, Susan Foster and Melissa Law for procurement of clinical samples, and Drs. Henry Lin, D. Todd Griffith and Kevin Boesel for work on the omalizumab clinical trial.
Footnotes
Funding: NIAID Division of Intramural Research Grant # 1Z01-AI-000761-07. Genentech/Novartis funded the omalizumab clinical study and one of the authors, TBC.
References
- 1.Bieber T, de la Salle H, Wollenberg A, Hakimi J, Chizzonite R, Ring J, Hanau D, de la Salle C. Human epidermal Langerhans cells express the high affinity receptor for immunoglobulin E (Fc epsilon RI) J Exp Med. 1992;175:1285–90. doi: 10.1084/jem.175.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang B, Rieger A, Kilgus O, Ochiai K, Maurer D, Fodinger D, Kinet JP, Stingl G. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. 1992;175:1353–65. doi: 10.1084/jem.175.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin MH, Kilgus O, Kinet JP, Stingl G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–50. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–8. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–54. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Novak N, Allam JP, Hagemann T, Jenneck C, Laffer S, Valenta R, Kochan J, Bieber T. Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2004;114:364–70. doi: 10.1016/j.jaci.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Maurer D, Fiebiger S, Ebner C, et al. Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha- and Fc epsilon RI gamma-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157:607–16. [PubMed] [Google Scholar]
- 8.Novak N, Kraft S, Bieber T. Unraveling the mission of FcepsilonRI on antigen-presenting cells. J Allergy Clin Immunol. 2003;111:38–44. doi: 10.1067/mai.2003.2. [DOI] [PubMed] [Google Scholar]
- 9.Kraft S, Novak N, Katoh N, Bieber T, Rupec RA. Aggregation of the high-affinity IgE receptor Fc(epsilon)RI on human monocytes and dendritic cells induces NF-kappaB activation. J Invest Dermatol. 2002;118:830–7. doi: 10.1046/j.1523-1747.2002.01757.x. [DOI] [PubMed] [Google Scholar]
- 10.Katoh N, Kraft S, Wessendorf JH, Bieber T. The high-affinity IgE receptor (FcepsilonRI) blocks apoptosis in normal human monocytes. J Clin Invest. 2000;105:183–90. doi: 10.1172/JCI6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 12.Reischl IG, Dubois GR, Peiritsch S, Brown KS, Wheat L, Woisetschlager M, Mudde GC. Regulation of Fc epsilonRI expression on human monocytic cells by ligand and IL-4. Clin Exp Allergy. 2000;30:1033–40. doi: 10.1046/j.1365-2222.2000.00859.x. [DOI] [PubMed] [Google Scholar]
- 13.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001;167:1290–6. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 14.Saini SS, MacGlashan D. How IgE upregulates the allergic response. Curr Opin Immunol. 2002;14:694–7. doi: 10.1016/s0952-7915(02)00404-1. [DOI] [PubMed] [Google Scholar]
- 15.Reischl IG, Corvaia N, Effenberger F, Wolff-Winiski B, Kromer E, Mudde GC. Function and regulation of Fc epsilon RI expression on monocytes from non-atopic donors. Clin Exp Allergy. 1996;26:630–41. [PubMed] [Google Scholar]
- 16.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (Fc epsilon RI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 17.Saini SS, Klion AD, Holland SM, Hamilton RG, Bochner BS, Macglashan DW., Jr The relationship between serum IgE and surface levels of FcepsilonR on human leukocytes in various diseases: correlation of expression with FcepsilonRI on basophils but not on monocytes or eosinophils. J Allergy Clin Immunol. 2000;106:514–20. doi: 10.1067/mai.2000.108431. [DOI] [PubMed] [Google Scholar]
- 18.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 19.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/s1074-7613(03)00178-x. [DOI] [PubMed] [Google Scholar]
- 21.Novak N, Allam P, Geiger E, Bieber T. Characterization of monocyte subtypes in the allergic form of atopic eczema/dermatitis syndrome. Allergy. 2002;57:931–5. doi: 10.1034/j.1398-9995.2002.23737.x. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa S, Pawankar R, Suzuki K, Nakahata T, Furukawa S, Okumura K, Ra C. Functional expression of the high affinity receptor for IgE (FcepsilonRI) in human platelets and its’ intracellular expression in human megakaryocytes. Blood. 1999;93:2543–51. [PubMed] [Google Scholar]
- 23.Kepley CL, Craig SS, Schwartz LB. Identification and partial characterization of a unique marker for human basophils. J Immunol. 1995;154:6548–55. [PubMed] [Google Scholar]
- 24.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136:225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 25.Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975;54:1–27. [PubMed] [Google Scholar]
- 26.Lin H, Boesel KM, Griffith DT, Prussin C, Foster B, Romero FA, Townley R, Casale TB. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Foster B, Prussin C. unit 6.24, Detection of Intracellular Cytokines by Flow Cytometry. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology; Wiley: 2003. pp. 6.24.1–6..16. [Google Scholar]
- 28.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–20. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 30.Takamizawa M, Rivas A, Fagnoni F, Benike C, Kosek J, Hyakawa H, Engleman EG. Dendritic cells that process and present nominal antigens to naive T lymphocytes are derived from CD2+ precursors. J Immunol. 1997;158:2134–42. [PubMed] [Google Scholar]
- 31.Di Pucchio T, Lapenta C, Santini SM, Logozzi M, Parlato S, Belardelli F. CD2+/CD14+ monocytes rapidly differentiate into CD83+ dendritic cells. Eur J Immunol. 2003;33:358–67. doi: 10.1002/immu.200310010. [DOI] [PubMed] [Google Scholar]
- 32.Crawford K, Gabuzda D, Pantazopoulos V, Xu J, Clement C, Reinherz E, Alper CA. Circulating CD2+ monocytes are dendritic cells. J Immunol. 1999;163:5920–8. [PubMed] [Google Scholar]
- 33.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–30. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 34.Novak N, Tepel C, Koch S, Brix K, Bieber T, Kraft S. Evidence for a differential expression of the FcepsilonRIgamma chain in dendritic cells of atopic and nonatopic donors. J Clin Invest. 2003;111:1047–56. doi: 10.1172/JCI15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35:408–16. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]


