Abstract
The exocytic pathway provides a physical route through which newly synthesized secretory and membrane proteins are deployed to the eukaryote cell surface. For newly synthesized α1-antitrypsin (AAT), the modification of its asparagine-linked oligosaccharides by a slow-acting mannosidase partitions the misfolded monomer into the proteasomal degradation pathway. Herein, we asked whether, and how, modification by endoplasmic reticulum mannosidase I (ERManI) contributes to the preferential selection of the misfolded AAT monomer for proteasomal degradation. Transiently expressed mutant and WT AAT variants underwent rapid destabilization in response to an artificially elevated ERManI concentration in the murine hepatoma cell line, Hepa1a. Based on the mannosidase- and lactacystin-sensitive properties of intracellular turnover, a stochastic model is proposed in which the delayed onset of the glycan modification, relative to the duration of nonnative protein structure, coordinates the preferential degradation of the misfolded monomer and spares the native molecule from destruction. Newly synthesized endogenous transferrin underwent degradation in response to an elevated concentration of ERManI, whereas the nonglycosylated secretory glycoprotein albumin was not affected. Taken together, these findings indicate that efficient conformational maturation might function as the initial quality control standard for a broad population of glycoproteins.
The majority of physiological systems that contribute to health or disease are, at their core, protein-driven processes. In fact, aberrant protein conformational rearrangement is the underlying etiologic agent of many genetic disorders. In the early exocytic pathway, secretory and membrane proteins unable to acquire native structure after biosynthesis are eliminated by a collection of processes designated “endoplasmic reticulum-associated degradation” (ERAD) (1–8). Prominent molecular events currently under intense investigation include the retrograde translocation of substrates into the cytosol via the Sec61p channel, conjugation with ubiquitin, and subsequent degradation by the multicatalytic 26S proteasome (for reviews, see refs. 3 and 8).
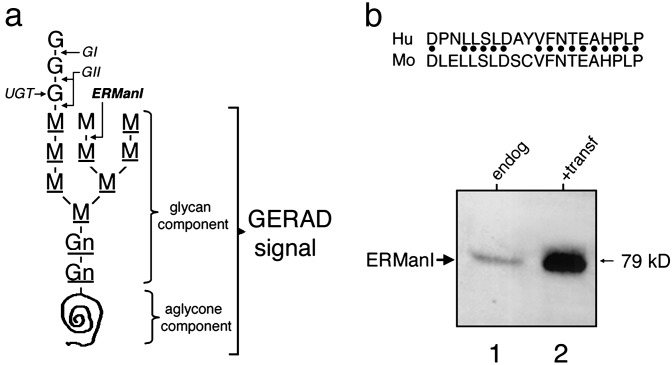
In recent years, the delineation of events functionally upstream of substrate recruitment in ERAD has become a focal point for biomedical investigations (4, 5). It is expected that the additional information will identify members of the global protein biosynthetic quality control network, most of which contribute to numerous loss- and gain-of-toxic-function disorders (6, 7). To this end, a picture has emerged in which asparagine-linked glycosylation (Fig. 1a) assists the productive folding of newly synthesized glycoproteins by facilitating their physical interaction with a small family of lectins that contribute to the glycoprotein folding machinery (9–11). Importantly, specific glycan modifications also provide a means by which an arsenal of oligosaccharide-processing enzymes and lectins mediate glycoprotein degradation in the absence of conformational maturation after biosynthesis (ref. 4 and references therein). The ability of α-1,2-mannosidase inhibition to arrest aberrant glycoprotein turnover indicates that glycoprotein ERAD (GERAD) is initiated through an early glycan-based proteasome-targeting event (4). The intracellular location of the glycan modification, its sensitivity to kifunensine (12, 13), and the results of yeast genetics (14) have implicated ER mannosidase I (ERManI) as the most likely cellular candidate responsible for generating the glycan-based component of a putative GERAD signal (4). ERManI generates the glycan-based signal determinant through the slow removal of a single mannose unit from asparagine-linked Man9GlcNAc2 (Fig. 1a). Nonnative protein structure is proposed to function as an additional, but aglycone (i.e., noncarbohydrate), GERAD signal determinant (4, 5). How the two components are used to accurately discriminate between slow folding and misfolded glycoproteins is not easily reconciled because nonnative structure persists during much of the biosynthetic maturation, even for WT glycoproteins (7). Currently, the conceptual understanding of how ERManI functions in GERAD, if it functions at all, is limited to a poorly defined timing mechanism, or molecular clock (ref. 4 and references therein).
Fig. 1.
Artificial elevation of the ERManI concentration in Hepa1a cells. (a) Shown is the 14-unit asparagine-linked oligosaccharide precursor that consists of glucose (G), mannose (M), and N-acetylglucosamine (Gn). The sites of hydrolysis by glucosidase I (GI), glucosidase II (GII), ER mannosidase I (ERManI), and the glucose transferred by UDP-glc:glycoprotein glucosyltransferase (UGT) are shown. Underlined sugar units depict the Man8B glycan-based component of the GERAD signal, whereas the aglycone (noncarbohydrate) determinant consists of nonnative protein structure. (b) Immunoblot of soluble Nonidet P-40 cell extracts 72 h posttransfection with ERManI/pCDNA3.1(Zeo+) (lane 2) or when mock transfected with the empty vector (lane 1). Shown above the panel is the human (Hu) ERManI synthetic peptide and homology with the mouse (Mo) homolog used for the production of rabbit polyclonal antiserum (ERMI-CC20).
Elucidation of the earliest steps in GERAD is expected to assist in the mechanism-based treatment of genetic disorders such as serum α1-antitrypsin (AAT) deficiency (15). Accumulation of the aberrant glycoprotein in the distended hepatocyte ER (16, 17), resulting from its inadequate elimination (18), is the most common genetic risk factor for childhood liver disease (19). Impaired AAT secretion is responsible for the elasolytic destruction of lung alveoli, which leads to chronic obstructive pulmonary emphysema. This loss-of-function phenotype reflects the role of serum AAT as the principal inhibitor of neutrophil elastase (20). Herein, elucidation of how the initial mannosidase-dependent targeting event contributes to GERAD substrate selection was investigated by transient expression studies in the murine hepatoma cell line, Hepa1a (4, 5, 17). A model is proposed in which the timing of the glycan modification, relative to the duration of nonnative glycoprotein structure, functions as an underlying stochastic mechanism by which ERManI coordinates completion of the putative two-component GERAD signal in a manner that targets only newly synthesized AAT conformers with permanent structural defects for degradation by the proteasome.
Materials and Methods
Reagents. Routine chemicals and buffers were purchased from Sigma. Lactacystin was purchased from the E. J. Corey laboratory (Harvard Medical School, Boston). Kifunensine was from Toronto Research Chemicals (Downsview, ON, Canada). Lipofectamine 2000 Reagent was a product of Invitrogen. The murine hepatoma cell line Hepa1a (17, 21) was a kind gift from G. Darlington (Baylor College of Medicine). The cDNA for hemagglutinin-tagged murine ER degradation-enhancing α-mannosidase-like protein (EDEM), a generous gift from N. Hosokawa and K. Nagata (Kyoto University, Kyoto), was subcloned into the EcoRI site of pCDNA3.1 Zeo(+) for transient expression. An immunoglobulin fraction of rabbit polyclonal anti-human AAT was purchased from Boehringer Mannheim. Rabbit polyclonal antiserum against transferrin was purchased from Sigma.
Transient Transfection. The murine hepatoma cell line Hepa1a was grown as monolayers in standard growth medium (21) and split into 100-mm dishes at a cell density of 70% confluence. On the following day, cell monolayers were transfected according to the Lipofectamine 2000 protocol with hERMI/pCDNA3.1 Zeo(+), which is the human ERManI cDNA (12) subcloned into the EcoRI site of pCDNA3.1Zeo(+) (Invitrogen). Cotransfection was performed with the pCDNA3.1Zeo(+) expression vector constructs in which the full-length cDNAs that encode human AAT variants null(Hong Kong) (21), proteinase inhibitor (PI) Z (22), or PI M(Val-213) (21, 23) had been subcloned into the unique EcoRI site [i.e., hATZ/pCDNA3.1Zeo(+), hATNHK/pCDNA3.1Zeo(+), and hATM/pCDNA3.1 Zeo(+)]. Briefly, 0.030 mg of Qiagen-grade plasmid DNA, diluted in 1.5 ml of Opti-MEM I medium (Invitrogen), were combined with 0.030 ml of Lipofectamine 2000 reagent in an equal volume of Opti-MEM I medium. Subsequent incubation at room temperature allowed for the formation of the DNA–liposome complex. After addition of 3 ml of Opti-MEMI medium, the 6-ml medium containing DNA–Lipo2000 complex was applied to one 100-mm dish. At least three dishes were used for each transfection. After 5 h of incubation at 37°C, 6 ml of 2× complete medium were added to each dish for continuous cell growth. Forty-eight hours posttransfection, cells from all of the dishes were collected, mixed, and subcultured at identical cell densities in either 60-mm or 100-mm dishes to eliminate differences in transfection efficiency. Only in those experiments in which the transfection efficiency reached 65% was the analysis of the endogenous secretory glycoprotein transferrin performed.
Western Blotting. Approximately 48 h after transfection, 5 × 106 cells were lysed in 1 ml of buffered Nonidet P-40. Equivalent aliquots were resolved by SDS/PAGE and transferred to nitrocellulose before blotting with a rabbit polyclonal antibody (1:250) raised against a 20-aa synthetic peptide homologous to the C terminus of human ERMI (ERMI-CC20) that shares strong amino acid identity with the mouse homolog (Fig. 1b; Alpha Diagnostic, San Antonio, TX). Signals were detected with the recommended ECL Western blotting reagents (Amersham Pharmacia).
Metabolic Radiolabeling and Immunoprecipitation. Changes in the rate of target protein degradation were initiated 72 h posttransfection by pulse–chase metabolic radiolabeling with [35S]methionine in combination with immunoprecipitation (24). Monolayers of semiconfluent cells were incubated for 1 h at 37°C in complete medium with or without specific inhibitors (lactacystin, 0.025 mM; kifunensine, 0.2 mM), and then subjected to methionine starvation in methionine-free medium (ICN) with or without inhibitors for an additional 1 h. [35S]Methionine (ICN) was added [0.150 mCi (1 Ci = 37 GBq) per 100-mm dish] during a 10-min pulse, followed by up to 3 h of chase in serum-free DMEM (GIBCO/BRL) containing 0.2 mM unlabeled methionine with or without specific inhibitors at the same concentration used during the preincubation and pulse. At the designed time points, cells were lysed with buffered Nonidet P-40 detergent. Immunoprecipitates were generated from the cell lysates and medium as described (25), and resolved by SDS/PAGE for the detection of radiolabeled proteins by fluorography. A 1:100 dilution of rabbit polyclonal antiserum was used for the immunoprecipitation of endogenous transferrin. Quantitative analysis was performed by densitometric scanning by using the National Institutes of Health IMAGE program.
Results
Rapid Destabilization of Misfolded AAT in Response to the Overexpression of ERManI. Human AAT exhibits extensive allelic polymorphism (26). Many of the mutations are capable of hindering conformational maturation after biosynthesis such that the translated polypeptides become substrates for intracellular degradation (15). The consequences of elevated recombinant human ERManI overexpression on the fates of three allelic human AAT variants with distinct folding characteristics provided a model both to test the validity of the two-component GERAD signal hypothesis (Fig. 1a), and to investigate the manner by which its formation coordinates the selection of substrates for degradation by the proteasome. Oligosaccharide structural studies were purposefully omitted from the experimental design because the latent removal of additional mannose units by ERManI (27) is suspected to occur after recognition of the completed GERAD signal and would unnecessarily complicate the interpretation of our findings. Furthermore, no attempts were made to identify any signals that might elicit the degradation of nonglycosylated proteins, as these are not relevant to the study of GERAD or serum AAT deficiency. Our strategy was to use the murine hepatoma cell line Hepa1a as a host in transient expression experiments because mannosidase-dependent oligosaccharide processing is the rate-limiting step in the proteasomal degradation of mutant AAT (28, 29).
In mammalian cells, the role of ERManI in GERAD signal formation had originated from glycan inhibitor studies (ref. 4, and references therein). Transient expression of human recombinant ERManI under the transcriptional control of the cytomegalovirus promoter/enhancer routinely led to a 2- to 8-fold elevation of immunoreactive ERManI over the basal endogenous concentration and was a function of the DNA concentration in the transfection solution (Materials and Methods). In the present study, the fates of distinct AAT variants were studied under conditions in which transfection routinely elevated the overall ERManI concentration ≈5-fold (Fig. 1b). Under these conditions, the total ERManI concentration did not deviate by >20% in multiple experiments, or when cotransfected with the human AAT expression plasmids, or with pCDNA3.1(Zeo+) (not shown).
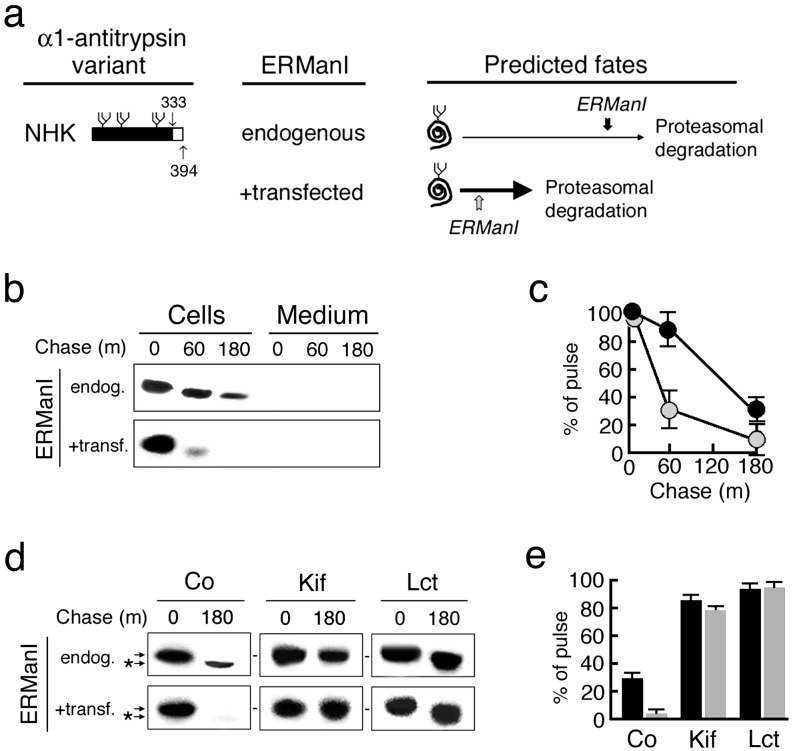
The truncation of 33 C-terminal amino acids from AAT variant null(Hong Kong) precludes conformational maturation after biosynthesis (Fig. 2a), ensuring the persistence of nonnative protein structure during intracellular retention. The entire cohort of pulse-radiolabeled null(Hong Kong) was degraded when transiently expressed in Hepa1a (Fig. 2 b and c), identical to that observed when stably expressed in this host cell line (24, 30). Cotransfection with the ERManI expression construct rapidly destabilized newly synthesized null(Hong Kong) (Fig. 2 b and c). That the anomaly represented accelerated degradation was confirmed by the capacity of lactacystin, a 26S proteasome inhibitor (31), to arrest intracellular turnover (Fig. 2 d and e).
Fig. 2.
The consequences of elevated ER mannosidase expression on the intracellular stability of human AAT variant null(Hong Kong) after biosynthesis. (a) Depicted are the C-terminal truncation and the predicted fates of newly synthesized variant null(Hong Kong) (NHK) in response to the basal (endogenous) and overexpressed (+transfected) ERManI concentrations in Hepa1a. The placement of vertical arrows depicts the predicted relative timing of the glycan modification under both conditions. (b) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated null(Hong Kong) (NHK) from cells and medium. (c) Quantitation of the results shown in b under endogenous (black circles) and elevated (shaded circles) ERManI concentrations. (d) Shown are the effects of kifunensine (Kif) and lactacystin (Lct) on the fate of newly synthesized null(Hong Kong), and on the discrete mobility shift (*) in SDS/PAGE. (e) Shown is quantitation of pulse-radiolabeled null(Hong Kong) in response to the endogenous (black bars) and elevated (shaded bars) concentrations of ERManI, and in response to specific treatments.
ERManI Plays a Direct Role in the Acceleration of AAT Degradation. That rapid destabilization of variant null(Hong Kong) had resulted from elevated ERManI activity was confirmed by the ability of kifunensine, a general α-1,2-mannosidase inhibitor (32), to arrest intracellular turnover (Fig. 2e) and prevent the discrete mobility shift in SDS/PAGE (Fig. 2d). The latter results from the processing of asparagine-linked glycans (28, 29). Cotransfection with inactive human ERManI, resulting from the epitope tagging of its C terminus ER luminal domain (K.W.M., unpublished observations), failed to alter the rate of cotransfected null(Hong Kong) degradation as compared with the WT mannosidase (Fig. 3a). That rapid degradation was not the result of an elevated intracellular concentration of EDEM, as previously published (33, 34), was supported by the results of cotransfection studies in which EDEM did not significantly alter the fate of newly synthesized null(Hong Kong) in Hepa1a, as compared with HEK293 (Fig. 3b). Importantly, a 5-fold range in the elevation of the EDEM concentration had no effect on null(HongKong) degradation in Hepa1a (not shown). This observation is consistent with the fact that GERAD signal recognition is mediated by EDEM as a rate-limiting step in the HEK293 (5, 33, 34), whereas formation of the signal by ERManI is rate-limiting in Hepa1a (28, 29).
Fig. 3.
Confirmation that elevated ERManI activity directly accelerates null(Hong Kong) degradation. (a) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated null(Hong Kong) (NHK) from cells and medium in response to endogenous (endog) ERManI, or after cotransfection with native (active) or myc-tagged (inactive) human ERManI. (b) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated null(Hong Kong) from soluble Nonidet P-40 cells extracts after cotransfection with EDEM (+transf) or with the empty vector (endog). In each of the studies, comparisons were made between identical numbers of cells in which the efficiency of transfection did not differ by >20%.
Evidence for a Stochastic Role Played by ERManI. The inhibitory function of AAT involves insertion of the cleaved reactive center loop to generate an additional strand for the A β-sheet (35). A mutation at the base of the reactive center loop (Fig. 4a) leads to a specific conformational rearrangement in variant PI Z (26) in which intermolecular loop insertion leads to spontaneous polymerization of the newly synthesized molecules (35). The anomaly functions as the predominant intracellular retention mechanism in liver hepatocytes (16), and is faithfully duplicated in Hepa1a (22, 30). We took advantage of the fact that PI Z polymers, all of which originate from a late-folding intermediate (36), are degraded in Hepa1a by a coexisting non-proteasomal disposal system (30). The prediction was that if ERManI plays a stochastic role in GERAD signal formation, then its elevated concentration would result in the modification of glycans attached to the upstream nonnative monomer, rather than the structured polymers, and therefore lead to degradation by the proteasome (Fig. 4a). At the endogenous ERManI concentration, <10% of newly synthesized PI Z was secreted into the medium (Fig. 4c), and the remainder was degraded by a kifunensine-sensitive, but lactacystin-resistant, system (Fig. 4 d and e), as previously observed in stable expression experiments (30).
Fig. 4.
The consequences of elevated ER mannosidase expression on the intracellular stability of human AAT variant PI Z after biosynthesis. (a) Depicted are the single point mutation and predicted fates of newly synthesized variant null(Hong Kong) (NHK) in Hepa1a in response to the basal (endogenous) and overexpressed (+transfected) ERManI concentrations. Loop-sheet polymerization of a late folding intermediate is depicted with brackets. The predicted relative timing of the glycan modification in relation to the formation of polymers is shown for both conditions. (b) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated PI Z from cells and medium. (c) Quantitation of the results shown in b under endogenous (black circles) and elevated (shaded circles) ERManI concentrations. (d) Shown are the effects of kifunensine (Kif) and lactacystin (Lct) on the fate of newly synthesized Pi Z, and on the discrete mobility shift (*) in SDS/PAGE. (e) Shown is quantitation of pulse-radiolabeled PI Z in response to the endogenous (black bars) and elevated (shaded bars) concentrations of ERManI, and in response to specific treatments.
Overexpression of ERManI accelerated the degradation of newly synthesized PI Z >3-fold over control (Fig. 4 b and c). Under these conditions, degradation of almost the entire cohort of radiolabeled PI Z was mediated by the proteasome, as demonstrated by an acquired sensitivity to lactacystin (Fig. 4 d and e). The PI Z degradation was also susceptible to treatment with kifunensine (Fig. d and e), which ablated the electrophoretic shift of the radiolabeled molecules in SDS/PAGE (Fig. 4d). The findings are consistent with the notion that the ERManI concentration plays a stochastic role in timing the onset of the glycan modification, which leads to degradation.
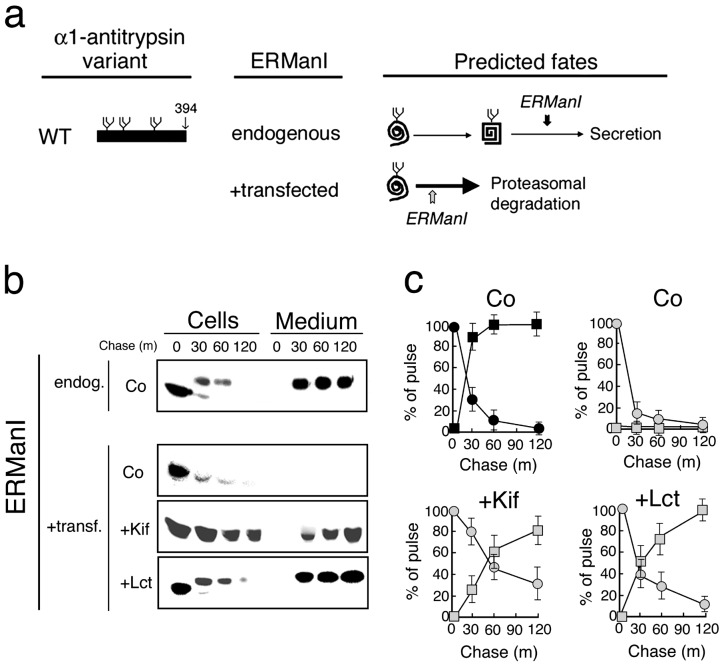
Conformational Maturation Prevents Completion of the GERAD Signal. Next, we asked whether conformational maturation protects newly synthesized WT AAT from degradation through the attenuation of nonnative protein structure. At the basal endogenous ERManI concentration, the entire cohort of the newly synthesized WT AAT was quantitatively secreted into the medium (Fig. 5b), identical to our previous observations in the stably transfected cells (21). Intracellular conversion to the slower electrophoretic mobility, identical to that secreted into the medium (Fig. 5b), reflects the conjugation of charged sialic acid residues to asparagine-linked glycans during transit through the trans Golgi compartment (24). The prediction was that newly synthesized WT AAT would be diverted to the proteasome in response to ERManI overexpression in the event that the accelerated onset of the modification preferentially tags glycans attached to the early nonnative monomer (Fig. 5a). Consistent with the prediction, ERManI overexpression led to rapid intracellular disappearance of newly synthesized WT AAT and concomitant loss of the radiolabeled molecules from the chase medium (Fig. 5 b and c).
Fig. 5.
Changes in the intracellular stability and secretion of newly synthesized WT human AAT. (a) Depicted are the structure of WT AAT, its conformational maturation, and the predicted fates in response to ERManI overexpression (+transfected) or when transfected with the empty vector (endogenous). Shown is the predicted relative timing of the glycan modification relative to conformational maturation. (b) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated WT AAT from cells and medium under the endogenous (endog.) and elevated (+transf.) ERManI concentrations, plus the effects of kifunensine (+Kif), lactacystin (+Lct), or no additional treatments (Co). (c) Shown is quantitation of pulse-radiolabeled WT AAT in cells (circles) and medium (squares) in response to the endogenous (black) and elevated (shaded) concentrations of ERManI, and in response to the specific treatments. Results from more than four experiments are shown.
That rapid destabilization of newly synthesized WT AAT resulted from intracellular degradation, rather than insolubility, was confirmed by the absence of radiolabeled AAT in the insoluble detergent cell extracts (not shown). This idea was also confirmed by the complete restoration of WT AAT secretion in response to treatment with kifunensine (Fig. 5 b and c). Here, the electrophoretic migration of the secreted molecules was similar to that of the intracellular species (Fig. 5b) resulting from the absence of sialic acid conjugation in the trans Golgi in response to mannosidase inhibition. That the up-regulation of EDEM is not involved in this phenomenon is supported by the prior observation that EDEM is incapable of eliciting the degradation of newly synthesized WT AAT (34).
It is noteworthy that WT AAT secretion was also rescued in response to the ablation of a late step in the disposal process with the proteasome inhibitor lactacystin (Fig. 5 b and c). The latter findings imply that one, or more, downstream steps that either mediate recognition of the completed GERAD signal or facilitate substrate recruitment are not necessarily committed steps in the disposal process in the event that conformational maturation is attainable. Taken together, the results are consistent with the proposed two-component GERAD signal hypothesis in which newly synthesized WT AAT is protected from degradation in response to the eventual loss of a GERAD signal component under the basal ERManI concentration.
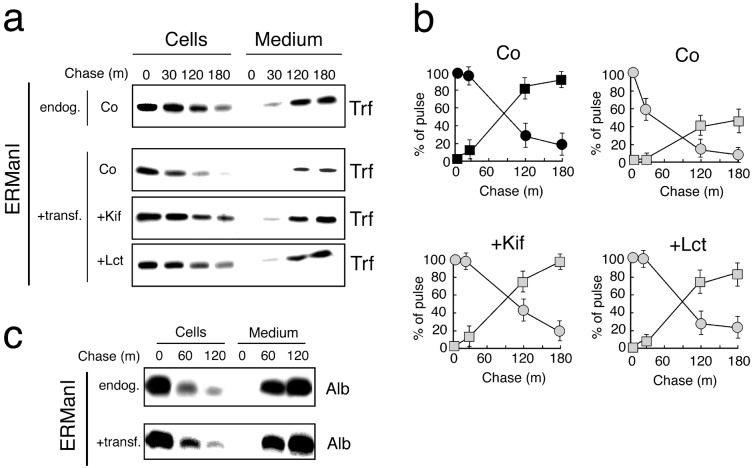
Next, the fate of newly synthesized transferrin was investigated to determine whether an endogenous secretory glycoprotein (37) would be subject to the effects of an elevated ERManI concentration. In pulse–chase studies, newly synthesized transferrin was secreted at a rate equivalent to its loss from cells (Fig. 6 a and b). Under normal conditions, significant intracellular loss was not detected until 120 min of chase, and ≈90% of the original cohort was secreted by 180 min (Fig. 6 a and b). However, in cells transfected with ERManI, intracellular loss was detected at 30 min of chase (Fig. 6a), and only 45% of newly synthesized molecules were secreted at 180 min (Fig. 6a). That the anomalies reflected rapid ERManI-mediated degradation was confirmed by the restoration of transferrin secretion when transfected cells were incubated with either kifunensine or lactacystin (Fig. 6 a and b). That the entire cohort of newly synthesized transferrin was not subject to rapid degradation probably reflects the fact that the transfection efficiency was 65%, such that the remaining cells remained under control conditions. Under an identical set of conditions, the secretion of endogenous mouse albumin was unaffected (Fig. 6c), indicating that the manipulation did not interfere with the secretion of a nonglycosylated secretory protein. Considering these findings, plus the fact that in no experiment did we detect the secretion of radiolabeled null(Hong Kong), it can be concluded that the collection of intracellular retention mechanisms were not affected by any of the manipulations. Taken together, these findings support the notion that ERManI may play a broad role in directing the fate of newly synthesized glycoproteins.
Fig. 6.
The effect of ERManI overexpression on endogenous transferrin and albumin. (a) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated transferrin (Trf) from cells and medium under the endogenous (endog.) and elevated (+transf.) ERManI concentrations, plus the effects of kifunensine (+Kif), lactacystin (+Lct), or no additional treatments (Co). (b) Shown is quantitation of pulse-radiolabeled transferrin in cells (circles) and the medium (squares) in response to the endogenous (black) and elevated (shaded) concentrations of ERManI, and in response to the specified treatments. The results from more than three experiments are shown. (c) Pulse–chase radiolabeling and fluorographic detection of immunoprecipitated murine albumin (Alb) from cells and medium after cotransfection with ERManI (+transf.) or with the empty vector (endog). In each of the studies, comparisons were made between identical numbers of cells in which the efficiency of transfection was ≈65%.
Discussion
Our (4) recent classification of GERAD as a distinct branch of the global ERAD network is based on several lines of evidence including: the observation that several aberrant glycoproteins are not substrates for degradation in response to de-glycosylation (ref. 4, and references therein), the recent identification of a specific ubiquitin ligase capable of recognizing sugar chains (38), and the molecular cloning of EDEM (33), which is suspected to function in recognizing the GERAD signal (33, 34, 39, 40).
A primary objective of the present study was to unmask the “molecular logic” by which the combination of GERAD signal determinants are used to preferentially target misfolded AAT for proteasomal degradation in a manner that spares early nonnative WT intermediates. The goal was to further our understanding of the rules that govern AAT biosynthetic quality control as a means to eventually uncover new disease markers and novel avenues for the treatment of serum AAT deficiency in which detailed structural data are available for both the WT and mutant variants (26). This work is particularly relevant to the analysis of AAT deficiency in which biosynthetic quality control systems are reported to function as a conspicuous modifier of the associated loss-of-function (i.e., chronic obstructive pulmonary emphysema) and gain-of-toxic function (i.e., liver cirrhosis) (5, 41) disorders.
In the present study, the collective fates of both mutant and WT AAT variants revealed that the concentration of ERManI plays a central role in coordinating the initial discriminatory step in the disposal process. By delaying the onset of the glycan modification, relative to the duration of nonnative protein structure, the low basal ERManI concentration is responsible for coordinating the preferential degradation of the misfolded AAT monomer. That WT endogenous transferrin was subject to degradation by ERManI overexpression indicates that the system is not restricted to moderating the biosynthetic maturation of only AAT. Our contention is that coevolution of the secretory pathway with the nuclear envelope (4) reflects the temporal and spatial expansion of the eukaryote genome expression surveillance system in which ERManI functions as a GERAD gatekeeper, perhaps to a broad population of glycoproteins.
It is well known that physical interaction with ER lectins and traditional molecular chaperones can significantly influence productive polypeptide folding (42), and may even alter the susceptibility of asparagine-linked glycans to ERManI (29). As such, it is conceivable that, in any given cell, evolutionary factors have balanced the relative protein folding efficiency and quality control capacity to prevent the random and unwarranted elimination of endogenous newly synthesized glycoproteins. To this end, cell-specific adherence to a particular set of quality control standards, and the functional knit between coevolved folding and quality control pathways, might explain the altered stability of some proteins in heterologous expression systems (5, 11, 43).
The present findings provide a mechanistic understanding as to how efficient conformational maturation can function as the initial quality control standard encountered by newly synthesized glycoproteins. However, because distinct folding and degradation programs apparently operate in different cell lines (5), it is premature to conclude that all glycoproteins are subject to the proposed model. Nevertheless, it seems that noncompliance with the established quality control standard underlies the decision to degrade terminally misfolded variant null(Hong Kong), whereas stability of WT AAT and transferrin is indicative of their involvement in efficient folding pathways. If correct, then the instability of newly synthesized WT AAT and transferrin reported in the present study likely reflects the inadequacy of the folding mechanisms to comply with the new standard set by artificial elevation of ERManI.
It is intriguing to speculate that cells might be able to regulate the quality control standard in response to changes in the environment, or physiological conditions, simply by altering the intracellular concentration of ERManI. In this manner, ERManI would have the capacity to function as an adjustable “timer” capable of influencing the glycoprotein structural maturation process. Because the mammalian ERManI gene is not under the transcriptional control by the unfolded protein response (36), in contrast to EDEM (44), it will be useful to investigate alternative strategies by which its intracellular concentration might be regulated.
Acknowledgments
This work was supported, in part, by National Institutes of Health Grants HL62553 (to R.N.S.), GM47533 (to K.W.M.), and RR05351 (to K.W.M.); by Research Grant RO2-5 from the Alpha-1 Foundation (to R.N.S.); and by funds from the Moran Foundation (to Y.W.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ER, endoplasmic reticulum; ERManI, ER mannosidase I; ERAD, ER-associated degradation; GERAD, glycoprotein ERAD; AAT, α1-antitrypsin; EDEM, ER degradation-enhancing α-mannosidase-like protein; PI, proteinase inhibitor.
References
- 1.McCracken, A. A. & Brodsky, J. L. (1996) J. Cell Biol. 132 291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer, T. & Wolf, D. H. (1997) FASEB J. 11 1227-1233. [DOI] [PubMed] [Google Scholar]
- 3.Fewell, S. W., Travers, K. J., Weissman, J. S. & Brodsky, J. L. (2001) Annu. Rev. Genet. 35 149-191. [DOI] [PubMed] [Google Scholar]
- 4.Cabral, C. M., Liu, Y. & Sifers, R. N. (2001) Trends Biochem. Sci. 26 619-624. [DOI] [PubMed] [Google Scholar]
- 5.Cabral, C. M., Liu, Y., Moremen, K. W. & Sifers, R. N. (2002) Mol. Biol. Cell 13 2639-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas, P. J., Qu, B.-H. & Pedersen, P. L. (1995) Trends Biochem. Sci. 20 456-459. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury, P., Liu, Y. & Sifers, R. N. (1997) News Physiol. Sci. 12 162-165. [Google Scholar]
- 8.Bonifacino, J. S. & Weissman, A. M. (1998) Annu. Rev. Cell Dev. Biol. 14 19-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellgaard, L. & Helenius, A. (2001) Curr. Opin. Cell Biol. 13 431-437. [DOI] [PubMed] [Google Scholar]
- 10.Ellgaard, L., Molinari, M. & Helenius, A. (1999) Science 286 1882-1888. [DOI] [PubMed] [Google Scholar]
- 11.Hammond, C., Braakman, I. & Helenius, A. (1994) Proc. Natl. Acad. Sci. USA 91 913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez, D. S., Karaveg, K., Vandersall-Nairn, A. S., Lal. A. & Moremen, K. W. (1999) J. Biol. Chem. 274 21375-21386. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay, L. O. & Herscovics, A. (1999) Glycobiology 9 1073-1078. [DOI] [PubMed] [Google Scholar]
- 14.Jakob, C. A., Burda, P., Rothe, J. & Aebi, M. (1998) J. Cell Biol. 142 1223-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sifers, R. N., Finegold, M. J. & Woo, S. L. C. (1992) Semin. Liver Dis. 12 301-310. [DOI] [PubMed] [Google Scholar]
- 16.Sharp, H. L. (1971) Hosp. Pract. 6 83-96. [Google Scholar]
- 17.Graham, K. S., Le, A. & Sifers, R. N. (1990) J. Biol. Chem. 265 20463-20468. [PubMed] [Google Scholar]
- 18.Wu, Y., Whitman, I., Molmenti, E., Moore, K., Hippenmeyer, P. & Perlmutter, D. H. (1994) Proc. Natl. Acad. Sci. USA 91 9014-9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpert, D., Molleston, J. P. & Perlmutter, D. H. (2000) J. Pediatr. Gastroenterol. Nutr. 31 258-263. [DOI] [PubMed] [Google Scholar]
- 20.Culliton, B. J. (1989) Science 246 750-751. [DOI] [PubMed] [Google Scholar]
- 21.Sifers, R. N., Brashears-Macatee, S., Kidd, V. J., Muensch, H. & Woo, S. L. C. (1988) J. Biol. Chem. 263 7330-7335. [PubMed] [Google Scholar]
- 22.Le, A., Ferrell, G. A., Dishon, D. S., Le, Q.-Q. & Sifers, R. N. (1992) ) J. Biol. Chem. 267 1072-1080. [PubMed] [Google Scholar]
- 23.Long, G. L., Chandra, T., Woo, S. L., Davie, E. W. & Kurachi, K. (1984) Biochemistry 23 4828-4837. [DOI] [PubMed] [Google Scholar]
- 24.Le, A., Graham, K. S. & Sifers, R. N. (1990) J. Biol. Chem. 265 14001-14007. [PubMed] [Google Scholar]
- 25.Le, A., Steiner, J. L., Ferrell, G. A., Shaker, J. C. & Sifers, R. N. (1994) J. Biol. Chem. 269 7514-7519. [PubMed] [Google Scholar]
- 26.Stein, P. E. & Carrell, R. W. (1995) Nat. Struct. Biol. 2 96-113. [DOI] [PubMed] [Google Scholar]
- 27.Herscovics, A., Romero, P. A. & Tremblay, L. O. (2002) Glycobiology 12 4G-15G. [PubMed] [Google Scholar]
- 28.Liu, Y., Choudhury, P., Cabral, C. M. & Sifers, R. N. (1997) J. Biol. Chem. 272 7946-7951. [DOI] [PubMed] [Google Scholar]
- 29.Liu, Y., Choudhury, P., Cabral, C. M. & Sifers, R. N. (1999) J. Biol. Chem. 274 5861-5867. [DOI] [PubMed] [Google Scholar]
- 30.Cabral, C. M., Choudhury, P., Liu, Y. & Sifers, R. N. (2000) J. Biol. Chem. 275 25015-25022. [DOI] [PubMed] [Google Scholar]
- 31.Fenteany, G., Standaert, R. F., Lane, W. S., Choi, S. Corey, E. J. & Schreiber, S. L. (1995) Science 268 726-731. [DOI] [PubMed] [Google Scholar]
- 32.Elbein, A. D. (1991) FASEB J. 5 3055-3063. [DOI] [PubMed] [Google Scholar]
- 33.Hosokawa, N., Wada, I., Hasegawa, K., Yorihuzi, T., Tremblay, L. O., Herscovics, A. & Nagata, K. (2001) EMBO Rep. 2 415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda, Y., Hosokawa, N., Wada, I. & Nagata, K. (2003) Science 299 1394-1397. [DOI] [PubMed] [Google Scholar]
- 35.Lomas, D. A., Evans, D. L. I., Finch, J. T. & Carrell, R. W. (1992) Nature 357 605-607. [DOI] [PubMed] [Google Scholar]
- 36.Yu, M.-H., Lee, K. N. & Kim, J. (1995) Nat. Struct. Biol. 2 363-367. [DOI] [PubMed] [Google Scholar]
- 37.Lodish, H. F., Kong, N., Snider, M. & Strous, G. J. (1983) Nature 304 80-83. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida, Y., Chiba, T., Tokunaga, F., Kawasaki, H., Iwai, K., Suzuki, T., Ito, Y., Matsuoka, K., Yoshida, M., Tanaka, K. & Tai, T. (2002) Nature 418 483-442. [DOI] [PubMed] [Google Scholar]
- 39.Molinari, M., Calance, V., Galli, C., Lucca, P. & Paganetti. (2003) Science 299 1397-1400. [DOI] [PubMed] [Google Scholar]
- 40.Sifers, R. N. (2003) Science 299 1330-1331. [DOI] [PubMed] [Google Scholar]
- 41.Kopito, R. R. & Ron, D. (2000) Nat. Cell Biol. 2 E207-E209. [DOI] [PubMed] [Google Scholar]
- 42.Gething, M. J. & Sambrook, J. (1992) Nature 355 33-45. [DOI] [PubMed] [Google Scholar]
- 43.Su, K., Stoller, T., Rocco, J., Zemsky, J. & Green, R. (1993) J. Biol. Chem. 268 14301-14309. [PubMed] [Google Scholar]
- 44.Yoshida H., Matsui, T., Hosokawa, N., Kaufman, R. J., Nagata, K. & Mori, K. (2003) Dev. Cell 4 265-271. [DOI] [PubMed] [Google Scholar]