Abstract
Coat protein I (COPI)-coated transport vesicles mediate protein and lipid transport in the early secretory pathway. The basic machinery required for the formation of these transport intermediates has been elucidated based on the reconstitution of COPI-coated vesicle formation from chemically defined liposomes. In this experimental system, the coat components coatomer and GTP-bound ADP-ribosylation factor (ARF), as well as p23 as a membrane-bound receptor for COPI coat proteins, were shown to be both necessary and sufficient to promote COPI-coated vesicle formation. Based on biochemical and ultrastructural analyses, we now demonstrate that the catalytic domain of ARF-GTPase-activating protein (GAP) alone is sufficient to initiate uncoating of liposome-derived COPI-coated vesicles. By contrast, ARF-GAP activity is not required for COPI coat assembly and, therefore, does not seem to represent an essential coat component of COPI vesicles as suggested recently [Yang, J. S., Lee, S. Y., Gao, M., Bourgoin, S., Randazzo, P. A., et al. (2002) J. Cell Biol. 159, 69–78]. Thus, a complete round of COPI coat assembly and disassembly has been reconstituted with purified components defining the core machinery of COPI vesicle biogenesis.
Keywords: coatomer, ADP-ribosylation factor, ARF-GAP, p24 protein family
The biogenesis of intracellular transport intermediates involves the membrane recruitment of cytoplasmic coat proteins (1–5). Coat proteins have been shown to polymerize on the membrane surface, a process that is likely to provide a mechanical force that induces membrane curvature (1, 2, 5). The products of this process are coated transport vesicles that are directed to specific target organelles to release their cargo molecules. Thus, cargo sorting is achieved by the enrichment in or exclusion from transport vesicles and proper targeting of transport vesicles to specific organelles.
Three kinds of transport vesicle have been functionally characterized at a molecular level and can be defined by both their membrane origin and their coat proteins (1). Clathrin-coated vesicles are formed from both the plasma membrane and the trans-Golgi network and mediate vesicular trafficking within the endosomal membrane system (5). Coat protein I (COPI)- and COPII-coated vesicles are transport intermediates of the secretory pathway (4, 6, 7). COPII vesicles emerge from the endoplasmic reticulum to export newly synthesized secretory proteins toward the Golgi (4, 6). COPI vesicles instead seem to be involved in both biosynthetic (anterograde) and retrograde transport within the Golgi complex (8), as well as mediating the recycling of proteins from the Golgi to the endoplasmic reticulum (9–11).
COPI vesicles have widely been used as a model system to study the molecular mechanism of transport vesicle biogenesis because they can be generated from purified Golgi membranes in vitro and isolated in a highly purified form (3, 12). Their coat structure consists of the heptameric coatomer complex (13) and the small GTPase ADP-ribosylation factor (ARF) 1 (14). More recently, the core machinery of COPI vesicle formation has been defined based on the development of an experimental system that reconstitutes this process employing chemically defined components (15). In this system, COPI vesicle budding occurs from liposomes with a defined lipid composition that contain the cytoplasmic domain of the integral Golgi membrane protein p23 attached to a lipid anchor (15). From these studies, it can be concluded that the coat proteins coatomer and ARF, the latter being activated by GTP, are the only soluble components required for COPI vesicle formation (15), a view consistent with earlier conclusions based on experiments employing Golgi fractions as donor membranes for COPI budding (16). Whereas other studies suggested particular lipids such as those with acidic properties to function as coat protein receptors (17), the presentation of the cytoplasmic domains of p23 on the surface of liposomes is equally sufficient to promote coat protein binding and results in independence of COPI vesicle budding from particular lipids (15). Because p23 is an abundant component of Golgi membranes (18–20) and the lipid mixtures used by Spang et al. (17) are not closely resembling the lipid composition of the Golgi complex, it has been concluded that coat protein binding to p23 is the principal interaction that initiates COPI coat assembly (2). In this context, both ARF1 in its GDP-bound form and coatomer have been shown to directly interact with p23 (21, 22).
Whereas COPI vesicles were originally identified under conditions that promote intra-Golgi transport (23), they became accessible for a thorough biochemical analysis only because they were found to accumulate in the presence of nonhydrolyzable analogues of GTP such as guanosine 5′-[γ-thio]triphosphate (GTPγS) (12, 24). In analogy to the Ras Q61L mutant (25, 26), an ARF mutant protein (ARF-Q71L) that is incapable of hydrolyzing GTP was generated and used to demonstrate that ARF-mediated GTP hydrolysis is required for COPI coat disassembly (27). ARF-GTPases have a very low intrinsic activity; therefore, ARF-GTPase-activating proteins (ARF-GAPs) are required to trigger appreciable rates of ARF-dependent GTP hydrolysis. A Golgi-localized ARF-GAP specific for ARF1 was discovered whose catalytic domain alone is sufficient to induce the GTPase activity of ARF1 (28). Intriguingly, coatomer is capable of increasing this activity >1,000-fold by forming a ternary complex with ARF1 and the catalytic domain of ARF-GAP (29). However, it remains uncertain how ARF-mediated GTP hydrolysis is regulated during a single round of vesicular transport; i.e., when exactly is the coat removed during the process of vesicle targeting and fusion? This problem is even more complex because it has been shown that ARF-mediated GTP hydrolysis is already required at an early stage of COPI vesicle formation, namely in the proper sorting of cargo proteins during vesicle formation (30–33).
In the present study, we made use of the liposome-based COPI budding assay described in ref. 15 to analyze the minimal requirements for a complete round of COPI coat assembly and disassembly. Based on both biochemical and ultrastructural analyses, we find that the catalytic domain of ARF-GAP is both necessary and sufficient to initiate uncoating of liposome-derived COPI-coated vesicles. By contrast, ARF-GAP does not appear to exert an essential function in the formation of liposome-derived COPI-coated vesicles, a finding that is discussed with special attention to a recent report that proposes an essential role for ARF-GAP as a coat protein (34). In conclusion, a complete round of COPI coat assembly and disassembly has been reconstituted employing chemically defined components defining the core machinery of COPI vesicle biogenesis.
Materials and Methods
Antibodies. For Western blot analyses, the antipeptide antibodies C1PL (β′-COP) (35, 36) and ARF1-C1 were used. The ARF1-C1 antibody is directed against the C-terminal ARF1 sequence CDWLSNQLRNQK that was coupled to keyhole limpet hemocyanin using glutaraldehyde crosslinking. Polyclonal antibodies were raised in rabbits according to standard protocols.
Lipids. All phospholipids and cholesterol were purchased from Avanti Polar Lipids except phosphatidic acid, which was from Sigma. The purified lipids were derived from natural sources (phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol, bovine liver; phosphatidylserin and sphingomyelin, bovine brain; phosphatidic acid, yolk; and cholesterol, wool grease).
Purification of Coatomer, N-myristoylated ARF1, and the Catalytic Domain of ARF-GAP. Rabbit liver coatomer was purified as described (37, 38). Recombinant N-myristoylated human ARF1 as well as the mutant form ARF-Q71L were purified to near homogeneity according to Franco et al. (39). The catalytic domain of ARF-GAP was purified according to Goldberg (29).
Lipopeptide Synthesis and Generation of Liposomes. p23 lipopeptide was synthesized as described (37). Lipopeptide (5 mol%) and lipids (95 mol%, Golgi-like composition; ref. 15) were dissolved in chloroform. A lipid film was generated by evaporation of the solvent applying a gentle N2 stream followed by rehydration of lipids in buffer (25 mM Hepes, pH 7.4/100 mM KCl) at room temperature. After 10 cycles of rapid freezing and thawing with occasional mixing, the resulting suspension of multilammellar liposomes was extruded through 800-nm polycarbonate membranes (Avestin, Mannheim, Germany) 21 times. Extruded liposomes were centrifuged at 18,000 × g for 5 min to remove insoluble material and stored at 4°C.
COPI Budding Assay from Liposomes. A complete incubation (final volume, 100 μl) consisted of liposomes (final lipid concentration, 500 μM), 9 μM coatomer, 3 μM ARF1, and 100 μM GTP or GMPPNP. As opposed to earlier protocols (15, 37), nucleotide exchange efficiency was improved by preincubating ARF1 and liposomes in low magnesium buffer (25 mM Hepes, pH 7.4/100 mM KCl/0.5 mM MgCl2/1 mM EDTA/100 μM GTP) for 30 min at 37°C to promote spontaneous nucleotide exchange (40). Thereafter, magnesium chloride was added at a final concentration of 2.5 mM to stabilize the GTP-bound form of ARF1. In a second step, the budding reaction was initiated by adding coatomer (final concentration 9 μM) followed by incubation of the sample for 30 min at 37°C.
Where indicated, the catalytic domain of ARF1-GAP was added at a final concentration of 3 μM. After incubation for 30 min at 37°C, the samples were adjusted to 55% sucrose (wt/wt) in a final volume of 650 μl (load) and placed on the bottom of a Beckman SW55 centrifugation tube (SCI-Laborgeräte, Weinheim, Germany). The gradients were prepared by adding 400 μl of 50% (wt/wt), 40% (wt/wt), 30% (wt/wt), 20% (wt/wt) sucrose (each prepared with gradient buffer), and 1 ml of gradient buffer (25 mM Hepes, pH 7.4/2.5 mM MgCl2/100 mM KCl). After ultracentrifugation at 150,000 × gav for 4 h at 4°C, the sucrose gradients were fractionated from the top starting with one fraction of 1.2 ml, one fraction of 200 μl, 15 fractions of 100 μl (termed 1–15), and one fraction of 350 μl (fraction 16). The sucrose concentrations of all fractions were determined, and aliquots of the fractions between 20 and 50% sucrose (fractions 3–13) were separated on 7.5–15% SDS gradient gels. Western blot analysis was conducted employing anticoatomer (C1PL) and anti-ARF1 antibodies (ARF1-C1).
Electron Microscopy. Purified COPI vesicles (with or without GAP treatment) were diluted in phosphate buffer (100 mM, pH 7.4) and fixed for 2 h at 4°C by the addition of glutaraldehyde at a final concentration of 2% (wt/vol). Fixed material was then collected by ultracentrifugation at 100,000 × gav for 60 min at 4°C. Sediments were washed with 0.1% tannic acid followed by phosphate buffer (2×). Postfixation with 1% osmium tetroxide was carried out at room temperature for 1 h. For dehydration, samples were treated with increasing concentrations of acetone (30, 50, 70, 90, and 100%; 15 min each) followed by an additional incubation with 100% acetone for 15 min. Subsequently, fixed material was embedded in Spurr's resin (Sigma) and incubated for 48 h at 60°C to allow polymerization. Ultrathin sections were prepared using a Leica UCT ultracut device and stained with 1% aqueous uranyl acetate and 1% lead citrate (41). Samples were analyzed with a Zeiss EM10 transmission electron microscope.
Results
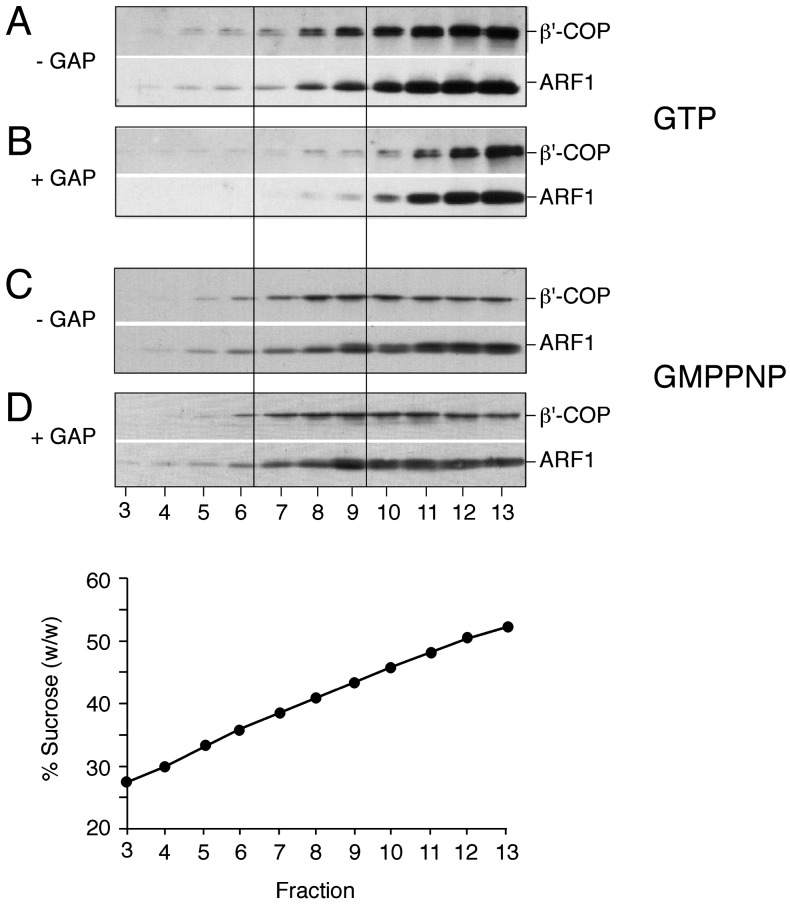
Preincubation of COPI Budding Reactions with the Catalytic Domain of ARF-GAP Results in a Reduced Yield of Liposome-Derived Coated Vesicles. To initiate studies on the molecular requirements for COPI coat disassembly, we first asked whether the addition of a recombinant form of the catalytic domain of ARF-GAP (28) affects the liposome-based COPI budding assay described before (15). Standard mixtures containing p23 lipopeptide-containing liposomes were incubated in the presence of ARF-GTP and coatomer for 30 min at 37°C. Coated vesicle formation was analyzed by flotation in sucrose density gradients based on the immunological detection of ARF and coatomer in fractions 7–9 that are characterized by a buoyant density corresponding to ≈40% sucrose (wt/wt) (Fig. 1A). As shown in Fig. 1B, preincubation of standard mixtures with the catalytic domain of ARF-GAP resulted in a marked decrease of ARF and coatomer immunostaining in fractions 7–9. Thus, the presence of GAP activity dramatically reduces the yield of coated vesicles in this experimental system. To exclude unspecific effects, the experiments shown in Fig. 1 A and B were repeated in the presence of GMPPNP, a nonhydrolyzable analogue of GTP. Under these conditions, GAP activity added before the incubation procedure did not affect the yield of coated vesicles (Fig. 1 C and D). These results establish that ARF-mediated GTP hydrolysis stimulated by the catalytic domain of ARF-GAP results in a decreased yield of coated vesicles as determined with the liposome-based COPI budding assay.
Fig. 1.
COPI budding from liposomes in the presence or absence of the catalytic domain of ARF-GAP. COPI budding assays using Golgi-like liposomes containing p23-lipopeptide, recombinant ARF1, and coatomer were performed in the presence (B and D) or absence (A and C) of the catalytic domain of ARF-GAP. The experiments shown in A and B were performed in the presence of GTP; those shown in C and D are in the presence of GMPPNP. After incubation for 30 min at 37°C, samples were separated by flotation in sucrose density gradients. Fractions 3–13 (see Materials and Methods for details) were separated on SDS gels, followed by immunodetection of β′-COP and ARF1 based on Western blotting. Liposome-derived COPI-coated vesicles migrate in fractions 7–9 corresponding to a sucrose density of ≈40% (wt/wt).
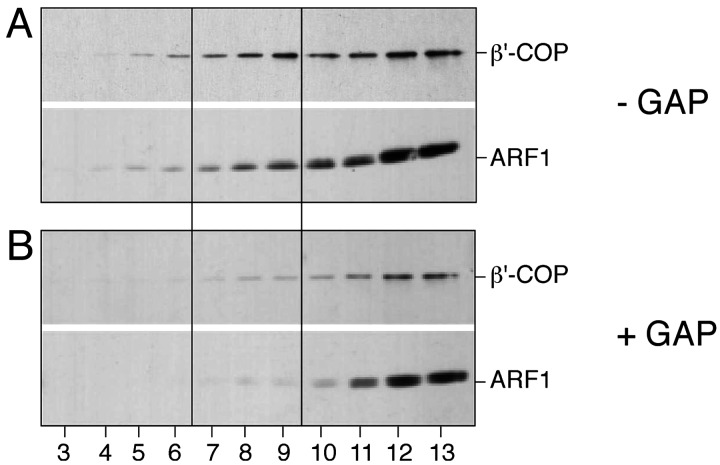
Preformed COPI-Coated Vesicles Get Uncoated in the Presence of the Catalytic Domain of ARF-GAP. The experiments shown in Fig. 1 do not unambiguously demonstrate that GAP activity results in the uncoating of fully formed COPI-coated vesicles, as the formal possibility exists that, under the conditions used, coated vesicles do not form at all. To analyze whether the catalytic domain of ARF-GAP is sufficient to trigger uncoating of preformed COPI vesicles, we conducted standard COPI budding assays in the presence of GTP followed by the addition of ARF-GAP at the end of the incubation procedure. Following 30 min of incubation at 37°C to allow COPI vesicle formation, the sample was split to treat one half with ARF-GAP, whereas the other half was left untreated as a control. Based on ARF and coatomer immunoreactivity in fractions 7 to 9 of the sucrose density gradient, appreciable amounts of coated vesicles could only be detected in mock-treated samples (Fig. 2A), whereas no significant amounts of coated vesicles were detectable after ARF-GAP treatment at the end of the incubation (Fig. 2B). Thus, the yield of coated vesicles in the presence of ARF-GAP activity is consistently low independent of the time point of its addition. Although it remains unresolved whether COPI vesicles can form in the presence of ARF-GAP, these results demonstrate that preformed coated vesicles can be efficiently uncoated after addition of ARF-GAP activity.
Fig. 2.
Postincubation of COPI budding samples with ARF-GAP activity. Liposome-derived COPI-coated vesicles were generated in the presence of GTP as described in the legend of Fig. 1. Thereafter, the sample was split, with one half being mock-treated (A) and the other half incubated with the catalytic domain of ARF-GAP (B). Samples were analyzed by flotation in sucrose density gradients and SDS/PAGE–Western blotting as described in the legend to Fig. 1. For details, see Materials and Methods.
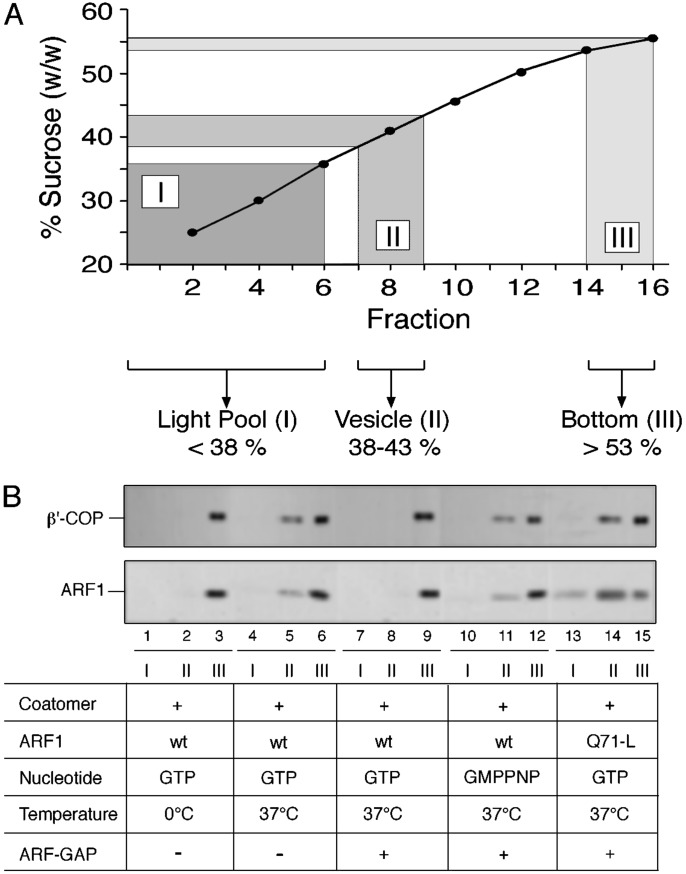
To further substantiate this conclusion biochemically, various control conditions were applied. To minimize loss of material, the sucrose density gradient was divided into three pools (Fig. 3A). Pool I represents the light fractions of the gradient (fractions 1–6) that contain the donor liposomes. Pool II contains coated vesicles (fractions 7–9), whereas pool III represents the load (fractions 14–16) consisting of both free proteins and protein aggregates. To confirm these assignments under various control conditions described earlier (15), budding assays were conducted followed by the analysis of pool I, II, and III for the presence of ARF and coatomer. When standard mixtures were incubated at 0°C, both pool I and pool II were found not to contain any coat proteins (Fig. 3B, lanes 1–3). By contrast, shifting the incubation temperature to 37°C resulted in the appearance of immunoreactivity for ARF and coatomer in pool II (Fig. 3B, lanes 4–6). These results are consistent with the temperature-dependent formation of coated vesicles from p23 lipopeptide-containing liposomes. Consistent with the results depicted in Figs. 1 and 2, immunoreactivity in pool II for ARF and coatomer disappears upon addition of ARF-GAP at the end of the budding reaction (Fig. 3B, lanes 7–9). As expected, this effect is reversed when the budding reaction is performed in the presence of GMPPNP, a condition that prevents GAP-triggered GTP hydrolysis mediated by ARF (Fig. 3B, lanes 10–12). Most strikingly, when a mutant form of ARF, ARF-Q71L (27), that is incapable of hydrolyzing GTP, was used during the budding reaction, coated vesicles are detected in pool II despite the addition of GAP activity at the end of the incubation (Fig. 3B, lanes 13–15). Based on this biochemical analysis, it can be concluded that the catalytic domain of ARF-GAP alone is both necessary and sufficient to initiate uncoating of liposome-derived COPI-coated vesicles.
Fig. 3.
Uncoating of liposome-derived COPI vesicles depends on ARF1-GTP hydrolysis triggered by the catalytic domain of ARF-GAP. Liposome-derived COPI-coated vesicles were generated as described in the legend of Fig. 1. As indicated, a number of parameters such as the use of GTP versus GMPPNP, ARF1 wild-type versus ARF-Q71L, and temperature (37°C versus 4°C) were varied. Samples were separated by flotation in sucrose density gradients as described in the legend to Fig. 1. Fractions were collected as pool I (donor liposomes), pool II (coated vesicles), and pool III (load) as indicated in A. For each experimental condition shown in B, 2.5% of pool I and II as well as 0.5% of pool III were analyzed by SDS/PAGE and Western blotting using anti-β′-COP and anti-ARF1 antibodies as described in the legend to Fig. 1.
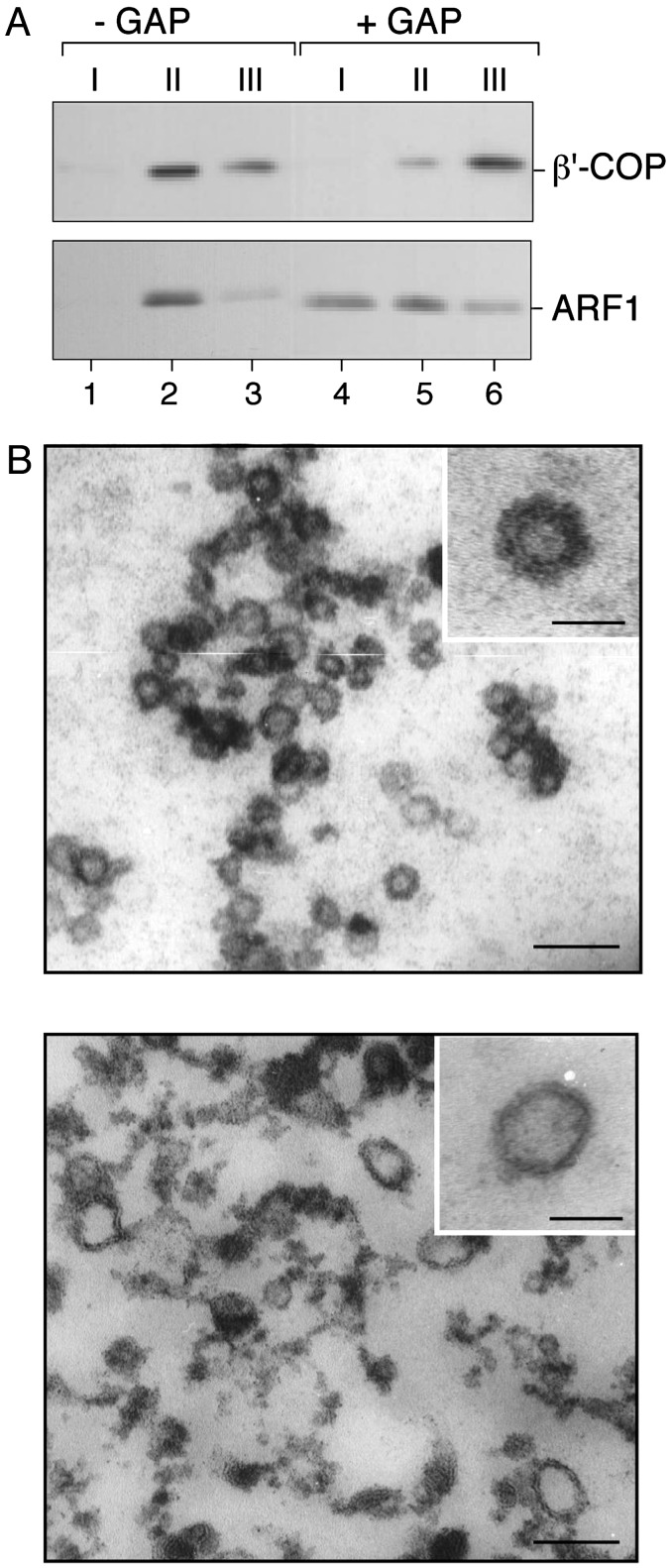
Ultrastructural Analysis of COPI Coat Disassembly. To corroborate the biochemical results described above, we went on to analyze by electron microscopy liposome-derived COPI-coated vesicles before and after addition of GAP activity. To remove excess amounts of coat proteins present in the incubation mixture, liposome-derived coated vesicles were first isolated as described in the experiments depicted in Fig. 3. After treatment of pool II with ARF-GAP activity, this pool once again was fractionated by flotation in the sucrose density gradient to reisolate coated vesicles. As shown in Fig. 4A, appreciable amounts of ARF and coatomer immunoreactivity are detectable in pool II in case ARF-GAP was not added before reisolation of coated vesicles (Fig. 4A, lanes 1–3). By contrast, the amount of coated vesicles was strongly decreased in case ARF-GAP was added after the first flotation gradient, as indicated by a much weaker immunoreactivity for ARF and coatomer in pool II (Fig. 4A, lanes 4–6). Strikingly, the ratio of coatomer in pool II versus pool III is reversed when comparing conditions in the absence and presence of GAP activity, indicating that vesicle-bound coatomer was redistributed into the supernatant that fractionates in pool III after the second flotation gradient.
Fig. 4.
Ultrastructural analysis of ARF-GAP-induced uncoating of COPI-coated vesicles. Liposome-derived COPI-coated vesicles were generated in the presence of GTP as described in the legend of Fig. 1. (A) After isolation by flotation in sucrose density gradients the coated vesicle fraction (pool II, see legend of Fig. 3) was split, with one half being mock-treated and the other half incubated with the catalytic domain of ARF-GAP. Thereafter, coated vesicles were reisolated based on a second flotation gradient that was again fractionated into three pools as described in the legend to Fig. 3. For each experimental condition shown in A, 25% of pool I and II as well as 5% of pool III were analyzed by SDS/PAGE and Western blotting using anti-β′-COP and anti-ARF1 antibodies as described in the legend to Fig. 1. (B) COPI vesicles were generated in the presence of GTP and isolated as described in the legend to Fig. 3. After incubation in the presence or absence of the catalytic domain of ARF-GAP, samples were processed for electron microscopy as described under Materials and Methods. The bars in the main panels correspond to 100 nm; the bars in the Insets correspond to 50 nm.
Although it was difficult to directly analyze COPI budding and uncoating reactions by electron microscopy due to the large amounts of soluble coat proteins present, the experimental setup described in Fig. 4A allowed us to monitor vesicle uncoating by ultrastructural methods. As shown in Fig. 4B, numerous small vesicles containing a thick coat structure can be observed in samples that are derived from incubations in the absence of GAP activity (Fig. 4B Upper). As can be seen in the Inset, these vesicles are characterized by a diameter of ≈50 nm, which is consistent with earlier findings (15). By contrast, samples derived from incubations in the presence of GAP activity seem more heterogeneous because they contain both naked vesicles and protein aggregates (Fig. 4B Lower). The latter material is obviously derived from the preexisting coated vesicles that lose their coat proteins upon addition of GAP activity. A representative uncoated vesicular structure is shown in the Fig. 4B Lower Inset. Based on these ultrastructural studies, it can be concluded that the addition of the catalytic domain of ARF-GAP to synthetic liposome-derived COPI-coated vesicles is both necessary and sufficient to trigger COPI coat disassembly.
Discussion
In the current study, we define the molecular core machinery that is required for COPI coat assembly and disassembly. On the basis of an experimental system that functionally reconstitutes the formation of COPI-coated vesicles from liposomes (15), it was possible to study a complete round of (i) coat protein recruitment to a synthetic membrane, (ii) budding, and (iii) uncoating, by using chemically defined components. Our findings demonstrate that the COPI coat proteins ARF1 and coatomer, the coat receptor p23, and the catalytic domain of ARF-GAP (28), a protein that increases the intrinsic GTPase activity of ARF1, are the only components required for the biogenesis of COPI-coated vesicles including subsequent coat disassembly. These findings confirm the conclusions of previous studies by Tanigawa et al. (27), who demonstrated that Golgi-derived COPI-coated vesicles accumulate in the presence of ARF1-Q71L, a mutant GTPase that is restricted to its GTP form. As our results were obtained with purified components, i.e., in the absence of any other factors, it can now be excluded that proteins other than those mentioned above contribute to the basic process of COPI coat assembly and disassembly in an essential manner.
Recently, it has been suggested that ARF-GAP plays an essential role in the formation of COPI-coated vesicles (34). One key finding of this study was that GTPγS inhibits COPI budding from native Golgi membranes. Moreover, the authors suggest that ARF-GAP functions as a coat component of COPI-coated vesicles, which is discussed in the light of findings from other groups in that ARF-mediated GTP hydrolysis is required for cargo uptake during COPI vesicle formation (30–33). Although it is certainly true that mechanisms must exist that allow ARF-mediated GTP hydrolysis at an early stage of COPI budding without compromising the formation of COPI-coated vesicles, our findings suggest that ARF-GAP is not required for coated vesicle formation. Clearly, ARF-GAP is neither essential for COPI budding in the in vitro system (15) used in this study nor is it required in the COPI budding assay established by Schekman and coworkers (17). Moreover, COPI-coated vesicles can be formed from salt-washed Golgi membranes, a treatment that removes ARF-GAP activity. This experimental setup has actually allowed the accumulation of COPI-coated vesicles in the presence of GTP (9, 42). Finally, the interpretation of the data from Yang et al. (34) regarding the inhibitory effect of GTPγS on COPI budding is in striking contrast to the original observations by Rothman and coworkers, who first demonstrated that COPI-coated vesicles actually accumulate in the presence of nonhydrolyzable analogues of GTP (12, 24). These results were confirmed by studies that demonstrate uncoupling of biosynthetic cargo sorting from vesicle formation in the presence of GTPγS (31, 32). Here, it was clearly shown that COPI vesicles are normally produced; however, they are depleted of biosynthetic cargo molecules (31, 32). Based on these considerations, it seems unlikely that ARF-GAP can be regarded as a coat component of COPI vesicles essential for COPI coat assembly.
Nevertheless, the future challenge is now to elucidate the spatial and temporal regulation of ARF-mediated GTP hydrolysis during a complete round of vesicle budding, targeting, and fusion with specific organelles. Besides the core machinery of COPI coat assembly and disassembly as defined in this study, other factors must be operational under in vivo conditions to achieve the coordinated interplay that results in a productive pathway of cargo sorting during COPI budding. Moreover, ARF-mediated GTP hydrolysis at early stages of COPI budding must be restricted in a way that does not compromise the formation of coated vesicles. At this time, it remains unclear whether this is achieved by multiple distinct ARF-GAP activities, by proteins regulating ARF-GAP activity, or by a combination of both mechanisms. In this context, it will be interesting to analyze the functional relevance of ARF-GAP interactions with SNAREs (43) and the KDEL receptor (44, 45).
Acknowledgments
This work was supported by German Research Foundation Grant SFB 352 (to F.T.W. and W.N.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GTPγS, guanosine 5′-[γ-thio]triphosphate; ARF, ADP-ribosylation factor; GAP, GTPase-activating protein.
References
- 1.Kirchhausen, T. (2000) Nat. Rev. Mol. Cell Biol. 1 187-198. [DOI] [PubMed] [Google Scholar]
- 2.Nickel, W., Brügger, B. & Wieland, F. T. (2002) J. Cell Sci. 115 3235-3240. [DOI] [PubMed] [Google Scholar]
- 3.Rothman, J. E. (1994) Nature 372 55-63. [DOI] [PubMed] [Google Scholar]
- 4.Schekman, R. & Orci, L. (1996) Science 271 1526-1533. [DOI] [PubMed] [Google Scholar]
- 5.Schmid, S. L. (1997) Annu. Rev. Biochem. 66 511-548. [DOI] [PubMed] [Google Scholar]
- 6.Barlowe, C. (1998) Biochim. Biophys. Acta 1404 67-76. [DOI] [PubMed] [Google Scholar]
- 7.Rothman, J. E. & Wieland, F. T. (1996) Science 272 227-234. [DOI] [PubMed] [Google Scholar]
- 8.Orci, L., Stamnes, M., Ravazzola, M., Amherdt, M., Perrelet, A., Söllner, T. H. & Rothman, J. E. (1997) Cell 90 335-349. [DOI] [PubMed] [Google Scholar]
- 9.Sönnichsen, B., Watson, R., Clausen, H., Misteli, T. & Warren, G. (1996) J. Cell Biol. 134 1411-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letourneur, F., Gaynor, E. C., Hennecke, S., Demolliere, C., Duden, R., Emr, S. D., Riezman, H. & Cosson, P. (1994) Cell 79 1199-1207. [DOI] [PubMed] [Google Scholar]
- 11.Cosson, P. & Letourneur, F. (1994) Science 263 1629-1631. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra, V., Serafini, T., Orci, L., Shepherd, J. C. & Rothman, J. E. (1989) Cell 58 329-336. [DOI] [PubMed] [Google Scholar]
- 13.Waters, M. G., Serafini, T. & Rothman, J. E. (1991) Nature 349 248-251. [DOI] [PubMed] [Google Scholar]
- 14.Serafini, T., Orci, L., Amherdt, M., Brunner, M., Kahn, R. A. & Rothman, J. E. (1991) Cell 67 239-253. [DOI] [PubMed] [Google Scholar]
- 15.Bremser, M., Nickel, W., Schweikert, M., Ravazzola, M., Amherdt, M., Hughes, C. A., Sollner, T. H., Rothman, J. E. & Wieland, F. T. (1999) Cell 96 495-506. [DOI] [PubMed] [Google Scholar]
- 16.Orci, L., Palmer, D. J., Amherdt, M. & Rothman, J. E. (1993) Nature 364 732-734. [DOI] [PubMed] [Google Scholar]
- 17.Spang, A., Matsuoka, K., Hamamoto, S., Schekman, R. & Orci, L. (1998) Proc. Natl. Acad. Sci. USA 95 11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn, K., Orci, L., Ravazzola, M., Amherdt, M., Bremser, M., Lottspeich, F., Fiedler, K., Helms, J. B. & Wieland, F. T. (1996) J. Cell Biol. 135 1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojo, M., Pepperkok, R., Emery, G., Kellner, R., Stang, E., Parton, R. G. & Gruenberg, J. (1997) J. Cell Biol. 139 1119-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez, M., Dejgaard, K., Füllekrug, J., Dahan, S., Fazel, A., Paccaud, J. P., Thomas, D. Y., Bergeron, J. J. & Nilsson, T. (1998) J. Cell Biol. 140 751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harter, C. & Wieland, F. T. (1998) Proc. Natl. Acad. Sci. USA 95 11649-11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gommel, D. U., Memon, A. R., Heiss, A., Lottspeich, F., Pfannstiel, J., Lechner, J., Reinhard, C., Helms, J. B., Nickel, W. & Wieland, F. T. (2001) EMBO J. 20 6751-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orci, L., Glick, B. S. & Rothman, J. E. (1986) Cell 46 171-184. [DOI] [PubMed] [Google Scholar]
- 24.Melancon, P., Glick, B. S., Malhotra, V., Weidman, P. J., Serafini, T., Gleason, M. L., Orci, L. & Rothman, J. E. (1987) Cell 51 1053-1062. [DOI] [PubMed] [Google Scholar]
- 25.Krengel, U., Schlichting, L., Scherer, A., Schumann, R., Frech, M., John, J., Kabsch, W., Pai, E. F. & Wittinghofer, A. (1990) Cell 62 539-548. [DOI] [PubMed] [Google Scholar]
- 26.Der, C. J., Finkel, T. & Cooper, G. M. (1986) Cell 44 167-176. [DOI] [PubMed] [Google Scholar]
- 27.Tanigawa, G., Orci, L., Amherdt, M., Ravazzola, M., Helms, J. B. & Rothman, J. E. (1993) J. Cell Biol. 123 1365-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cukierman, E., Huber, I., Rotman, M. & Cassel, D. (1995) Science 270 1999-2002. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg, J. (1999) Cell 96 893-902. [DOI] [PubMed] [Google Scholar]
- 30.Lanoix, J., Ouwendijk, J., Lin, C. C., Stark, A., Love, H. D., Ostermann, J. & Nilsson, T. (1999) EMBO J. 18 4935-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malsam, J., Gommel, D., Wieland, F. T. & Nickel, W. (1999) FEBS Lett. 462 267-272. [DOI] [PubMed] [Google Scholar]
- 32.Nickel, W., Malsam, J., Gorgas, K., Ravazzola, M., Jenne, N., Helms, J. B. & Wieland, F. T. (1998) J. Cell Sci. 111 3081-3090. [DOI] [PubMed] [Google Scholar]
- 33.Pepperkok, R., Whitney, J. A., Gomez, M. & Kreis, T. E. (2000) J. Cell Sci. 113 135-144. [DOI] [PubMed] [Google Scholar]
- 34.Yang, J. S., Lee, S. Y., Gao, M., Bourgoin, S., Randazzo, P. A., Premont, R. T. & Hsu, V. W. (2002) J. Cell Biol. 159 69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenbeck, G., Harter, C., Brecht, A., Herrmann, D., Lottspeich, F., Orci, L. & Wieland, F. T. (1993) EMBO J. 12 2841-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harter, C., Draken, E., Lottspeich, F. & Wieland, F. T. (1993) FEBS Lett. 332 71-73. [DOI] [PubMed] [Google Scholar]
- 37.Nickel, W. & Wieland, F. T. (2001) Methods Enzymol. 329 388-404. [DOI] [PubMed] [Google Scholar]
- 38.Pavel, J., Harter, C. & Wieland, F. T. (1998) Proc. Natl. Acad. Sci. USA 95 2140-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franco, M., Chardin, P., Chabre, M. & Paris, S. (1995) J. Biol. Chem. 270 1337-1341. [DOI] [PubMed] [Google Scholar]
- 40.Randazzo, P. A., Terui, T., Sturch, S., Fales, H. M., Ferrige, A. G. & Kahn, R. A. (1995) J. Biol. Chem. 270 14809-14815. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds, E. (1963) J. Cell Biol. 17 208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ostermann, J., Orci, L., Tani, K., Amherdt, M., Ravazzola, M., Elazar, Z. & Rothman, J. E. (1993) Cell 75 1015-1025. [DOI] [PubMed] [Google Scholar]
- 43.Rein, U., Andag, U., Duden, R., Schmitt, H. D. & Spang, A. (2002) J. Cell Biol. 157 395-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoe, T., Huber, I., Vasudevan, C., Watkins, S. C., Romero, G., Cassel, D. & Hsu, V. W. (1999) J. Biol. Chem. 274 20545-20549. [DOI] [PubMed] [Google Scholar]
- 45.Aoe, T., Cukierman, E., Lee, A., Cassel, D., Peters, P. J. & Hsu, V. W. (1997) EMBO J. 16 7305-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]