Abstract
Hematopoietic cells have long been defined as round, nonpolar cells that show uniform distribution of cell surface-associated molecules. However, recent analyses of the immunological synapse and the importance of lipid microdomains in signaling have shed new light on the aspect of lymphocyte polarization during the activation processes, but none of the molecules implicated so far in either the activation process or the microdomain residency are known to have a preferential localization in nonactivated cells. Chemical crosslinking and fluorescence resonance energy transfer methods have allowed the visualization of certain glycosylphosphatidylinositol-anchored proteins in lipid rafts but so far no microdomain resident protein has been shown to exist as visible stable platforms in the membrane. We report here that two lipid microdomain resident proteins, flotillins/reggies, form preassembled platforms in hematopoietic cells. These platforms recruit signaling molecules upon activation through lipid rafts. The preassembled platforms significantly differ from the canonical cholesterol-dependent “lipid rafts,” as they are resistant to cholesterol-disrupting agents. Most evidence for the functional relevance of microdomains in living cells remains indirect. Using laser scanning confocal microscopy, we show that these proteins exist as stable, microscopically patent domains localizing asymmetrically to one pole of the cell. We present evidence that the asymmetric concentration of these microdomain resident proteins is built up during cytokinesis.
Keywords: lipid rafts, microdomains, lymphocyte signaling, polarization, cytokinesis
According to the hitherto accepted view, lymphocytes and monocytes are round, nonpolarized cells (1–3) that become polarized and recruit certain molecules to the polarized edge only upon activation (4, 5). Several recent studies (6–8) have emphasized the importance of certain proteins in activation-dependent cellular polarization and subsequent immunological synapse formation. These studies also underscored the importance of lipid microdomains in the polarization and the activation processes (5, 7). Molecules of the ezrin, radixin, and moesin (ERM) family (6, 8–10), PKC-θ (7), several signaling molecules like lck (11), fyn (12), LAT, and vav, and several coreceptors like CD28 (11), CD21, and CD19 (13) (in the case of B cells) have been shown to be translocated upon activation to the supramolecular activation clusters in a polarized fashion. However, under nonactivated conditions none of these molecules showed any preferential localization that could act as a predetermined platform for activation.
The existence of these lipid microdomains in live cells has been a disputatious subject because the demonstration of these microdomains in nontreated cells has been extremely difficult (14). The concept of rafts itself has been very controversial, and the definite proof of their existence in living cells has been very elusive. Crosslinking with bifunctional reagents (15) and fluorescence resonance energy transfer (16) have allowed the visualization of certain glycosylphosphatidylinositol (GPI)-anchored proteins in lipid rafts, but so far no microdomain resident proteins have been shown to exist as visible stable platforms in the membrane. We show here that the lipid microdomain marker proteins flotillin-1 and -2 form stable, microscopically visible preassembled large platforms in hematopoietic cells and possibly serve as scaffolds for activation. Flotillins are christened variously as reggies (17), cavatellins (18), and epidermal surface antigen (19). For the sake of simplicity, the term flotillin-1 is used to refer to reggie-2 or cavatellins and flotillin-2 is used to refer to reggie-1 throughout this article. Flotillins are highly related proteins (20), which were originally identified as lipid raft-associated morphogenetic proteins (17, 21). Previous studies on these proteins and their morphogenetic function in neuronal (17) and epithelial (22) cells have led us to investigate the role of these proteins in lymphocyte signaling.
Materials and Methods
Cells and Reagents. The B cell lines were cultured and maintained as described (23). The Ramos B cell line (kindly provided by T. Wirth, University of Ulm, Ulm, Germany) was maintained in 10% FCS containing RPMI medium 1640 (GIBCO/BRL) with 5% CO2. The T cell lines Jurkat and Molt4 were maintained in 5% FCS containing RPMI medium 1640 at 5% CO2. The U937 promonoytic cell line was maintained in Iscove's medium (Invitrogen) containing 10% FCS in 8% CO2. The antibodies against reggies/flotillins were as described (23). Phospho-extracellular signal-regulated kinase (ERK) antibodies were from Cell Signal Technology (Frankfurt). Polyclonal antibodies against lyn, CD71, and CD55 were from Santa Cruz Biotechnology. mAbs against lck, CD3, and antiphosphotyrosine antibody conjugated to horseradish peroxidase (HRP) were from BD Biosciences (Heidelberg). Goat anti-human IgM conjugated with HRP was from Southern Biotechnology Associates. Biotinylated mouse mAb against CD21 (clone BU32) was from Cymbus Biotechnology (Dianova, Hamburg, Germany). Cholera toxin (CTx) B subunit unconjugated and conjugated with HRP, rabbit CTx antibodies, methyl-B-cyclodextrin (CDX), nocodazole, phorbol 12-myristate 13-acetate (PMA), calcium ionophore all were from Sigma. Cy3 and Alexa green-conjugated secondary antibodies were from Jackson Laboratories and Molecular Probes, respectively. Phalloidin-conjugated with Alexa green was a kind gift from M. Bastmeyer (University of Konstanz).
Overexpression of Reggie-1-Enhanced GFP (EGFP) in Jurkat T Cells. Jurkat T cells were electroporated with 5 μg Reggie-1-EGFP (C. Neumann-Giessen, B. Falkenbach, L.R., G. Lüers, H.I., C.A.O.S., V. Herzog, and R.T., unpublished data), and the cells were analyzed after 36–48 h.
Isolation of Detergent-Insoluble Fractions. Detergent-insoluble fractions were isolated as described (23).
Activation of T Cells and ERK/Mitogen-Activated Protein Kinase Activation. A total of 5 × 107 cells per ml was incubated with 15 μg of CTx-B subunit and incubated in the cold for 30 min. After washing twice with serum-free medium, anti-CTx antibodies were added and incubated again in the cold for 30 min. The excess of antibody was removed by washing twice and the cells were incubated with 37°C prewarmed medium, incubated at 37°C for the indicated times, and immediately quenched with 5 vol of ice-cold serum free medium. Jurkat T cells (5 × 107) were activated for the times indicated with 50 ng/ml PMA and 1 μM ionomycin in serum-free medium. After spinning down the cells at 800 × g in the cold, cells were lysed in 1% Nonidet P-40 lysis buffer, and an aliquot of each sample was used for Western blotting.
Immunoprecipitation. The CTx-B crosslinked and activated T cells were lysed as described, and the lysate was precleared with protein-G coupled magnetic beads (Dynal, Oslo) for 2 h at 4°C. The precleared lysate was separated from the beads by magnetic separation. Protein G-coupled magnetic beads were incubated with either the antiflotillin-1/reggie-2 antibodies or the mouse IgG antibodies as control for 50 min at room temperature. The beads were washed twice with 0.1% Nonidet P-40 lysis buffer and added to the precleared lysate and incubated overnight in the cold. The antibody-bound beads were magnetically separated and washed once with 1% Nonidet P-40 lysis buffer and thrice with 0.1% Nonidet P-40 lysis buffer. The immunoprecipitates were analyzed by Western blotting as described below.
CDX Treatment. Cells were treated with 12.5 mM CDX for 45 min at 37°C in serum-free medium. At the end of the incubation, cells were washed thoroughly to remove excess CDX. Also cells were incubated with trypan blue to check viability at the end of the treatment. Most of the cells excluded the dye. An aliquot was processed for immunofluorescence and ≈108 cells were lysed in 1% Triton X-100 lysis buffer for preparation of detergent-insoluble fractions. Control cells did not receive any CDX treatment.
Western Blotting. The sucrose gradient fractions and whole-cell lysates of activated or nonactivated cells or immunoprecipitates were run on 4–12% Bis-Tris gradient gels (Novex, Invitrogen) and blotted onto nitrocellulose membranes (Protran, Schleicher & Schuell) according to the manufacturer's instructions. The blots were blocked twice with 1.5% BSA/TBST (TBS containing 0.02% Tween) for 1 h each and incubated with the primary antibodies either for 1 h at room temperature or overnight in cold and washed six times with TBST for 10 min each. HRP-conjugated secondary antibodies (Pierce) were incubated for 45 min at room temperature, and the chemiluminescence was detected by a West Pico Super Signal Chemiluminescence kit (Pierce).
Cell Cycle Analysis by the Nocodazole Wash Method. Jurkat cells were treated with 40 ng/ml nocodazole for 12 h to synchronize cells in G2/M transition phase. The cells were washed and further cultured in fresh medium without nocodazole for another 1–2 h to allow the cells to proceed into mitosis, and immunofluorescence costaining experiments were performed by using antibodies against flotillin-2 and β-tubulin.
Immunofluorescence and Laser Scanning Confocal Microscopy. For all fluorescence microscopy experiments, treated or untreated cells were washed and attached to polylysine-coated coverslips (≈105 cells per slip). The cells were then fixed either with ice-cold methanol for 5 min in -20°C or 4% paraformaldehyde for 5 min at room temperature. After fixation, the cells were washed three times with 0.1% Triton X-100 containing PBS and then the primary antibodies in 1% BSA/PBS were added for 1 h at room temperature. After primary antibody binding, the cells were washed four times with 0.1% Triton X-100 containing PBS, and the fluorescent-conjugated secondary antibodies were added for 45 min at room temperature. The cells were washed thrice with PBS and mounted on microscopic slides by using Mowiol (Calbiochem) with n-propylgallate as antifading agent. After 24 h, the stainings were analyzed in a LSM 510 microscope (Zeiss) equipped with a Plan-Apochromat ×100 oil immersion lens by using the LSM 510 image browser software.
Results and Discussion
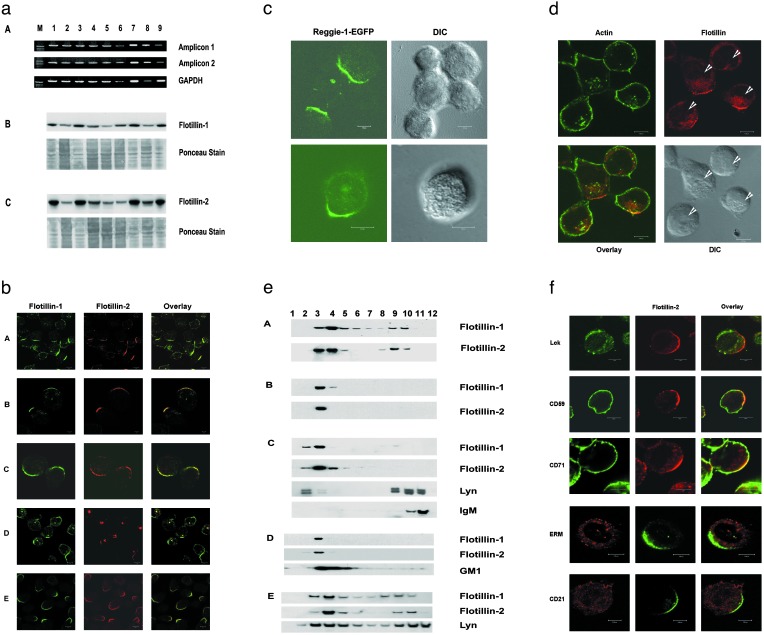
Flotillin-Defined Preassembled Platforms (PAPs) Are Present in All of the Hematopoietic Cells. Recently, we showed the expression and the lipid raft association of flotillin-2 in different stages of B cell development (23). All of the cells expressed both proteins and contained these proteins in high amounts (Fig. 1a). B cells, when stained with antiflotillin-1 and -2 antibodies, showed a distinct “cap”-like staining (Fig. 1b). Because most of the resting B cells exhibited this striking cap-like staining, we extended our studies to other cells of hematopoietic origin. Jurkat T cells (Fig. 1b), Molt4 T cells (data not shown), and the promonocytic U937 cells (Fig. 1b) also showed a cap-like staining. This striking feature was shown by both flotillins, and both of them showed extensive colocalization in these PAPs (Fig. 1b). In different stages of B cell development, represented by the different B cell lines, Nalm6 (pre-B cell stage), Raji and Ramos (mature B cell stage), ≈80% of the cells showed PAPs, and in the Jurkat T cells, ≈70% of the cells showed PAPs, whereas in the U937 monocytic cell line, we observed that >90% of the cells exhibited this feature.
Fig. 1.
Exclusive asymmetric localization of flotillins in PAPs and their DRM partitioning in resting cells. (a) Expression of flotillins in different hematopoietic cells. Lanes 1–9: pro-B cell line KM3, pre-B cell Nalm6, Epstein–Barr virus-transformed mature B cell lines Dakiki, Raji, plasma B cell line HSSultan, peripheral blood B lymphocytes, T lymphocytes, T cell leukemic Jurkat cells, and the promonocytic cell line, U937, respectively. M, marker lane. (A) PCR amplification of flotillin-1. GAPDH amplification serves as control. (B and C) Detection of flotillin-1 and -2 by Western blotting. Both flotillins run at 48 kDa under reducing conditions and the Ponceau stainings serve as loading controls. (b) Nonactivated pre-B cell line Nalm6 (A), mature B cell lines Raji (B), Ramos (C), T leukemic Jurkat cells (D), and promonocytic cell line U937 (E) were stained with mouse monoclonal antiflotillin-1 (green) costained with affinity-purified rabbit polyclonal anti-reggie-1/flotillin-2 (red) antibodies. All of the cells exhibited the cap-like staining. (c) Reggie-1-EGFP is selectively targeted to the PAPs. Jurkat cells were electroporated with the plasmid and the cells were analyzed by laser scanning confocal microscopy. The overexpressed fusion protein was also targeted to the PAPs. (d) Jurkat cells were stained with antiflotillin-1 antibodies and costained with phalloidin green. Intense intracellular staining of flotillins shows the outlines of nuclei (arrowheads) and helps perceive the orientation of the PAPs with respect to the nucleus. Bright-field images (differential interference contrast microscopy, DIC) show the intact, round morphology of these cells. (e) Detergent insolubility of flotillins. Cells (A–E as in b) were lysed in cold 1% Triton X-100 and a sucrose gradient was overlaid onto the lysate as mentioned in Materials and Methods. The detergent-insoluble fractions 2–5 were identified by CTx-B-HRP and anti-lyn antibody staining. Note that the B cell receptor (IgM) under nonactivating conditions was excluded from the detergent-insoluble fractions. (f) The first three rows represent nonactivated Jurkat T cells stained with flotillin-2/reggie-1 antibodies (red) and anti-lck, anti-CD59, and anti-CD71 antibodies (green). The fourth row shows anti-ezrin, radixin, and moesin (ERM) (red) and antiflotillin-2 (green) costaining in U937 promonocytic cells. The bottom row represents the B cell line Raji stained with anti-CD21 (red) and antiflotillin-2 (green) antibodies. None of the molecules except flotillins showed a polarized localization. (Bars = 5 μm.)
Overexpressed Reggie-1/Flotillin-2 Is Targeted to PAPs. To check whether flotillins could be targeted to the PAPs, we overexpressed reggie-1(flotillin-2)-EGFP in Jurkat T cells. Remarkably, we found that overexpressed flotillins were also targeted to the PAPs (Fig. 1c), indicating that flotillins carry a selective PAP-targeting signal. Overexpression also led to the formation of filopodia- and lamellipodia-like structures in accordance with earlier reports on forced expression of flotillins in epithelial cells leading to enhanced growth of subcellular structures such as filopodia (22).
The PAPs mostly were 5–10 μm in size and composed of smaller clusters of ≈100 nm each (Supporting Materials and Methods and Fig. 5, which are published as supporting information on the PNAS web site, www.pnas.org). Furthermore, bright-field images and actin staining showed that these cells were round, intact, and morphologically undisturbed (Fig. 1d). Fig. 1d also shows the orientation of these PAPs with respect to the nucleus. In most of the cases, we observed that the caps are polarized just opposite to the nuclear constriction coinciding with the microtubule organizing centers (see Fig. 4). With widely different fixation procedures (methanol, paraformaldehyde, and methanol/paraformaldehyde) the PAPs were observed, and the flotillin-defined PAPs exist in living cells. Also the transfected cells with the EGFP construct, even without spinning or settling the cells on polylysine, showed flotillin PAPs, excluding the possibility that flotillin PAPs were formed either because of the extracellular matrix contact or the mechanical stress on the cells (data not shown).
Flotillins Exclusively Partition into Detergent-Resistant Microdomains of Hematopoietic Cells. Rafts, or the membrane microdomain components, could be isolated by cold detergent extraction methods (14, 24) and are defined operationally as detergent-resistant membranes (DRMs) (3, 25). Flotillins are membrane-associated proteins (21, 26), and almost all of the membrane-associated flotillins fractionated in the detergent-resistant fractions of the immune cells (Fig. 1e). Centrifugation through sucrose gradients indicated that the PAPs were contributed by the lipid microdomain-associated flotillins, which cofractionated with the lipid raft markers like lyn and GM1 and excluded the nonlipid raft proteins like IgM (Fig. 1e). Purified plasma membrane fractions and surface biotinylation experiments also clearly demonstrated that the membrane-associated flotillin PAPs almost exclusively partition into DRMs (data not shown).
Flotillins Alone Show Asymmetric Localization in Resting Cells. We tested for the localization patterns of several of the molecules implicated in raft association and cellular polarization like CD59, CD71 (27), ezrin, radixin, and moesin (ERM) family proteins (6, 8, 10), lck (11), CD21 (13) (Fig. 1f), and flotillins (21, 23). None of the molecules except the flotillins localized extensively to a circumscript area of the cell surface. We also observed intracellular localization of flotillins concentrated near the centrosome (23) (Fig. 1b). These cells were not activated by any means and were devoid of any contamination. The caps seem to exist under resting conditions and are conserved throughout the hematopoietic system.
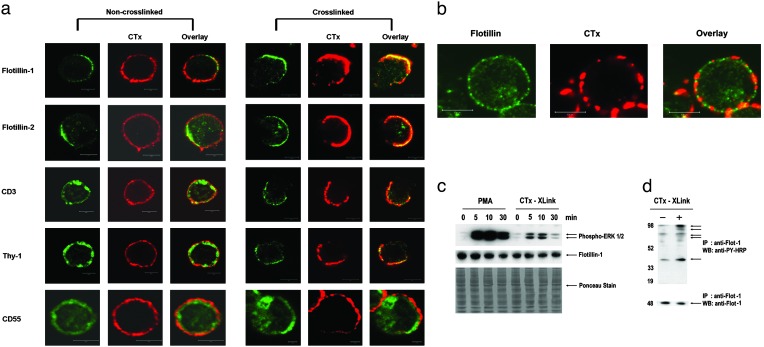
Lymphocyte Activation Recruits Signaling Components to PAPs. Here we investigated the possible role of these PAPs in lymphocyte activation and signaling. Crosslinking with CTx-B has been recently used to study the influence of raft-associated proteins in T cell receptor (TCR) signaling (27, 28). Noncrosslinked T cells showed uniform cell surface distribution of GM1, the GPI-anchored protein Thy1, TCR-CD3, and CD55 (the decay accelerating factor), but displayed a very polarized localization of flotillins-1 and -2 (Fig. 2a). Crosslinking with CTx-B induces patching or capping, a phenomenon associated with TCR signaling induced by raft clustering (27–29). CTx-B activation resulted in CTx-B caps that colocalized extensively with PAPs (Fig. 2a). As an internal control, a minor fraction of cells showed no GM1 polarization even after CTx-B crosslinking and these cells also lacked polarized flotillin staining (Fig. 2b). The CTx-B-induced caps also colocalized with Thy1 and CD3 (Fig. 2a), confirming earlier studies (27, 28) that lipid raft clustering induces recruitment of the signaling components of the TCR machinery. We show here that these caps induced by lipid raft clustering colocalize with the preexisting flotillin platforms but not with CD55 (30) (Fig. 2a) and the transferrin receptor CD71 (data not shown), which are excluded. None of the molecules except flotillins existed in polarized form before raft clustering. It is also interesting to note that CD55, despite being a GPI-anchored protein, did not copatch with GM1. A pioneering study by Madore et al. (31) showed that functionally different GPI-anchored proteins are structurally different and could cluster at different densities on different parts of the cell (30). This may explain why some GPI-anchored molecules copatch with GM1 and others do not.
Fig. 2.
Lipid raft clustering recruits raft-associated signaling molecules to the preassembled platforms and activates T cells. (a) CTx-B crosslinking (red) induces patching and recruits signaling molecules CD3 and Thy1 (green) to PAPs but not CD55. Noncrosslinked T cells (Left) show uniform distribution of GM1 (CTx-B, red), CD3, Thy1, and CD55 (green) but very polarized expressions of flotillins-1 and -2. (b) Some cells showed uniform distribution of GM1 in CTx-B crosslinked cells (red) and those cells also lacked flotillin (green) polarization. (c) Jurkat T cells were activated with PMA and CTx-B for the times indicated. The lysates were run on a reducing SDS/PAGE and blotted with antiphospho ERK-1/2 antibodies. The blots were stripped and blotted with antiflotillin-1 antibodies. Ponceau staining and flotillin levels showed loading of equal amounts of proteins. (d) Immunoprecipitation with antiflotillin-1 antibodies from CTx-B crosslinked and noncrosslinked Jurkat T cells. (Upper) Immunoprecipitates were blotted and stained with anti-phosphotyrosine (PY)-HRP antibodies. Arrows indicate flotillin-interacting phosphorylated protein bands enriched upon CTx-B crosslinking. (Lower) Loading control in the same immunoprecipitates by using antiflotillin-1 antibodies. (Bars = 5 μm.)
In addition to an accumulation of cell surface receptors and associated molecules in the PAPs after activation, we found a small, but significant, increase of flotillins in the PAPs after activation. This is most likely caused by a recruitment of flotillins from more remote sites of the membrane or from the intracellular pool (Figs. 2a and 5), suggesting that PAPs undergo a dynamic rearrangement of the flotillins upon activation rather than provide a mere static polarized scaffold for activation.
To address the question of whether raft clustering resulted in T cell activation, we crosslinked the T cells with CTx-B. In parallel, we treated T cells with the pan-specific activator PMA and the Ca2+ ionophore. Both CTx-B crosslinking (32) and PMA/Ca2+ ionophore treatment induced ERK phosphorylation (Fig. 2c) whereas levels of flotillin did not change. Immunoprecipitations from lysates of activated and nonactivated cells showed that flotillins preferentially interacted with specific phosphorylated proteins in activated, but not in resting, cells (Fig. 2d). Thus, our studies show that flotillins not only act as signaling platforms but also undergo dynamic rearrangement and participate in T cell signaling by interacting with specific phosphoproteins only upon activation. These studies are in agreement with recent studies on insulin signaling that have shown that flotillin binds to components of the insulin signaling pathway upon insulin binding to the receptor (33).
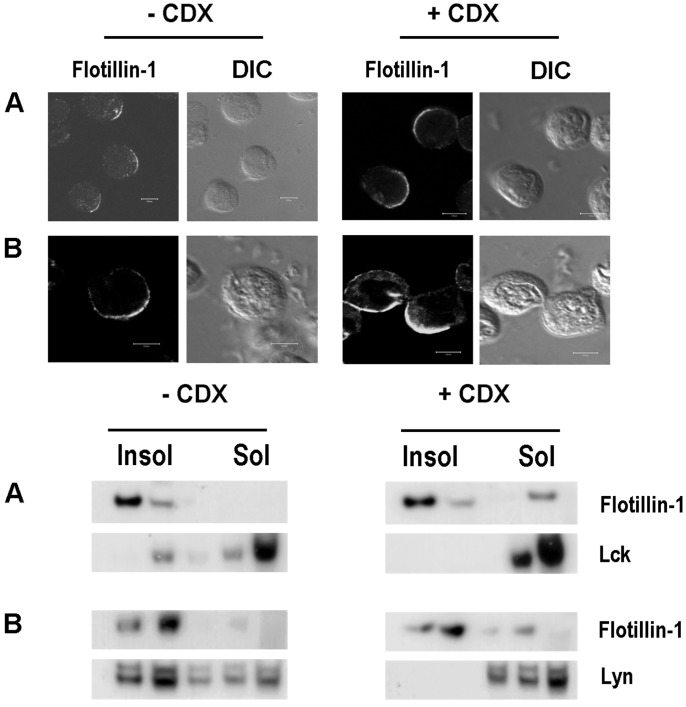
Flotillin PAPs Define a Subset of DRMs. Lipid rafts defined as DRMs are Triton X-100 insoluble and are enriched in cholesterol (3, 25). Any alteration in the cholesterol content leads to instability of these lipid patches in membranes, making most of the raft proteins vulnerable to Triton X-100 solubility (34, 35). Therefore we have investigated the effect of cholesterol disruption on the stability of the PAPs to determine their cholesterol dependency. We used CDX, an agent known to extract cholesterol, to study the effect of cholesterol disruption on the stability of PAPs. As shown by sucrose flotation gradients in Fig. 3, the lipid raft marker lck in Jurkat T cells and the src kinase lyn in U937 cells disappeared from the Triton X-100-insoluble fractions and appeared instead in the soluble fractions upon CDX treatment (Fig. 3 Lower). LAT, another lipid raft resident protein in T cells, completely moved out of the insoluble fraction upon CDX treatment and increased ERK phosphorylation (35) caused by CDX treatment (data not shown). This finding substantiates the fact that the CDX treatment indeed removes certain proteins out of the cholesterol-dependent lipid microdomains. In contrast, flotillins remained in the insoluble fractions and confocal staining showed that the integrity of the PAPs was conserved even after cholesterol disruption (Fig. 3 Upper). Cells starved of cholesterol also retained flotillins in the detergent-resistant fractions and displayed intact PAPs when analyzed through confocal microscopy (data not shown). It therefore appears that flotillins are enriched in detergent-resistant microdomains that are CDX resistant, thereby defining a subset of DRMs. Raft residency requirements are diverse and have been discussed controversially (36–38). GPI-anchored proteins, doubly acylated src-family kinases, and cholesterol-linked proteins are known to reside in the cholesterol-dependent rafts (3) but flotillins lack GPI-anchoring signals or homology to any raft-resident transmembrane or kinase family members. Recent studies (ref. 39 and C. Neumann-Giesen, B. Falkenbach, L.R., G. Lüers, H.I., C.A.O.S., V. Herzog, and R.T., unpublished data) show that flotillins could be palmitoylated and/or myristoylated and transported to the membrane via a Golgi-independent pathway. But what confers cholesterol independency on flotillins remains to be investigated.
Fig. 3.
Flotillins form stable cholesterol-independent preassembled microdomains and platforms. (Upper) Confocal staining of untreated (-CDX) and CDX-treated (+CDX) (12.5 mM, 45 min, 37°C) Jurkat (A) and U937 (B) cells with antiflotillin-1 antibodies. The corresponding bright-field images (differential interference contrast microscopy, DIC) are shown. (Lower) Western blotting of sucrose gradient fractions of detergent-lysed (1% Triton X-100, 4°C) untreated (-CDX) and CDX-treated (+CDX) Jurkat (A) and U937 (B) cells. Insol represents the detergent-insoluble fractions and Sol represents the soluble fractions. The lysates were processed as described in Materials and Methods and blotted with antiflotillin-1, lck, and lyn antibodies. (Bars = 5 μm.)
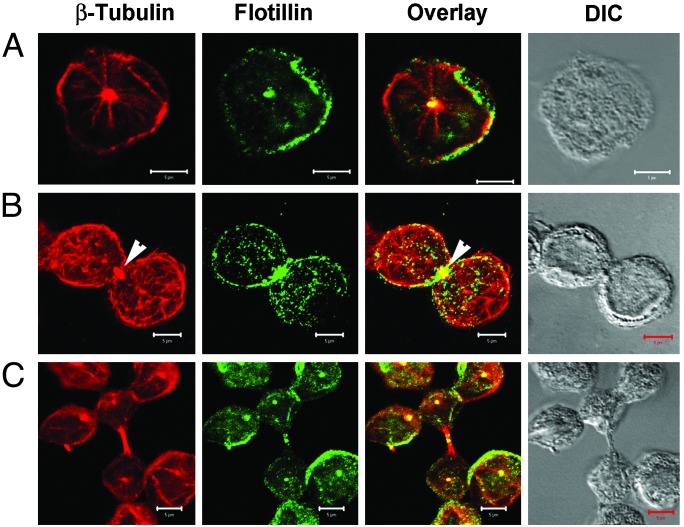
Polarization of Flotillins Is Built up During Cytokinesis. Some, but not all, lymphocytes showed an accumulation of flotillins at contact sites (Fig. 1b) (23). We investigated the possibility that PAPs might be established early in the cell cycle during cytokinesis. Indeed, the polarization of certain molecules appears to arise during cytokinesis when certain proteins preferentially assemble on specific sides of the dividing cell (40, 41). We arrested the cells in G2/M phase by the nocodazole wash procedure (42) and allowed the cells to progress through the different stages of the cell cycle. We found that significant amounts of flotillins accumulated at the site where the emerging cells separate during cytokinesis (Fig. 4). It therefore seems that polarity of flotillin expression is determined during cytokinesis. Centrosomes have recently been implicated as being essential for initiating the S phase and completing cytokinesis (43). The microtubule association of flotillins in the centrosomal region (Fig. 1b) (23) also could explain the importance of cytokinesis in the polarized expression of these proteins or vice versa.
Fig. 4.
Cytokinesis in correlation with polarized expression of flotillins. Jurkat T cells were treated with nocodazole for 12 h to synchronize cells in the G2/M transition phase, washed, and further cultured in fresh medium without nocodazole for another 1–2 h to allow the cells to proceed into mitosis. (A) The asynchronous culture showing the microtubule organizing centers stained with β-tubulin antibody (red) and flotillin-2 antibody (green). (B) A cell undergoing cytokinesis evidenced by the position of midbody in the division plane (arrowheads). Note the accumulation of flotillins in the division plane. (C) The cells after division. Most of the cells have flotillins polarized in PAPs. DIC (differential interference contrast microscopy) represents the bright-field images of the cells. (Bars = 5 μm.)
In conclusion, we have shown here that the lipid microdomain resident proteins flotillin-1 and -2 form visible, preassembled platforms of 5–10 μm and localize asymmetrically in resting hematopoietic cells. They also serve as signaling scaffolds for activation and participate in relaying the signal. Polarized cytokinesis, as shown here, seems to be responsible for the asymmetric localization of these proteins. Moreover, these PAPs are cholesterol-independent stable microdomains conferring structural and signaling polarity to hematopoietic cells.
Supplementary Material
Acknowledgments
We thank Dr. Rolf Knippers for critical reading of the manuscript and advice on cell cycle experiments. We thank Drs. Thomas Harder, Tony Magee, Susan Pierce, David Wallach, Marcus Groettrup, and Rudolf Lucas for their helpful suggestions throughout the work. We gratefully acknowledge the help of Monika Marx, Dirk Lehnert, and Sylvia Hannbeck with the confocal studies and Ulli Beck, Marianne Wiechers, and Elisabeth Naidoo for their technical assistance. This work was supported by grants from the Fonds der Chemischen Industrie, the Bundesministerium für Forschung und Technologie, and the Deutsche Forschungsgemeinschaft (to C.A.O.S.), the Ministry of Science and Culture of the state of Baden-Württemberg (to C.A.O.S., H.P., and H.I.), the Hans-Hench-Stiftung, the Bundesministerium für Forschung und Technologie (Lead Project 01GG9834), and the European Union (QLG1-CT-2001-01536 and QLG1-CT-2001-01407) (to H.I.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GPI, glycosylphosphatidylinositol; ERK, extracellular signal-regulated kinase; HRP, horseradish peroxidase; CTx, cholera toxin; CDX, cyclodextrin; PMA, phorbol 12-myristate 13-acetate; EGFP, enhanced GFP; PAP, preassembled platform; DRM, detergent-resistant membrane.
References
- 1.Anton van der Merwe, P., Davis, S. J., Shaw, A. S. & Dustin, M. L. (2000) Semin. Immunol. 12 5-21. [DOI] [PubMed] [Google Scholar]
- 2.Bromley, S. K., Burack, W. R., Johnson, K. G., Somersalo, K., Sims, T. N., Sumen, C., Davis, M. M., Shaw, A. S., Allen, P. M. & Dustin, M. L. (2001) Annu. Rev. Immunol. 19 375-396. [DOI] [PubMed] [Google Scholar]
- 3.Simons, K. & Toomre, D. (2000) Nat. Rev. Mol. Cell Biol. 1 31-39. [DOI] [PubMed] [Google Scholar]
- 4.Dustin, M. L. & Chan, A. C. (2000) Cell 103 283-294. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Mouton, C., Abad, J. L., Mira, E., Lacalle, R. A., Gallardo, E., Jimenez-Baranda, S., Illa, I., Bernad, A., Manes, S. & Martinez, A. C. (2001) Proc. Natl. Acad. Sci. USA 98 9642-9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allenspach, E. J., Cullinan, P., Tong, J., Tang, Q., Tesciuba, A. G., Cannon, J. L., Takahashi, S. M., Morgan, R., Burkhardt, J. K. & Sperling, A. I. (2001) Immunity 15 739-750. [DOI] [PubMed] [Google Scholar]
- 7.Bi, K., Tanaka, Y., Coudronniere, N., Sugie, K., Hong, S., van Stipdonk, M. J. & Altman, A. (2001) Nat. Immunol. 2 556-563. [DOI] [PubMed] [Google Scholar]
- 8.Roumier, A., Olivo-Marin, J. C., Arpin, M., Michel, F., Martin, M., Mangeat, P., Acuto, O., Dautry-Varsat, A. & Alcover, A. (2001) Immunity 15 715-728. [DOI] [PubMed] [Google Scholar]
- 9.Shaw, A. S. (2001) Immunity 15 683-686. [DOI] [PubMed] [Google Scholar]
- 10.Delon, J., Kaibuchi, K. & Germain, R. N. (2001) Immunity 15 691-701. [DOI] [PubMed] [Google Scholar]
- 11.Holdorf, A. D., Lee, K. H., Burack, W. R., Allen, P. M. & Shaw, A. S. (2002) Nat. Immunol. 3 259-264. [DOI] [PubMed] [Google Scholar]
- 12.Stuermer, C. A., Lang, D. M., Kirsch, F., Wiechers, M., Deininger, S. O. & Plattner, H. (2001) Mol. Biol. Cell 12 3031-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherukuri, A., Cheng, P. C., Sohn, H. W. & Pierce, S. K. (2001) Immunity 14 169-179. [DOI] [PubMed] [Google Scholar]
- 14.Edidin, M. (2001) Trends Cell Biol. 11 492-496. [DOI] [PubMed] [Google Scholar]
- 15.Friedrichson, T. & Kurzchalia, T. V. (1998) Nature 394 802-805. [DOI] [PubMed] [Google Scholar]
- 16.Varma, R. & Mayor, S. (1998) Nature 394 798-801. [DOI] [PubMed] [Google Scholar]
- 17.Schulte, T., Paschke, K. A., Laessing, U., Lottspeich, F. & Stuermer, C. A. (1997) Development (Cambridge, U.K.) 124 577-587. [DOI] [PubMed] [Google Scholar]
- 18.Volonte, D., Galbiati, F., Li, S., Nishiyama, K., Okamoto, T. & Lisanti, M. P. (1999) J. Biol. Chem. 274 12702-12709. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder, W. T., Stewart-Galetka, S., Mandavilli, S., Parry, D. A., Goldsmith, L. & Duvic, M. (1994) J. Biol. Chem. 269 19983-19991. [PubMed] [Google Scholar]
- 20.Malaga-Trillo, E., Laessing, U., Lang, D. M., Meyer, A. & Stuermer, C. A. (2002) J. Mol. Evol. 54 235-245. [DOI] [PubMed] [Google Scholar]
- 21.Bickel, P. E., Scherer, P. E., Schnitzer, J. E., Oh, P., Lisanti, M. P. & Lodish, H. F. (1997) J. Biol. Chem. 272 13793-13802. [DOI] [PubMed] [Google Scholar]
- 22.Hazarika, P., Dham, N., Patel, P., Cho, M., Weidner, D., Goldsmith, L. & Duvic, M. (1999) J. Cell Biochem. 75 147-159. [PubMed] [Google Scholar]
- 23.Solomon, S., Masilamani, M., Rajendran, L., Bastmeyer, M., Stuermer, C. A. & Illges, H. (2002) Immunobiology 205 108-119. [DOI] [PubMed] [Google Scholar]
- 24.Brown, D. A. & Rose, J. K. (1992) Cell 68 533-544. [DOI] [PubMed] [Google Scholar]
- 25.Brown, D. A. & London, E. (1997) Biochem. Biophys. Res. Commun. 240 1-7. [DOI] [PubMed] [Google Scholar]
- 26.Lang, D. M., Lommel, S., Jung, M., Ankerhold, R., Petrausch, B., Laessing, U., Wiechers, M. F., Plattner, H. & Stuermer, C. A. (1998) J. Neurobiol. 37 502-523. [DOI] [PubMed] [Google Scholar]
- 27.Janes, P. W., Ley, S. C. & Magee, A. I. (1999) J. Cell Biol. 147 447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villalba, M., Bi, K., Rodriguez, F., Tanaka, Y., Schoenberger, S. & Altman, A. (2001) J. Cell Biol. 155 331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder, T., Scheiffele, P., Verkade, P. & Simons, K. (1998) J. Cell Biol. 141 929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gousset, K., Wolkers, W. F., Tsvetkova, N. M., Oliver, A. E., Field, C. L., Walker, N. J., Crowe, J. H. & Tablin, F. (2002) J. Cell. Physiol. 190 117-128. [DOI] [PubMed] [Google Scholar]
- 31.Madore, N., Smith, K. L., Graham, C. H., Jen, A., Brady, K., Hall, S. & Morris, R. (1999) EMBO J. 18 6917-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouy, H., Deterre, P., Debre, P. & Bismuth, G. (1994) J. Immunol. 152 3271-3281. [PubMed] [Google Scholar]
- 33.Baumann, C. A., Ribon, V., Kanzaki, M., Thurmond, D. C., Mora, S., Shigematsu, S., Bickel, P. E., Pessin, J. E. & Saltiel, A. R. (2000) Nature 407 202-207. [DOI] [PubMed] [Google Scholar]
- 34.Ilangumaran, S. & Hoessli, D. C. (1998) Biochem. J. 335 433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabouridis, P. S., Janzen, J., Magee, A. L. & Ley, S. C. (2000) Eur. J. Immunol. 30 954-963. [DOI] [PubMed] [Google Scholar]
- 36.Edidin, M. (2001) Science STKE 2001 PE1. [DOI] [PubMed] [Google Scholar]
- 37.Ostermeyer, A. G., Beckrich, B. T., Ivarson, K. A., Grove, K. E. & Brown, D. A. (1999) J. Biol. Chem. 274 34459-34466. [DOI] [PubMed] [Google Scholar]
- 38.Melkonian, K. A., Chu, T., Tortorella, L. B. & Brown, D. A. (1995) Biochemistry 34 16161-16170. [DOI] [PubMed] [Google Scholar]
- 39.Morrow, I. C., Rea, S., Martin, S., Prior, I. A., Prohaska, R., Hancock, J. F., James, D. E. & Parton, R. G. (2002) J. Biol. Chem. 277 48834-48841. [DOI] [PubMed] [Google Scholar]
- 40.Schenkman, L. R., Caruso, C., Page, N. & Pringle, J. R. (2002) J. Cell Biol. 156 829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drubin, D. G. & Nelson, W. J. (1996) Cell 84 335-344. [DOI] [PubMed] [Google Scholar]
- 42.Hung, D. T., Jamison, T. F. & Schreiber, S. L. (1996) Chem. Biol. 3 623-639. [DOI] [PubMed] [Google Scholar]
- 43.Doxsey, S. (2001) Nat. Rev. Mol. Cell Biol. 2 688-698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.