Abstract
Recently, p53 was demonstrated to affect the expression of the insulin-like growth factor 1 receptor (IGF-1R), a receptor tyrosine kinase that plays a crucial role in growth and survival of cancer cells. However, the underlying mechanisms for interaction between p53 and IGF-1R are still not fully understood. One of the challenging questions remaining to be answered is why the wild-type p53, which per se represses the transcription of the IGF-1R gene, in overexpressed form is necessary for a high IGF-1R expression. In this study, we show that inhibition of p53 causes ubiquitination and down-regulation, through increased degradation, of the IGF-1R in human malignant melanoma cells. This effect, which was independent of the p53 status (i.e., wild type or mutated), was prevented if Mdm2 was coinhibited. Similar results were obtained in UV-irradiated human melanocytes (harboring wild-type p53), in which level of the IGF-1R increased after up-regulation of p53. Interestingly, the basal ubiquitination of the IGF-1R in untreated cells also depended on Mdm2. We could prove that Mdm2 physically associates with IGF-1R and that Mdm2 causes IGF-1R ubiquitination in an in vitro assay. Taken together our data provide evidence that Mdm2 serves as a ligase in ubiquitination of the IGF-1R and thereby causes its degradation by the proteasome system. Consequently, by sequestering Mdm2 in the cell nuclei, the level of p53 may indirectly influence the expression of IGF-1R. This role of Mdm2 and p53 represents an unexpected mechanism for the regulation of IGF-1R and cell growth.
Keywords: cell growth, p53
The insulin-like growth factor 1 receptor (IGF-IR) has been shown to be crucial for tumor transformation, maintenance of tumorigenicity, promotion of cell growth, and prevention of apoptosis (1–3). IGF-1R is often overexpressed in malignant tumors (1–3). All of these circumstances make the IGF-1R an intriguing target in cancer. IGF-IR is a heterotetrameric receptor tyrosine kinase composed of two extracellular α-subunits, which are involved in ligand binding, and two transmembrane β-subunits that contain tyrosine kinase domains involved in signal transduction (4).
The mechanisms behind up-regulation of IGF-1R in tumor cells are still poorly understood. However, several studies during the last 8 years have reported intriguing connections between the IGF-1R and the p53 pathways (5–8). The actions of the IGF-1R and p53 pathways are distinctly opposite. Whereas IGF-1R promotes mitogenic and antiapoptotic signals, wild-type p53 induces cell-cycle arrest and apoptosis.
Along with the diversity of genetic modifications in cancer, p53 abnormalities are probably the most prevalent defects (9). The roles played by p53 in cancer can be greatly simplified in three main categories: (i) loss of normal p53 functions, mainly related to the role of p53 as a transcription factor and acquired by mutations; (ii) dominant negative effect of mutant p53, abrogation of wild-type p53 as a result of heteromerization with mutant p53; and (iii) gain of function, the ability of mutant p53 to acquire new functions (10, 11). However, an interesting observation on p53 mutations is that most of them do not result in loss of proteins that mostly is the case with other tumor suppressor proteins. Quite the opposite, cancer cells usually accumulate the mutant and even the wild-type p53 proteins (12). The mechanisms of gain-of-function are still poorly understood. The p53 protein level is principally regulated by its interaction with Mdm2 by means of a regulatory feedback loop. Mdm2 ubiquitinates both p53 and itself, contributing to the rapid turnover of both proteins (13). Like p53, Mdm2 shuttles from the nucleus to the cytoplasm (14), and the shuttling of Mdm2 may be important for p53 export in some cells.
Werner et al. (6) have shown that wild-type p53 represses the transcription of the IGF-1R gene, whereas mutant p53 had the opposite effect. These studies were performed on p53-negative cells transfected with wild-type or mutant p53 cDNA, using reporter gene strategy.
Recently, we demonstrated that treatment with antisense oligodeoxynucleotides for p53 leads to down-regulation of the IGF-1R at the plasma membrane of malignant melanoma cells (8). Unexpectedly, however, the same response was obtained in melanoma cells overexpressing wild-type p53. It should be added here that the incidence of p53 mutations in malignant melanoma is very low, whereas p53 overexpression is common in this disease (15, 16). Even though the mechanisms underlying the effect of p53 on IGF-1R expression are not fully known, these data suggest that posttranscriptional events might be involved in p53-dependent regulation of IGF-1R (8).
The aim of the present study was to search for posttranscriptional events involved in p53-dependent expression of IGF-1R. Our results provide evidence that p53/Mdm2 is involved in ubiquitination and degradation of the IGF-1R.
Materials and Methods
Reagents. A mouse monoclonal antibody against the human IGF-1R was purchased from Oncogene Science. Polyclonal IGF-1R antibodies (N-20, C-20, and H-60), a mouse monoclonal antibody against human p53 (DO1), a mouse monoclonal antibody to Mdm2 (including the p53-Mdm2 complex), a monoclonal antibody to phosphotyrosine (PY99), and an antibody to actin (H-196) were from Santa Cruz Biotechnology. The proteasome inhibitor MG 132 was from Calbiochem. All other reagents unless stated otherwise were from Sigma.
Cell Lines. The human melanoma cell lines SK-MEL-5 and SK-MEL-28 were from American Type Culture Collection. The melanoma cell lines BE and DFB were provided by Rolf Kiessling (Karolinska Hospital). The R- and P6 mouse cell lines were gifts from Renato Baserga (Thomas Jefferson University, Philadelphia). The R- fibroblasts are IGF-1R negative and are derived from an IGF-1R knockout mouse embryo (17). The P6 line is a 3T3 derivative that overexpresses the human IGF-1R (17). The cells were cultured in DMEM supplemented with 10% (R-) or 5% (P6) FBS. P6 and R- cell lines were cultured in the presence of G-418 (Promega). Neonatal epidermal melanocytes were from Clonetics (San Diego) and cultured in melanocyte cell growth medium-2 supplemented (for 500 ml) with 7.5 mg/ml bovine pituitary extract, 0.5 μg of human recombinant fibroblast growth factor, 5 μg of phorbol myristate acetate, 0.25 mg of hydrocortisone, 2.5 mg of insulin, 25 mg of gentamycin, 25 μg/ml Amphotericin-B, and 2.5 ml of FBS according to the manufacturer's protocol.
Antisense Experiments. The antisense experiments were essentially performed as described (8). Antisense and sense oligodeoxynucleotides were purchased from Interactiva (Ulm, Germany). The sequences of the oligodeoxynucleotides for p53 and human and murine Mdm2 have been described (8, 18, 19). All oligodeoxynucleotides used were phosphorothiolated to protect them from the cell nucleases. Lipofectin (Life Technologies, Grand Island, NY) was used to deliver antisense oligodeoxynucleotides to the cultured cells. Because antisense oligodeoxynucleotides induce RNase H cleavage and further degradation of target mRNA, we tested the specificity of the antisense oligodeoxynucleotides using semiquantitative RT-PCR as described (8).
SDS/PAGE and Western Blotting. Protein samples were dissolved in a sample buffer containing 0.0625 M Tris·HCl (pH 6.8), 20% glycerol, 2% SDS, bromphenol blue, and DTT. Samples corresponding to 50–100 μg of cell protein were analyzed by SDS/ PAGE with a 7.5% or 10% separation gel. Molecular weight markers (Bio-Rad) were run simultaneously. After SDS/PAGE, the proteins were transferred overnight to nitrocellulose membranes (Amersham Pharmacia) and then blocked for 1 hat room temperature in a solution of 5% (wt/vol) skimmed milk powder and 0.02% (wt/vol) Tween 20 in PBS, pH 7.5. Incubation with appropriate primary antibodies was performed for 1 h at room temperature. This was followed by washes with PBS and incubation with a biotinylated secondary antibody (Amersham Pharmacia) for 1 h. After incubation with streptavidin-labeled horseradish peroxidase, the detection was made (Hyperfilm-ECL, Amersham Pharmacia). The films were scanned by Fluor-S (Bio-Rad).
Assay of Cell Growth and Survival. We performed the determinations with the cell proliferation kit II (Roche Diagnostics), which is based on the colorimetric change of the yellow tetrazolium salt XTT to orange formazan dye by the respiratory chain of viable cells (20). All standards and experiments were performed in triplicates.
UV Light Treatment. Cells were treated with UV light essentially as described (21). Before UV irradiation, cells were washed twice with PBS, irradiated through a thin film of PBS, and refed with their own medium. Cells were exposed through the cover of the dish, filtering out residual UVC. The UV source was a bank of two Philips TL 20W01 tubes with a peak output ≈310 nm (UVB). This spectrum represents the solar radiation spectrum that most actively induces genotoxic and carcinogenic effects. The dose used for UVB was 5 J/m2 and was optimized to induce maximal p53 expression with <10% decrease in cell viability 24 h after UV exposure.
Immunoprecipitation. The isolated cells were lysed as described (22). Fifteen microliters of protein G plus-A/G agarose and 1 μg of antibody were added to 1 mg of protein material. After overnight incubation at 4°C on a rocker platform, the immunoprecipitates were collected by centrifugation in a microcentrifuge at 2,500 rpm for 2 min. The supernatant was discarded, whereupon the pellet was washed and then dissolved in a sample buffer for SDS/PAGE.
Analysis of IGF-1R Synthesis and Degradation. After indicated experimental procedures, cells were transferred to methionine-free DMEM supplemented with 10% FBS and 100 μCi/ml l-[35S]methionine (specific activity >1,000 Ci/mM, Amersham Pharmacia) for 6 or 24 h. To determine IGF-1R synthesis, the cells were quickly washed twice with ice-cold PBS and lysed in RIPA buffer (1 × PBS/1% Triton-X-100/0.5% sodium deoxycholate/0.1% SDS), supplemented with protease inhibitor tablets (Roche Diagnostics). An equal amount of protein from each sample was immunoprecipitated with antibodies for the IGF-1R β-subunit (H-60) collected by protein A Sepharose (CL-4B, Amersham Pharmacia), resolved by SDS/PAGE, and visualized by autoradiography.
IGF-1R degradation was determined by pulse–chase experiments. The cells were, after the labeling with [35S]methionine (see above), carefully washed and transferred to radioactive-free DMEM containing 10% FBS for the indicated time periods. Cells were then harvested for detection of radioactive IGF-1R as described above.
IGF-1R/Mdm2 Interaction in Vitro. Mdm2 glutathione Sepharose beads were mixed with total protein extracts (500 μg) from P6 or R- cells. After 60 min of incubation at room temperature, the beads were washed three times with PBS, dissolved in SDS sample buffer, loaded on a 7.5% gel, and visualized after transfer to a nitrocellulose membrane with an anti-IGF-1R antibody (C20).
In Vitro Ubiquitination. In vitro ubiquitination of IGF-1R was performed essentially as described (23). Recombinant GST-Mdm2 was expressed in Escherichia coli and purified by using glutathione-Sepharose (Pierce). IGF-1R was isolated from P6 cells by immunoprecipitation with a polyclonal rabbit antibody directed against β-subunit (H60) and protein G-Sepharose (Amersham Pharmacia). IGF-1R Sepharose beads were mixed with or without GST-Mdm2, rabbit E1 (Calbiochem), E2 bacterial recombinant UbcH5B (Calbiochem), and His-6-Ubiquitin (Calbiochem) in a 30-μl reaction. After a 1-h incubation period at 37°C, the reaction was stopped by addition of SDS sample buffer. Reaction products were loaded on a 7.5% polyacrylamide gel, transferred to nitrocellulose membrane, and detected by using either antibody against IGF-1R (C20) or an anti-His tag antibody.
Results
Effects of p53 and Mdm2 Inhibition on IGF-1R and Cell Growth. Two human melanoma cell lines harboring wild-type p53 (MEL-5 and DFB) and two with hot spot p53 mutations (BE and MEL-28), all of which exhibit high IGF-1R expression (8), were treated with antisense oligodeoxynucleotides to p53 (ASp53), Mdm2 (ASMdm2) or both. The detailed conditions for optimization of the antisense experiments, including all necessary controls, have been described (8, 24). The effects on protein levels of p53, Mdm2, and IGF-1R β-subunit detected by Western blots for MEL-5 and MEL-28 are shown in Fig. 1A, and densitometry data (for quantification) of all four cell lines are shown in Fig. 1B. Consistent with our previous study (8), disruption of p53 expression caused IGF-1R down-regulation in all four cell lines. However, Mdm2 inhibition also drastically decreased IGF-1R, but only in cells harboring wild-type p53. This finding is completely consistent with the notion that wild-type p53, no longer inactivated by Mdm2, decreases transcription of the IGF-1R gene (6, 8). Surprisingly, combined treatment with ASp53 and ASMdm2 substantially decreased the effect of p53 inhibition on IGF-1R (strongest in the p53 wild-type cells), although the protein levels of both p53 and Mdm2 remained adequately decreased (Fig. 1 A). Similar results were obtained in all four cell lines (Fig. 1B).
Fig. 1.
Effect of p53 and Mdm2 inhibition on IGF-1R and cell growth on malignant melanoma cells. (A and B) The indicated cell lines either remained untreated (C) or were treated with Lipofectin (Lipofectin control, L), L + ASp53 (1.0 μM), L + ASMdm2 (2.0 μM), or L + ASp53 + ASMdm2 and with corresponding sense oligodeoxynucleotides (Sp53 and SMdm2) as indicated. After incubations for 24 h, the protein expression of p53, Mdm2, IGF-1R, and actin (loading control) was determined. Western blots for MEL-5 and MEL-28 are shown in A, and densitometry data (expressed as % of Lipofectin control) for all four cell lines are shown in B.(C) All four cell lines were treated as described in A but for 48 h. The amounts of surviving cells were determined by the XTT assay. The values are means and SDs of triplicates. It was confirmed in all experiments that Sp53 and SMdm2 were without effects (not shown in B and C). The experiments were repeated three to four times with similar results.
We also tested the effects of the above conditions on cell growth and survival of the four cell lines (Fig. 1C). As shown, both ASp53 and ASMdm2 inhibited growth of the two p53 wild-type cell lines (DFB and MEL-5) after 48 h of incubation, whereas upon cotreatment, cell growth was hardly affected. The mutant cell lines (BE and MEL-28) responded similarly to p53 inhibition as the p53 wild-type cells did, but only marginally to ASMdm2. As was the case with wild-type cells, the combined treatment regimen significantly decreased the growth inhibitory effect of ASp53 (Fig. 1C). Thus, the effects of the AS treatments on IGF-1R expression and cell growth correlate well with each other (compare Fig. 1 B and C). These data support the hypothesis that p53-dependent control of the IGF-1R may involve the action of Mdm2.
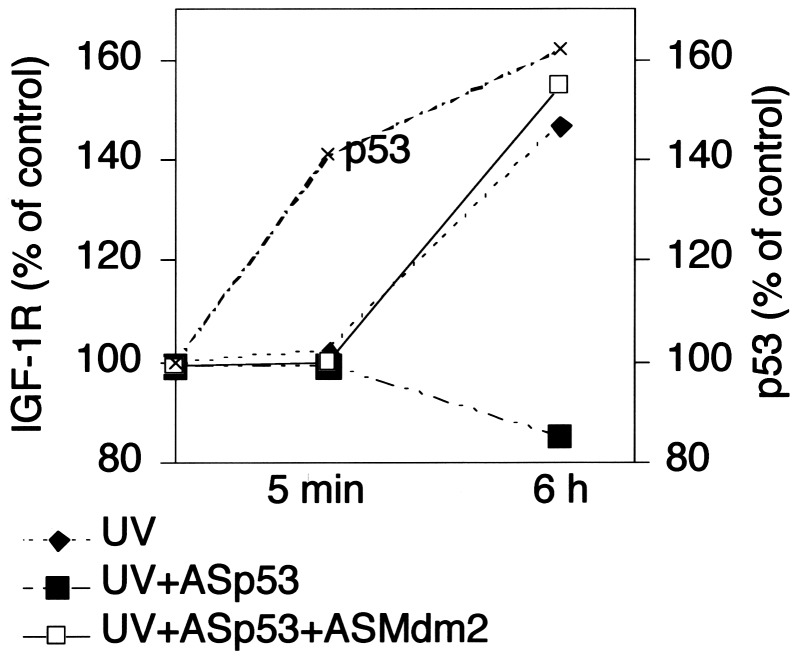
Effect of p53 on IGF-1R in UV-Irradiated Cells. We also studied the effect of p53 and Mdm2 abrogation on IGF-1R expression in UV-irradiated human melanocytes (harboring wild-type p53). Fig. 2 shows that 6 h after the irradiation the level of IGF-1R increased substantially. Inhibition of p53 expression, which increased immediately after irradiation, prevented the IGF-1R up-regulation. However, upon coinhibition of Mdm2, the inhibitory effect of the p53 down-regulation on IGF-1R expression was deleted.
Fig. 2.
Effect of UV irradiation on IGF-1R expression of cultured melanocytes and effect of inhibition of p53 and Mdm2. Control cells or cells treated with ASp53 (1 μM) or ASMdm2 (1 μM) were exposed to 5 J/m2 UVB. Western blotting was performed to measure IGF-1R, and quantification was made by densitometry. The p53 levels (also assayed by Western blotting and densitometry) of irradiated control (not treated with AS) cells are indicated in the graph. For further details, see Materials and Methods. The experiment was repeated twice with similar results.
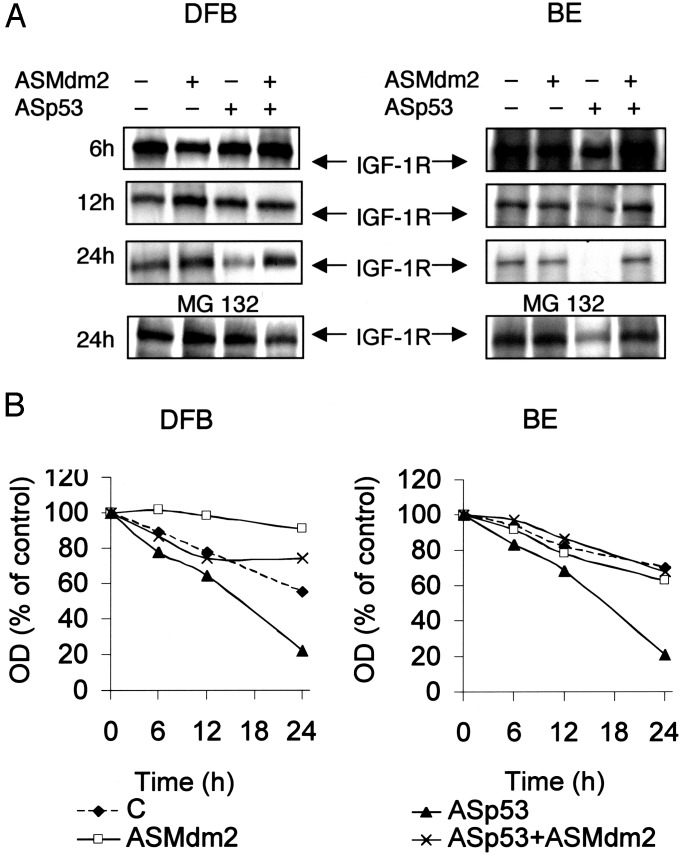
Effect on IGF-1R Degradation. We investigated the effects of p53 or Mdm2 inhibition (or both), obtained by antisense oligodeoxynucleotides, on the degradation of IGF-1R. This was performed on the DFB (p53 wild type) and BE (p53 mutant) cell lines by using pulse–chase experiments with [35S]methionine. Fig. 3A shows the autoradiographic detections of the IGF-1R β-subunit, and Fig. 3B shows the densitometry data obtained from the autoradiographs. The DFB control cells exhibited 22% and 45% degradation of IGF-1R after 12 and 24 h, respectively, whereas radiolabeled IGF-1R in the BE was slightly less decreased (18% and 30%, respectively) (Fig. 3B). The corresponding values for cells treated with ASp53 were 35% and 78%, and 31% and 79%, respectively, whereas there was no increased degradation (rather the opposite in DFB), compared with the controls, upon inhibition of Mdm2 and, notably, after coinhibition of Mdm2 and p53. The relatively late increase in IGF-1R degradation by p53-inhibited cells is most likely due to the delayed increase in the inhibitory effect on the p53 protein. First after 12 h of incubation with antisense oligodeoxynucleotides, there was an ≈50% decrease in the p53 protein levels (data not shown), which is consistent with other studies using ASp53 (25). As demonstrated in Fig. 3A Lower, the ASp53-induced increase (after a 24-h treatment) in IGF-1R degradation was largely counteracted by the 26S proteasome inhibitor MG 132, which was present during the last 6 h of the incubations in separate experiments.
Fig. 3.
Effect of p53 and Mdm2 inhibition on IGF-1R degradation. (A) Wild-type (DFB) and p53 mutant (BE) cells were labeled with [35S]methionine (100 μCi/ml) for 24 h in methionine-free medium, and thereafter transferred to radioactive-free methionine-supplemented medium containing ASp53, ASMdm2, or both, or corresponding Ss for the indicated times. In separate dishes, cells incubated for 24 h were treated with MG 132 (50 μM) during the last 6 h (Bottom). IGF-1R β-subunit was immunoprecipitated and resolved by SDS/PAGE and finally visualized by autoradiography. It was confirmed that the sense oligodeoxynucleotides (Sp53 and SMdm2) were without effects (not shown). (B) Densitometry data are shown. The results are representative of three independent experiments.
We also assessed de novo synthesis of the IGF-1R by using pulse labeling with [35S]methionine. After a 6-h pulse labeling, we could detect incorporation of [35S]methionine into the proreceptor, in line with the study of Sepp-Lorenzino et al. (26). Pretreatment with ASp53 did not change this incorporation (data not shown), indicating that the inhibition of p53 does not affect de novo synthesis of the IGF-1R.
Ubiquitination of IGF-1R and Association with Mdm2. Degradation of signal-transducing cell-surface receptors is mediated by internalization and endocytosis. Internalized receptors can either be transported to the lysosomes, where they are degraded, or be recycled to the plasma membrane (27). Recent experimental data have identified the ubiquitin proteasome pathways as a regulatory system for endocytosis (27–30). Ubiquitin is a polypeptide, 76 aa in length, playing many cellular functions (29). Ubiquitination of proteins requires the action of three enzymes: (i) ubiquitin-activating enzyme (E1), which binds to ubiquitin to generate a high energy E1-ubiquitin intermediate; (ii) ubiquitin-conjugating enzyme (E2), a ubiquitin carrier protein; and (iii) a ubiquitin ligase that transfers the ubiquitin to the target protein (31, 32). E3 plays a key role in the ubiquitin-mediated pathways because it serves as the specific recognition factor.
By considering the data in Figs. 1, 2, 3, it can be hypothesized that inhibition of p53 expression disrupts the balance between Mdm2 and p53; Mdm2 is no longer sequestered by p53 and may therefore exert others functions (33). Because Mdm2 has been defined as an E3 ligase, and moreover its involvement in ubiquitination of some membrane proteins has been clearly demonstrated (34), it would be attractive to investigate whether Mdm2 associates with and ubiquitinates the IGF-1R and thereby triggers its degradation.
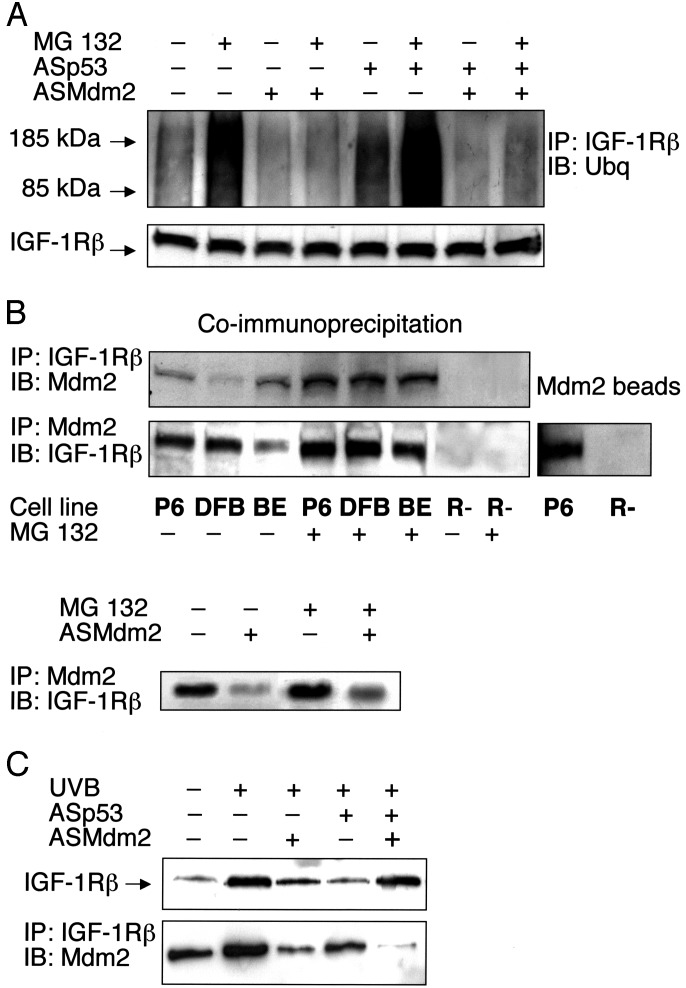
Based on the idea that IGF-1R may be ubiquitinated and degraded after inhibition of p53, we have now investigated whether treatment with ASp53 can induce ubiquitination of the IGF-1R. DFB cells were either untreated or transfected with antisense oligodeoxynucleotides for p53 or Mdm2 for a period of 18 h, with and without MG 132 present during the last 6 h. The β-subunit of IGF-1R was isolated by immunoprecipitation and analyzed by Western blotting using antibodies for ubiquitin. Fig. 4A shows that the IGF-1R β-subunit from ASp53- and 26S proteasome inhibitor-treated cells exhibits strong ubiquitin signals at sizes of 85–185 kDa. The high molecular smear-like bands are typical for multiubiquitinated proteins (30). This implies that ubiquitin is bound to the β-subunit of the IGF-1R and suggests that the receptor degradation is proteasome-dependent. However, upon coinhibition of p53 and Mdm2, there were no visible IGF-1R ubiquitination. Inhibition of only Mdm2 did not increase the amount of ubiquitinated IGF-1R compared with the controls, which in fact exhibited weak yet detectable ubiquitin signals (Fig. 4A). Rather, treatment with ASMdm2 reduced the baseline ubiquitination of the IGF-1R β-subunit. The results indicate that Mdm2 may be involved in IGF-1R ubiquitination.
Fig. 4.
Ubiquitination of IGF-1R and association with Mdm2. (A) DFB cells either remained untreated or were treated with ASp53, ASMdm2, or both as indicated for 12 h, after which the proteasome inhibitor MG 132 (50 μM) was added, or not, for an additional 6 h. IGF-1R was immunoprecipitated by a
β-subunit antibody. Equal amounts of purified IGF-1R immunoprecipitates (to compensate for decrease in ASp53-treated cells), verified in the lower panel, were resolved by SDS/PAGE and finally detected by Western blotting using an antibody to ubiquitin. (B Upper) Total proteins isolated from the indicated cell lines were immunoprecipitated with an IGF-1R β-subunit antibody and immunoblotted by an Mdm2 antibody, or vice versa. (B Lower Left) BE cells were pretreated with ASMdm2 for 12 h, and the proteasome inhibitor MG 132 was added, or not, for an additional 6 h before coimmunoprecipitation. (B Right) Total protein lysates from P6 and R- cells were mixed with Sepharose beads of recombinant Mdm2 (see Materials and Methods). The Mdm2 beads were analyzed by Western blotting using an IGF-1R β-subunit antibody. (C Upper) UV-irradiated human melanocytes were treated as indicated for 6 h. Thereafter, analysis of IGF-1R was performed as described for the experiments on the melanoma cell lines. (C Lower) UV-irradiated melanocytes were treated as indicated for 6 h. Immunoprecipitation using an IGF-1R β-subunit antibody and immunoblotting with an Mdm2 antibody was then done. The experiments were repeated two to four times with similar results.
Next, we investigated whether Mdm2 could be physically associated with the IGF-1R. For this purpose, we first immunoprecipitated the IGF-1R β-subunit or Mdm2 from protein extracts derived from DFB and BE cells, a cell line overexpressing IGF-1R (P6), and IGF-1R negative cells (R-) (serving as a negative control). We then probed with antibodies to Mdm2 or to the IGF-1R β-subunit, respectively. Irrespective of which antibody was used in the immunoprecipitation step, our data prove that the Mdm2 and IGF-1R β-subunit associates with each other (Fig. 4B Upper). The same results were obtained from all of the three IGF-1R positive cell lines. In a separate experiment, it was confirmed that ASMdm2 deleted the complex formation of IGF-1R and Mdm2 (Fig. 4B Lower Left). Consistent results were also obtained in a cell-free/antibody-free system, using recombinant Mdm2 beads to extract IGF-1R from P6 total protein lysate (Fig. 4B Right). We repeated the coimmunoprecipitation experiments using different antibodies several times with similar results.
As was already shown in Fig. 2, inhibition of p53 in UV-irradiated melanocytes resulted in deletion of the increase in IGF-1R expression. However, this effect was neutralized by Mdm2 coinhibition. In Fig. 4C Upper, the IGF-1R levels at 6 h after UV irradiation are shown. This also involves the effects of ASp53 and ASMdm2, or both. The results are similar to those found in melanoma cells harboring wild-type p53 (MEL-5, DFB) (compare Fig. 1 A and B). In Fig. 4C Lower, it is demonstrated that IGF-1R is associated with Mdm2. The IGF-1R/Mdm2 complex was increased in UV-irradiated cells, which is explained by the up-regulation of IGF-1R (Fig. 4C Upper), but also by an increase in Mdm2, which has been shown to reach a maximal level 6 h after a UV exposure (35). When the cells were treated with ASMdm2, there were almost no visible IGF-1R–Mdm2 complexes.
Taken together, the results presented in Fig. 4 demonstrate that Mdm2 is associated with the IGF-1R β-subunit and may be involved in ubiquitination of IGF-1R.
Mdm2-Dependent Ubiquitination of IGF-1R. Fig. 5A Upper Left demonstrates the effect of Mdm2 silencing on the basal ubiquitination of IGF-1R in P6 cells (which overexpress IGF-1R). Compared with other cells, basal IGF-1R ubiqutination is much higher in this cell line. The corresponding phenomenon was also shown in cells overexpressing the β2-adrenergic receptor (34). As shown, Mdm2 inhibition, induced by antisense oligodeoxynucleotides, causes a drastic decrease (75–80%) in the amount of ubiquitinated IGF-1R (Fig. 5A Lower Left). In Fig. 5A Right, it is shown that the IGF-1R from UV-treated melanocytes is ubiquitinated. When the cells have been treated with antisense oligodeoxynucleotides to Mdm2, this ubiquitination does not occur.
Fig. 5.
Mdm2-dependent ubiquitination of IGF-1R. (A Left) P6 cells either remained untreated or were treated with ASMdm2 for 18 h, with or without the proteasome inhibitor during the last 6 h. Equal amounts of purified immunoprecipitated IGF-1R β-subunit were analyzed by Western blotting using a ubiquitin antibody. The OD values for ubiquitinated IGF-1R are shown in A Lower.(A Right) UV-irradiated melanocytes either remained untreated or were treated with ASMdm2 as indicated for 6 h, whereupon immunoprecipitation and Western blot using ubiquitin antibodies were carried out. (B) In vitro ubiquitination of IGF-1R. Sepharose beads of IGF-1R, isolated from P6, were mixed with ubiquitin reagents with or without GST-Mdm2 as described in Materials and Methods. Equal amounts of purified IGF-1R immunoprecipitates (verified in Lower) were resolved by SDS/PAGE and finally detected by Western blotting using an antibody to His-tagged ubiquitin. The results are representative of three independent experiments.
To confirm the involvement of Mdm2 in ubiquitination of IGF-1R, we have investigated the effect of Mdm2 in vitro (cell-free) preparations by using a ubiquitin assay (Fig. 5B). As is clearly shown, the presence of Mdm2 (together with E1 and E2) in the reaction results in the ubiquitination of the IGF-1R β-subunit.
By comparing the in vitro ubiquitination of IGF-1R β-subunit (in Fig. 5B) with IGF-1R ubiquitination of cultured cells (especially in Fig. 5A), weak 85- to 95-kDa bands can be distinguished in the latter conditions. These bands do not appear in the in vitro ubiquitination. Presumably, they represent a fraction of mono- or oligo-ubiquitinated IGF-1R. Proteins being mono-ubiquitinated or ubiquitinated with less than four molecules are not degraded by the proteasome. To trigger proteasome degradation, the targeted proteins must be multiubiquitinated (27). Thus, in the cell systems it might be a relative overrepresentation of mono- and/or oligo-ubiquitinated proteins because multiubiquitinated proteins are degraded. The reason mono- or oligoubiquitinated IGF-1Rs do not occur in the cell-free system could be explained by the excess of ubiquitin reagents (allowing full ubiquitination) as well as the absence of the proteasome in the in vitro preparations.
Collectively, our findings show that Mdm2 associates with and ubiquitinates the IGF-1R. This points to an important function of p53/Mdm2 in regulation of this receptor tyrosine kinase. This function seems not to be limited to only malignant cells but may also play a regulatory role in IGF-1R regulation in normal cells.
Discussion
Our findings provide strong evidence that the oncoprotein Mdm2 serves as a ligase (ligase E3) in the ubiquitination of IGF-1R. First, we could demonstrate a physical association of IGF-1R to Mdm2; second, inhibition of p53 expression with maintained expression of Mdm2 led to ubiquitination of IGF-1R; third, coinhibition of Mdm2 expression rescued the cells from ubiquitination and down-regulation of IGF-1R; fourth, addition of Mdm2 together with IGF-1R in an in vitro ubiquitin assay resulted in ubiquitination of IGF-1R.
It is well known that Mdm2 does not exclusively interact with p53. Mdm2 has been demonstrated to bind to Rb and E2F, with resulting increase in E2F gene transactivation (36). Mdm2 also associates with ubiquitin conjugase-like protein TSG101, which has been found to degrade rapidly under conditions when the level of Mdm2 is enhanced (37). There is also evidence that Mdm2 increases degradation of other proteins (36). Recently, it was demonstrated that Mdm2 can ubiquitinate and cause proteasomal degradation of β-arrestin and the β2-adrenergic receptor (34).
It is tempting to speculate that Mdm2 may control the switch between the growth arrest and apoptotic signals of p53 and the cell-cycle progression and antiapoptotic signals of IGF-1R. This switch may depend on the Mdm2 location and/or posttranslational modification. Probably, the major function of nuclear Mdm2 is to control the p53 activity, a function that depends strictly on protein–protein interactions. After detachment from p53, Mdm2 may shuttle back to the cytosol, where it may be degraded by the proteasome or be available for other tasks. Alternatively, mutant type p53 might sequester Mdm2 into the cytoplasm. However, the distribution between the nucleus and cytosol is probably strictly controlled, rendering the cytosol concentration comparably low (33). The Mdm2 concentration in the cytosol depends on its synthesis/degradation rate, and on the phosphatidylinositol-3 kinase-Akt pathway and its interaction with p53 (33). The balance among all of these processes may determine the amount of cytoplasmic Mdm2 that is available for the IGF-1R. A massive p53 expression would deplete the cytoplasmic Mdm2 pool, and as a consequence of this increase, the expression of IGF-1R would be enhanced due to decreased receptor degradation. Furthermore, the phosphatidylinositol-3 kinase-Akt activation after increased IGF-1R expression would accentuate the nuclear Mdm2 localization. For cells expressing wild-type p53, this process would affect p53 activity.
In a broader term, our data point to an intriguing p53-dependent mechanism for IGF-1R regulation. The p53/Mdm2-dependent IGF-1R ubiquitination might decide the fate of the receptor by controlling receptor internalization and the endosomal sorting process. Such a scenario has in fact been observed in epidermal growth factor receptors, in which the putative E3 ubiquitin ligase c-Cbl is involved in receptor ubiquitination (36). Because of various conditions, the ErbBs are either recycled or degraded after ubiquitination by c-Cbl (36). In the case of IGF-1R, Mdm2-dependent ubiquitination may direct the receptor to the degradation pathways instead to the recycling pathways.
An interesting question regarding ligand-induced ubiquitination of plasma membrane receptors is whether this modification induces lysosomal versus proteasomal degradation. Degradation of several mammalian receptors, known to be ubiquitinated, is impaired by inhibitors of proteasome as well as by agents blocking the lysosomal degradation (31, 32). It is possible that a fraction of these receptors is degraded by proteasome, whereas another fraction is degraded by the lysosome. Alternatively, the proteasome and lysosome might destroy different parts of the receptor. A third possibility is that the proteasome mediates degradation of another protein, which in turn is required for an efficient targeting of the receptor to the lysosome (31, 32).
The findings of the present paper suggest a posttranscriptional p53 activity that is grossly exaggerated in cancer cells. Using systems with a strong expression of p53, i.e., malignant melanoma and UV-irradiated cells, we show that the high p53 level (independent of whether p53 is mutated or wild type) is necessary to maintain or induce a high expression of the IGF-1R, which in turn favors survival of both tumor cells and irradiated cells (37).
Acknowledgments
We thank Dr. Galina Selivanova, Karolinska Institute, for generously supplying the MDM-GST construct. We also thank Drs. Rolf Lewensohn and Ulf Wester at the Swedish Radiation Protection Authority for help with the UV source. This study was supported by grants from the Swedish Cancer Society, the Cancer Society in Stockholm, the Jubilee Fund of King Gustaf V, the Swedish Children Cancer Society, and the Karolinska Institute.
This paper was submitted directly (Track II) to the PNAS office. Abbreviation: IGF-IR, insulin-like growth factor 1 receptor.
References
- 1.Baserga, R. (1995) Cancer Res. 55 249-252. [PubMed] [Google Scholar]
- 2.Baserga, R., Resnicoff, M. & Dews, M. (1997) Endocrine 7 99-102. [DOI] [PubMed] [Google Scholar]
- 3.Werner, H. & Le Roith, D. (1997) Crit. Rev. Oncog. 8 71-92. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich, A., Gray, A., Tam, A. W., Yang-Feng, T., Tsubokawa, M., Collins, C., Henzel, W., Le Bon, T., Kathuria, S., Chen, E., et al. (1986) EMBO J. 5 2503-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckbinder, L., Talbott, R., Velasco-Miguel, S., Takenaka, I., Faha, B., Seizinger, B. R. & Kley, N. (1995) Nature 377 646-649. [DOI] [PubMed] [Google Scholar]
- 6.Werner, H., Karnieli, E., Rauscher, F. J. & LeRoith, D. (1996) Proc. Natl. Acad. Sci. USA 93 8318-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prisco, M., Hongo, A., Rizzo, M. G., Sacchi, A. & Baserga, R. (1997) Mol. Cell. Biol. 17 1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girnita, L., Girnita, A., Brodin, B., Xie, Y., Nilsson, G., Dricu, A., Lundeberg, J., Wejde, J., Bartolazzi, A., Wiman, K. G. & Larsson, O. (2000) Cancer Res. 60 5278-5283. [PubMed] [Google Scholar]
- 9.Prives, C. & Hall, P. A. (1999) J. Pathol. 187 112-126. [DOI] [PubMed] [Google Scholar]
- 10.Blagosklonny, M. V. (2000) FASEB J. 14 1901-1907. [DOI] [PubMed] [Google Scholar]
- 11.Cadwell, C. & Zambetti, G. P. (2001) Gene 277 15-30. [DOI] [PubMed] [Google Scholar]
- 12.Oren, M., Damalas, A., Gottlieb, T., Michael, D., Taplick, J., Leal, J., Maya, R., Moas, M., Seger, R., Taya, Y. & Ben-Ze'ev, A. (2002) Biochem. Pharmacol. 64 865. [DOI] [PubMed] [Google Scholar]
- 13.Woods, D. B. & Vousden, K. H. (2001) Exp. Cell Res. 264 56-66. [DOI] [PubMed] [Google Scholar]
- 14.Roth, J., Dobbelstein, M., Freedman, D. A., Shenk, T. & Levine, A. J. (1998) EMBO J. 17 554-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss, J., Schwechheimer, K., Cavenee, W. K., Herlyn, M. & Arden, K. C. (1993) Int. J. Cancer 54 693-699. [DOI] [PubMed] [Google Scholar]
- 16.Weiss, J., Heine, M., Arden, K. C., Korner, B., Pilch, H., Herbst, R. A. & Jung, E. G. (1995) Recent Res. Cancer Res. 139 137-154. [DOI] [PubMed] [Google Scholar]
- 17.Rubini, M., Hongo, A., D'Ambrosio, C. & Baserga, R. (1997) Exp. Cell Res. 230 284-292. [DOI] [PubMed] [Google Scholar]
- 18.Goetz, A. W., van der Kuip, H., Maya, R., Oren, M. & Aulitzky, W. E. (2001) Cancer Res. 61 7635-7641. [PubMed] [Google Scholar]
- 19.Chen, L., Agrawal, S., Zhou, W., Zhang, R. & Chen, J. (1998) Proc. Natl. Acad. Sci. USA 95 195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehm, N. W., Rodgers, G. H., Hatfield, S. M. & Glasebrook, A. L. (1991) J. Immunol. Methods 142 257-265. [DOI] [PubMed] [Google Scholar]
- 21.Maltzman, W. & Czyzyk, L. (1984) Mol. Cell. Biol. 4 1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girnita, L., Wang, M., Xie, Y., Nilsson, G., Dricu, A., Wejde, J. & Larsson, O. (2000) Anticancer Drug Des. 15 67-72. [PubMed] [Google Scholar]
- 23.Fang, S., Jensen, J. P., Ludwig, R. L., Vousden, K. H. & Weissman, A. M. (2000) J. Biol. Chem. 275 8945-8951. [DOI] [PubMed] [Google Scholar]
- 24.Xie, Y., Skytting, B., Nilsson, G., Gasbarri, A., Haslam, K., Bartolazzi, A., Brodin, B., Mandahl, N. & Larsson, O. (2002) Cancer Res. 62 3861-3867. [PubMed] [Google Scholar]
- 25.Hirota, Y., Horiuchi, T. & Akahane, K. (1996) Jpn. J. Cancer Res. 87 735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepp-Lorenzino, L., Ma, Z., Lebwohl, D. E., Vinitsky, A. & Rosen, N. (1995) J. Biol. Chem. 270 16580-16587. [DOI] [PubMed] [Google Scholar]
- 27.Hicke, L. (1999) Trends Cell Biol. 9 107-112. [DOI] [PubMed] [Google Scholar]
- 28.Hicke, L. (2001) Nat. Rev. Mol. Cell Biol. 2 195-201. [DOI] [PubMed] [Google Scholar]
- 29.Hicke, L. (1997) FASEB J. 11 1215-1226. [DOI] [PubMed] [Google Scholar]
- 30.Shih, S. C., Sloper-Mould, K. E. & Hicke, L. (2000) EMBO J. 19 187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonifacino, J. S. & Weissman, A. M. (1998) Annu. Rev. Cell Dev. Biol. 14 19-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glickman, M. H. & Ciechanover, A. (2002) Physiol. Rev. 82 373-428. [DOI] [PubMed] [Google Scholar]
- 33.Strous, G. J. & Schantl, J. A. (2001) Science STKE 2001 PE41. [DOI] [PubMed] [Google Scholar]
- 34.Shenoy, S. K., McDonald, P. H., Kohout, T. A. & Lefkowitz, R. J. (2001) Science 294 1307-1313. [DOI] [PubMed] [Google Scholar]
- 35.Perry, M. E., Piette, J., Zawadzki, J. A., Harvey, D. & Levine, A. J. (1993) Proc. Natl. Acad. Sci. USA 90 11623-11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daujat, S., Neel, H. & Piette, J. (2001) Trends Genet. 17 459-464. [DOI] [PubMed] [Google Scholar]
- 37.Li, L., Liao, J., Ruland, J., Mak, T. W. & Cohen, S. N. (2001) Proc. Natl. Acad. Sci. USA 98 1619-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levkowitz, G., Waterman, H., Zamir, E., Kam, Z., Oved, S., Langdon, W. Y., Beguinot, L., Geiger, B. & Yarden, Y. (1998) Genes Dev. 12 3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decraene, D., Agostinis, P., Bouillon, R., Degreef, H. & Garmyn, M. (2002) J. Biol. Chem. 277 32587-32595. [DOI] [PubMed] [Google Scholar]