Abstract
The renin–angiotensin system plays a critical role in blood pressure control and body fluid and electrolyte homeostasis. Besides angiotensin (Ang) II, other Ang peptides, such as Ang III [Ang-(2–8)], Ang IV [Ang-(3–8)], and Ang-(1–7) may also have important biological activities. Ang-(1–7) has become an angiotensin of interest in the past few years, because its cardiovascular and baroreflex actions counteract those of Ang II. Unique angiotensin-binding sites specific for this heptapeptide and studies with a selective Ang-(1–7) antagonist indicated the existence of a distinct Ang-(1–7) receptor. We demonstrate that genetic deletion of the G protein-coupled receptor encoded by the Mas protooncogene abolishes the binding of Ang-(1–7) to mouse kidneys. Accordingly, Mas-deficient mice completely lack the antidiuretic action of Ang-(1–7) after an acute water load. Ang-(1–7) binds to Mas-transfected cells and elicits arachidonic acid release. Furthermore, Mas-deficient aortas lose their Ang-(1–7)-induced relaxation response. Collectively, these findings identify Mas as a functional receptor for Ang-(1–7) and provide a clear molecular basis for the physiological actions of this biologically active peptide.
Keywords: binding, Mas protooncogene, renin angiotensin system
The renin angiotensin system (RAS), a potent regulator of blood pressure, plays a major role in the pathogenesis of cardiovascular diseases (1, 2). Emerging evidence suggests that angiotensin (Ang) II is not the only active peptide of the RAS. Other members of the system; Ang III [Ang-(2–8)], Ang IV [Ang-(3–8)], and Ang-(1–7) may also mediate the actions of the RAS (3). The vascular and baroreflex actions of Ang-(1–7) counteract those of Ang II (4, 5). Studies using the selective Ang-(1–7) antagonist A-779 (6, 7) provide evidence for an Ang-(1–7) receptor distinct from the classical Ang II receptors AT1 and AT2 (4, 7).
The Mas protooncogene, first detected in vivo by tumorigenic properties originating from rearrangement of its 5′ flanking region (8, 9), encodes a protein with seven hydrophobic transmembrane domains, considered to be an “orphan” G protein-coupled receptor (10). Whereas the tumorigenic properties of Mas seem to be negligible, transfection studies suggested that the Mas gene encodes an Ang II receptor (11). But, Ang II-induced intracellular Ca2+ responses in Mas-transfected cells occurred only in cells endogenously expressing the Ang II receptor AT1 (12). Other experiments indicated that Mas modulates intracellular signaling of AT1 after Ang II stimulation (13).
In this study, we performed radioligand binding with autoradiography on mouse kidneys, cell-specific binding, and functional studies in vitro, physiological and pharmacological ex vivo and in vivo experiments in Mas-deficient mice to demonstrate that the G protein-coupled receptor Mas binds Ang-(1–7) and is involved in the biologic actions of this heptapeptide.
Materials and Methods
In Vitro Receptor Autoradiography of 125I-Angiotensin Binding to Mouse Kidneys. Kidneys of Mas-deficient mice (14) and Mas WT animals were snap-frozen in isopentane cooled with liquid nitrogen. Sections (10 μm) were serially cut starting from the central area of the kidney, mounted onto 1%-gelatinized slides and dried at 4°C. Sections were stored frozen at –80°C.
The sections were thawed to ambient (22–24°C) temperature and preincubated in the following assay buffers. For Ang II receptor binding: 150 mM NaCl/5 mM EDTA/0.1 mM bacitracin/50 mM NaPO4, pH 7.2; for Ang-(1–7) binding: 10 mM Na-phosphate buffer, pH 7.4/120 mM NaCl/5 mM MgCl2/0.2% BSA/0.005% bacitracin; and for Ang IV binding: 150 mM NaCl/5 mM EDTA/50 μM Plummer's inhibitor (DL-2-mercaptomethyl-3-guanidoethylthiopropanoic acid)/100 μM phenylmethylsulfonyl f luoride/20 μM bestatin/1 mg/ml BSA/50 mM Tris·HCl, pH 7.4 for 30 min.
Sections were subsequently incubated with radioligand. For Ang II receptors: assay buffer with 0.5 nM 125I-sarcosine1, isoleucine8-Ang II (125I-[Sar-1, Ile-8]Ang II) and either 3 μM Ang II (nonspecific binding)/10 μM of the selective AT2 receptor antagonist PD123319 (for AT1 receptor binding)/10 μM of the selective AT1 receptor antagonist losartan (for AT2 receptor binding) for 2 h at 22–24°C; for Ang-(1–7) binding: assay buffer containing 1 nM 125I-Ang-(1–7)/100 μM phenylmethylsulfonyl fluoride/1 μM indomethacin/1 μM leupeptin/1 μM aprotinin/1 μM Ang (1–7) (nonspecific binding) for 1 h at 22–24°C; for Ang IV binding: assay buffer containing 1 nM of 125I-Ang IV and 10 μM Ang IV (nonspecific binding) for 1 h at 22–24°C.
After incubation, sections were rinsed (five times for 1 min each in assay buffer) preceded and succeeded by two quick dips in distilled water. Sections were dried under a stream of air at 22–24°C and exposed to autoradiographic film (Kodak Biomax MR-1) for 3 (Ang II), 14 [Ang-(1–7)], and 3 (Ang IV) days at -20°C. Film images were visualized and analyzed from an analog video image captured by an imaging program (AIS, Imaging Research, St. Catherine's, ON, Canada). Film exposure corresponding to tissue sections was quantitated by densitometry and converted to units of fmol/g tissue wet weight by using calibrated standards (125I-Microscales, Amersham Pharmacia Biosciences). Specific 125I-[Sar-1, Ile-8]Ang II, 125I-Ang-(1–7), and 125I-Ang IV binding was derived by subtracting nonspecific binding from total binding. To determine 125I-[Sar-1, Ile-8]Ang II binding to AT1 and AT2 receptor subtypes, nonspecific binding was subtracted from the binding in the presence of either PD123319 or losartan, respectively.
[Sar-1, Ile-8]Ang II, Ang-(1–7), and Ang IV were labeled with 125I by the chloramine T method (15) and purified by HPLC, as described (16).
Cell Culture. Cells. Commercially available cell lines Chinese hamster ovary (CHO) and COS, obtained from the American Type Culture Collection, Manassas, VA, were used for cell culture experiments. Cells were cultured 3 days or more as a monolayer (94/16-mm Petri dish) in culture medium recommended for each cell type. Cells were stably transfected with Mas cDNA driven by a cytomegalovirus promoter, as described by Pesquero et al. (17) and selected by neomycin.
Binding Studies. A. Competition experiments. 125I-Ang-(1–7) (0.5 nM) was incubated in 24-well plates for 60 min at 4°C in 300 μl of serum-free medium (DMEM) supplemented with 0.2% BSA, 0.005% bacitracin, 100 μM phenylmethylsulfonyl fluoride, and 500 μM o-phenanthroline with Mas-transfected cells in the presence or absence of Ang-(1–7) (10-11 to 10-5 M), the selective Ang-(1–7) antagonist d-Ala-7-Ang-(1–7) (A-779, 10-10 to 10-6 M), the AT2 antagonist PD123319 (10-10 to 10-5 M), the AT1 antagonist CV11974 (10-10 to 10-5 M), Ang II, Ang III, or Ang IV (each 10-10 to 10-5 M). After two washes with ice-cold serum-free DMEM, cells were disrupted with 0.1% Triton X-100 in water at 22–24°C. Bound radioactivity in the cell lysate was measured in a γ-counter. Each data point represents the mean of three to six experiments. Curve fit and analysis were performed by using graphpad prism (Graphpad Software. San Diego, CA).
B. Saturation binding experiments. Total binding of ligand (0.20–4 nmol/liter) to control or Mas-transfected COS cells was determined in duplicate wells. One micromol/liter of cold Ang-(1–7) was added to matched wells to determine nonspecific binding. After 60 min incubation at 4°C, cells were rinsed (×3) with ice-cold PBS, the supernatant removed, and the cells disrupted with 0.1% Triton X-100 in water at room temperature. Each data point is represented as the mean of three to six experiments. Curve fit and analysis were performed by using graphpad prism. Untransfected COS cells showed no specific 125I-Ang-(1–7) binding.
Arachidonic Acid (AA) Release. WT and Mas-transfected CHO or COS cells, preloaded with 0.2 μCi/well of [3H]AA for 18 h, were incubated with Ang II (10-8 M) or Ang-(1–7) (10-11 to 10-6 M) for 15 min at 37°C in Hanks' balanced salt solution. When the effects of A-779, irbesartan (AT1 receptor antagonist), or PD123319 on Ang-(1–7) actions were investigated, antagonists (10-8 M) were added to the well 10 min before Ang-(1–7). The amount of [3H]AA released into the medium and that remaining in the cells was measured by liquid scintillation spectrometry. The [3H]AA released into the medium was expressed as percent of the total cellular [3H]AA, referred to as fractional release.
Water Diuresis. Water diuresis was induced by i.p. injection of distilled water (0.5 ml/10 g). WT and Mas-knockout mice received a water load combined in the same injection with vehicle (0.9% NaCl 0.005 ml/10 g, n = 8 for each group) or Ang-(1–7) (4 pmol/10 g, n = 8 for Mas-knockout and n = 9 for WT mice).
In other experiments, the two subsets of mice were subjected to water load combined with i.p. injection of vehicle (0.9% NaCl 0.005 ml/10 g, n = 8 for each group) or arginine-vasopressin (AVP; 2 pmol/10 g, n = 6 for each group). Immediately after i.p. injection, mice were placed in metabolic cages and their urine output measured for 60 min (urine recovery rate evaluated in previous experiments was >85%).
Mouse Aortic Ring Preparation and Mounting. Rings (2–3 mm) from descending thoracic aorta containing a functional endothelium, cleared of adipose and connective tissue, were equilibrated in gassed (95% O2/5% CO2) Krebs–Henseleit solution for 1 h. During this time, the incubation medium was changed every 15 min. After equilibration, two contractile responses were evoked by submaximal concentrations of phenylephrine (0.3 μM) to elicit reproducible responses. The vasorelaxant effect of Ang-(1–7) was measured in rings precontracted with 0.1 μM phenylephrine. Ang-(1–7) (0.0001–0.3 μM) was added in increasing cumulative concentrations once the response to phenylephrine stabilized. The presence of a functional endothelium was assessed by the ability of acetylcholine (10 μM) to induce >70% relaxation of vessels precontracted with phenylephrine (0.3 μM). Mechanical activity, recorded isometrically by a force transducer (World Precision Instruments, Sarasota, FL), was fed to an amplifier-recorder (Model TMB-4; World Precision Instruments) and to a computer equipped with an analog-to-digital converter board (AD16JR; World Precision Instruments), using cvms data acquisition/recording software (World Precision Instruments).
Statistics. Statistical analyses for radioligand-binding studies used paired or unpaired t tests, with Aspin–Welch correction for heterogeneity of variance. Statistical analyses of the water diuresis experiments used a nonpaired t test. Comparison of the effects of peptides on AA release was analyzed by a nonpaired t test or one-way ANOVA followed by Newman–Keuls test. Two-way ANOVA was used to compare the relaxation produced by Ang-(1–7) in mice aortic rings.
Results
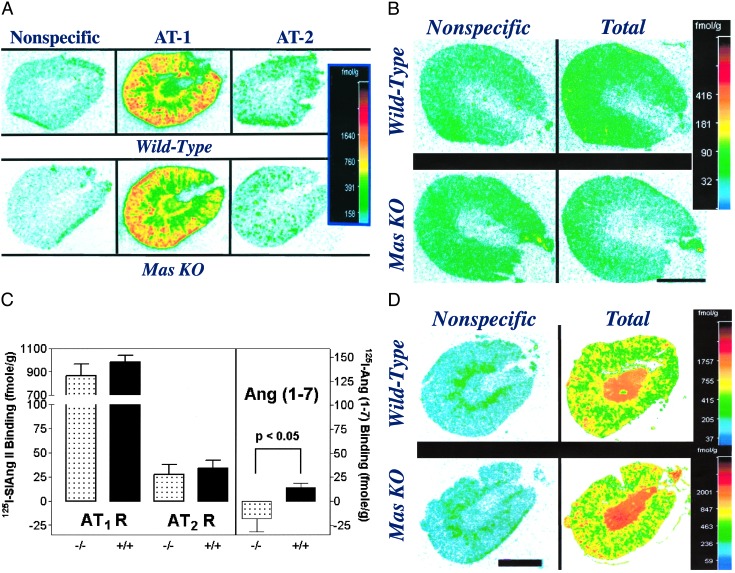
As summarized in Fig. 1C, the binding of 125I-[Sar-1, Ile-8]Ang II to kidney slices of WT mice was similar to that observed in kidneys of Mas-deficient mice (14). 125I-[Sar-1, Ile-8]Ang II bound predominantly to the AT1 receptor subtype, in both WT and Mas-knockout kidneys (Fig. 1 A Center and C), with low binding to the AT2 receptor subtype (Fig. 1 A Right and C). AT1 and AT2 specific 125I-[Sar-1, Ile-8]Ang II binding was similar in both WT and Mas-deficient kidneys (t = 0.98 and 0.46, respectively, df = 10).
Fig. 1.
Angiotensin receptor binding in WT and Mas knockout (KO) mouse kidneys. (A) Nonspecific, total AT1, and total AT2 binding of 125I-[Sar-1, Ile-8]Ang II binding in WT (Upper) and Mas-knockout (Lower) kidneys. (B) Nonspecific and total 125I-Ang (1–7) binding in WT (Upper) and Mas-knockout (Lower) kidneys. (C) Specific binding of 125I-[Sar-1, Ile-8] Ang II to AT1 (AT1 R) and AT2 (AT2 R) receptors in WT (+/+) and Mas-deficient (-/-) kidneys (Left) and specific binding of 125I-Ang (1–7) binding to WT (+/+) and Mas-knockout (-/-) kidneys (Right). Bars represent mean of six kidneys ± SEM. (D) Representative autoradiographic localizations of 125I-angiotensin IV binding in WT and Mas knockout mouse kidneys. Color bars (Right)reflect pseudocolor imaging of different levels of exposure of the autoradiogram converted to units of fmol/g as described in Materials and Methods. (Bar = 2 mm.)
Representative autoradiograms of 125I-Ang-(1–7) binding are shown in Fig. 1B. Low-level specific 125I-Ang (1–7) binding was observed in kidneys of Mas-WT mice. Specific 125I-Ang-(1–7) binding for the entire WT group was 14.1 ± 4.5 fmol/g (mean ± SEM). Nonspecific binding in Mas-deficient kidney sections tended to be higher than total binding in these kidneys and the average specific binding was <0 (Fig. 1C). However, this difference was not statistically significant (paired t = 1.39, df = 5). Specific 125I-Ang (1–7) binding was significantly greater in the kidneys of WT compared with the Mas-deficient mice (P = 0.029, one-tailed t test with Aspin–Welch correction). Total 125I-Ang-(1–7) binding in WT kidneys was significantly greater than nonspecific binding (paired t = 3.12, df = 5, P = 0.0262).
Binding of 125I-Ang-(1–7) was preserved in kidney slices of AT1 (18) and AT2 (19) knockout mice (data not shown). Additionally, slices of normal and Mas-knockout mice showed no major differences in the binding of 125I-Ang IV (Fig. 1D).
To further examine the specificity of Ang-(1–7) binding to Mas, binding studies were performed by using Mas-transfected CHO cells. [125I]-Ang-(1–7) bound with high affinity to Mas-transfected cells (KD = 0.83 ± 0.10 nM, Bmax = 58.8 fmol/mg protein in Mas-transfected CHO cells, Fig. 2A). Subsequent experiments examined specific [125I]-Ang-(1–7) binding in the presence or absence of Ang-(1–7) (10-11 to 10-5 M), its specific antagonist A-779 (6), CV 11974 and PD 123319. As shown in Fig. 2B, Ang-(1–7) and A-779 displaced 125I-Ang-(1–7) binding to Mas-transfected CHO cells with high affinity (IC50 = 6.9 nM and 0.3 nM, respectively), whereas other Ang metabolites competed with the 125I-Ang-(1–7) binding only in higher concentrations (Ang II: IC50 = 53.3 nM; Ang III: IC50 = 452 nM; Ang IV: IC50 = 1,238 nM). No significant displacement was observed with CV 11974 or PD 123319 (IC50 > 10 μM).
Fig. 2.
(A) Saturation isotherm and scatchard plot (Inset) of specific 125I-Ang-(1–7) binding to Mas-transfected COS cells. Cells were incubated with increasing concentrations of 125I-Ang-(1–7). No specific binding was determined in the presence of 1 μmol/liter Ang-(1–7). These data are represented as mean ± SEM of three different experiments. In the conditions used, the nonspecific binding averaged 40–60% of the total binding. (B) Competition for 125I-Ang-(1–7) binding to Mas-transfected CHO cells by Ang-(1–7) and receptor antagonists. Competition curves were generated by adding increasing concentrations of CV-9174, PD 123319, A-779, and Ang-(1–7) to the incubation buffer containing 0.4 nmol/liter of 125I-Ang-(1–7). Data are presented as mean ± SEM of three to six independent experiments.
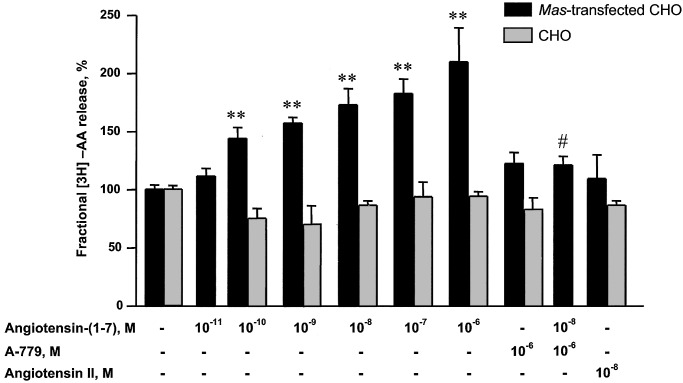
To evaluate the functional significance of Ang-(1–7), CHO and COS cells transfected with Mas were treated with varying concentrations of Ang-(1–7) and examined for AA release. Ang (1–7) caused a concentration-dependent increase in 3H-AA release from Mas-transfected CHO cells over a range of 10-11 to 10-6 M (Fig. 3). This effect was blocked by the Ang (1–7) antagonist A779. Ang II at 10-8 M produced no increase in AA release, whereas nontransfected CHO cells did not respond either to Ang-(1–7) or Ang II. Ang-(1–7) also stimulated AA release in Mas-transfected COS cells (Fig. 4). This release was not affected by irbesartan or PD 123319 (10-8 M).
Fig. 3.
Effect of the Ang-(1–7) antagonist A-779 on the Ang-(1–7)-induced [3H]AA release from Mas-transfected CHO cells. Data are presented as mean ± SEM of three to six independent experiments performed in triplicate. **, P < 0.01 compared with untreated Mas-transfected CHO, ANOVA followed by Newman–Keuls test; #, P < 0.05 compared with Ang-(1–7) 10-8 M, nonpaired t test.
Fig. 4.
Effect of the Ang-(1–7) antagonist A-779, irbesartan (AT1 receptor antagonist) or PD123319 (AT2 receptor antagonist) on Ang-(1–7)-induced [3H]AA release from Mas-transfected COS cells. Data are presented as mean ± SEM of three to six independent experiments performed in triplicate.
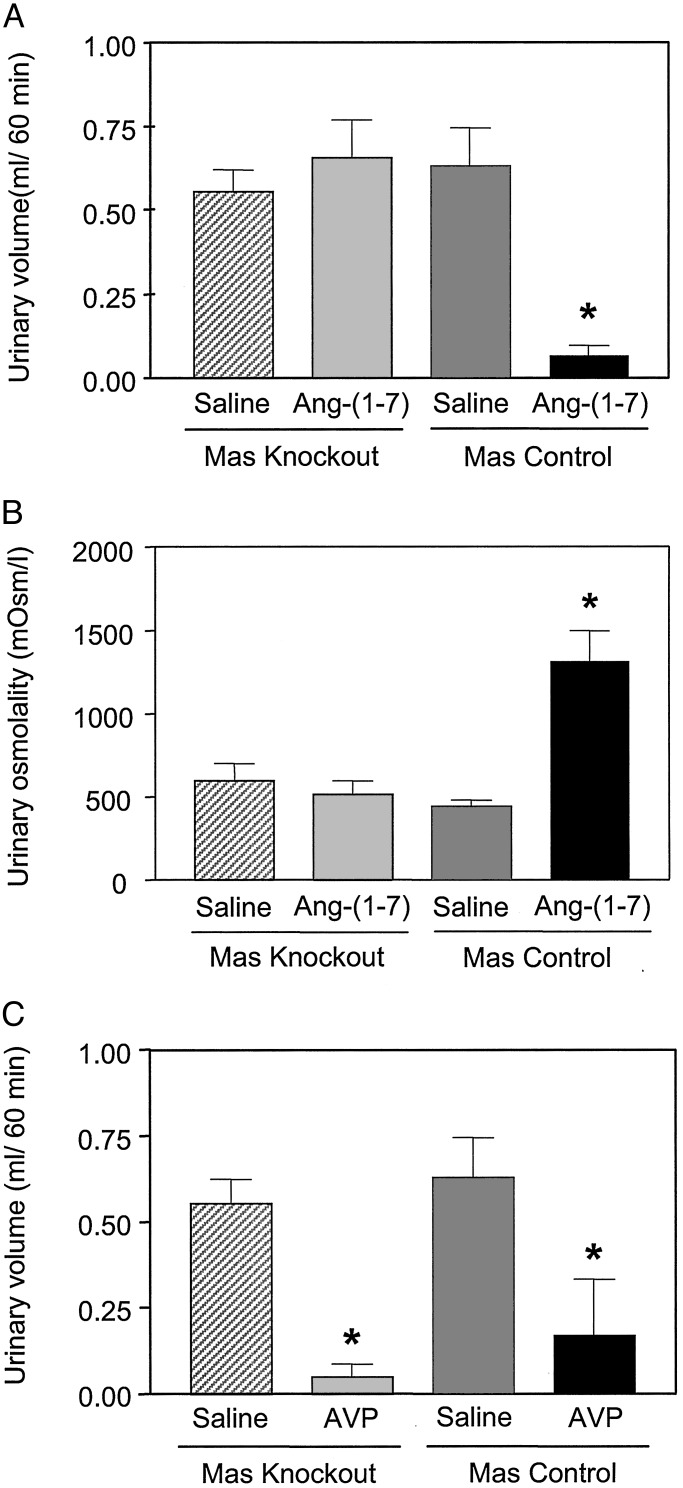
To further determine the functional significance of Ang-(1–7), we examined its antidiuretic effect after water loading in mice, because it exerts potent antidiuresis in water-loaded rats (20). Antidiuretic activity also was seen in Mas WT mice (Fig. 5A). The reduced urine volume in control mice was associated with an increase in urine osmolality (Fig. 5B). Corroborating the binding and AA release studies, the antidiuretic effect of Ang-(1–7), at a dose reducing 80% of the urinary flow in water-loaded control mice, was completely abolished in Mas-knockout animals. In contrast, AVP retained its antidiuretic activity in Mas-knockout mice (Fig. 5C). Mas-deficient mice showed no significant changes in basal renal function parameters (data not shown).
Fig. 5.
Antidiuretic effect of Ang-(1–7) and AVP in water-loaded mice. Male control (n = 25) and Mas-deficient (n = 24) mice (25–35 g) were used. (A) Effect of Ang-(1–7) on water diuresis. (B) Effect of Ang-(1–7) on urine osmolality. (C) Effect of AVP on water diuresis (the same experimental protocol was used). Data are presented as mean ± SEM. *, P < 0.05 compared with the vehicle-treated mice (ANOVA followed by Newman–Keuls test).
Because Ang-(1–7) causes vasorelaxation in a number of vascular beds (5), we examined the effects of Ang-(1–7) on relaxation of aortic rings derived from WT and Mas-deficient mice. The endothelium-dependent relaxation in WT animals [Emax = 40.8 ± 8.9%; (P < 0.001)] was abolished in the aortic rings of Mas-knockout mice (Fig. 6).
Fig. 6.
Vasodilator effect of Ang-(1–7) in endothelium-containing aortic rings from WT (control) and Mas-knockout mice (knockout). (A) Tracing illustrating the effect of Ang-(1–7) on preconstricted aorta rings. Vessels were preconstricted by incubation with 0.3 mM phenylephrine. Numbers below the arrows indicate log of the peptide concentration (0.0001–0.3 μM). The arrows without numbers indicate concentrations 3-fold higher than the previous addition. (B) Diagram summarizing the vasodilator effect of Ang-(1–7) in the aortic rings of both animal models. Each point represents mean ± SEM generated from five separated experiments. P < 0.001 (two-way ANOVA).
Discussion
To clarify the role of Mas in Ang II binding and signaling, we performed radioligand-binding studies with Ang II in kidney slices of WT and Mas-deficient mice (14). Ang II binding (mainly to AT1 receptors) was unaltered in kidneys of Mas-knockout mice compared with that of WT, indicating no influence of Mas on AT1 binding. However, Ang-(1–7) binding was absent in Mas-knockout mice, indicating that Mas is an Ang-(1–7) receptor. Furthermore, low-level specific 125I-Ang (1–7) binding observed in kidneys of Mas WT mice correlates with recent findings of weak Mas-mRNA expression in murine kidneys (21). That 125I-Ang-(1–7) binding was preserved in kidneys from AT1- and AT2-knockout mice excluded an indirect effect of Mas deficiency on the ability of these receptors to bind Ang-(1–7). In addition, the finding of comparable 125I-Ang IV binding in kidney slices of normal and Mas-knockout mice distinguishes Mas from the putative AT4 receptor (22).
Experiments using two different cell types transfected with Mas provided additional evidence for the binding of Ang-(1–7) to Mas. First, binding studies in Mas-transfected CHO cells demonstrated high affinity and specific binding of 125I-Ang-(1–7). Further, specific 125I-Ang-(1–7) binding to Mas-transfected CHO cells was displaced with high affinity both by unlabeled Ang-(1–7) and by A-779 and, to a much smaller degree, by Ang II and other Ang II metabolites. That no significant displacement was observed with similar concentrations of CV 11974 (candesartan) or PD 123319 excluded binding of Ang-(1–7) to AT1 and AT2. More importantly, Ang-(1–7) induced a significant increase in 3H-AA release that was completely abolished with A-779 in both COS and CHO cells transfected with Mas. The specificity of the Ang-(1–7)-Mas axis is confirmed by the fact that neither an AT1 nor an AT2 antagonist blocked this release. These results are consistent with previously reported findings in rabbit smooth muscle cells (23), in which the PLA2/ cyclooxygenase pathway contributed to the physiological effects of Ang-(1–7) through an interaction with its own receptor subtype (5, 24).
Our data in mice reproduced our previous findings of an antidiuretic action of Ang-(1–7) in water-loaded rats (20). The reduced urinary volume in control mice corresponded to a rise in its osmolality, indicating that the antidiuretic effect of Ang-(1–7) results from increased water reabsorption. This antidiuretic effect is blunted in Mas-deficient animals. Furthermore, the antidiuretic activity of vasopressin was preserved in Mas-knockout mice, excluding nonspecific changes in their renal function. Thus, the failure of Ang-(1–7) to display its antidiuretic activity in Mas-deficient mice establishes a physiological relevance to the binding and cell-culture studies identifying Mas as a functional receptor for Ang-(1–7).
To demonstrate that the interaction of Ang-(1–7) and Mas is not restricted to the kidney, we investigated its relaxant ability in aortic rings of Mas-deficient animals. As previously shown in dogs and rats (25, 26), vessels derived from WT animals relaxed after Ang-(1–7) treatment in a dose-dependent manner. However, Ang-(1–7) relaxation, but not endothelium-dependent relaxation of aortas by acetylcholine of Mas-knockout mice, was abolished.
This is a previously undescribed demonstration of a molecular basis for the physiological actions of Ang-(1–7). Our findings will help to elucidate its interaction with Ang II (27, 28), bradykinin (29, 30), and angiotensin-converting enzyme (31).
Further studies are underway to clarify to what extent other Ang-(1–7) actions, e.g., increased baroreflex sensitivity (32), are mediated by its interaction with Mas. These studies will also determine whether phenotypic alterations in Mas-deficient mice, e.g., changes in heart rate, blood pressure variability (33, 34), and behavioral anomalies (14, 35), result from the absence of Ang-(1–7) actions.
Although our data cannot exclude indirect interaction of Mas and Ang II via the AT1 receptor, they clearly demonstrate a direct interaction of Mas and Ang-(1–7). On the basis of our findings, we conclude that Mas binds Ang-(1–7) and is involved in mediating the biologic actions of this angiotensin peptide. These findings have clinical implications, because Ang-(1–7) counteracts Ang II and accumulates in patients treated with angiotensin-converting enzyme inhibitors (36) and thus may explain well demonstrated beneficial effects of these drugs. Our findings clearly widen the possibilities for treating cardiovascular diseases by using agonists for the Ang-(1–7)-Mas axis.
Acknowledgments
We thank Jose R. da Silva, Soraia S. Silva, Helmut Würdemann, and Cathy Knoeber for technical assistance. This work was supported in part by a Deutscher Akademischer Austauschdienst/Comissao de Aperfeiçoamento de Pessoal de Nival Superior PROBRAL project, Financiadora de Estudos e Projetos-Conselho Nacional de Pesquisas PRONEX, and a grant of the Humboldt Foundation (to S.H.-W.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ang, angiotensin; AVP, arginine-vasopressin; AA, arachidonic acid; CHO, Chinese hamster ovary.
References
- 1.Burnier, M. & Brunner, H. R. (2000) Lancet 355 637-645. [DOI] [PubMed] [Google Scholar]
- 2.Kim, S. & Iwao, H. (2000) Pharmacol. Rev. 52 11-34. [PubMed] [Google Scholar]
- 3.Ardaillou, R. (1997) Curr. Opin. Nephrol. Hypertens. 6 28-34. [DOI] [PubMed] [Google Scholar]
- 4.Ferrario, C. M., Chappell, M. C., Dean, R. H. & Iyer, S. N. (1998) J. Am. Soc. Nephrol. 9 1716-1722. [DOI] [PubMed] [Google Scholar]
- 5.Santos, R. A., Campagnole-Santos, M. J. & Andrade, S. P. (2000) Regul. Pept. 91 45-62. [DOI] [PubMed] [Google Scholar]
- 6.Fontes, M. A. P., Silva, L. C. S., Campagnole-Santos, M. J., Khosla, M. C., Guertzenstein, P. G. & Santos, R. A. S. (1994) Brain Res. 665 175-180. [DOI] [PubMed] [Google Scholar]
- 7.Santos, R. A. S. & Campagnole-Santos, M. J. (1994) Braz. J. Med. Biol. Res. 27 1033-1047. [PubMed] [Google Scholar]
- 8.Young, D., Waitches, G., Birchmeier, C., Fasano, O. & Wigler, M. (1986) Cell 45 711-719. [DOI] [PubMed] [Google Scholar]
- 9.Rabin, M., Birnbaum, D., Young, D., Birchmeier, C., Wingler, M. & Ruddle, F. H. (1987) Oncogene Res. 1 169-178. [PubMed] [Google Scholar]
- 10.Zohn, I. E., Symons, M., Chrzanowska-Wodnicka, M., Westwick, J. K. & Der, C. J. (1998) Mol. Cell. Biol. 18 1225-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, T. R., Blair, A. C., Marshall, J., Goedert, M. & Hanley, M. R. (1988) Nature 335 437-440. [DOI] [PubMed] [Google Scholar]
- 12.Ambroz, C., Clark, A. J. L. & Catt, K. J. (1991) Biochem. Biophys. Acta 1133 107-111. [DOI] [PubMed] [Google Scholar]
- 13.von Bohlen und Halbach, O., Walther, T., Bader, M. & Albrecht, D. (2000) J. Neurophysiol. 83 2012-2020. [DOI] [PubMed] [Google Scholar]
- 14.Walther, T., Balschun, D., Voigt, J. P., Fink, H., Zuschratter, W., Birchmeier, C., Ganten, D. & Bader, M. (1998) J. Biol. Chem. 273 11867-11873. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, W. M. & Greenwood. F. C. (1962) Nature 194 495-496. [DOI] [PubMed] [Google Scholar]
- 16.Speth, R. C. & Harding, J. W. (2000) in Angiotensin Protocols, Methods in Molecular Biology, ed. Wang, D. (Humana, Totawa, NJ), pp. 275-296.
- 17.Pesquero, J. B., Lindsey, C. J., Zeh, K., Paiva, A. C. M., Ganten, D. & Bader, M. (1994) J. Biol. Chem. 269 26920-26925. [PubMed] [Google Scholar]
- 18.Ito, M., Oliverio, M. I., Mannon, P. J., Best, C. F., Maeda, N., Smithies, O. & Coffman, T. M. (1995) Proc. Natl. Acad. Sci. USA 92 3521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichiki, T., Labosky, P. A., Shiota, C., Okuyama, S., Imagawa, Y., Fogo, A., Niimura, F., Ichikawa, I., Hogan, B. L. & Inagami, T. (1995) Nature 377 748-750. [DOI] [PubMed] [Google Scholar]
- 20.Santos, R. A. S., Simões e Silva, A. C., Magaldi, A. J., Klosla, M. C., César, K. R., Passaglio, K. T. & Baracho, N. C. V. (1996) Hypertension 27 875-884. [DOI] [PubMed] [Google Scholar]
- 21.Alenina, N., Bader, M. & Walther, T. (2002) Biochem. Biophys. Res. Commun. 290 1072-1078. [DOI] [PubMed] [Google Scholar]
- 22.Handa, R. K. (1999) Am. J. Physiol. 277 F75-F83. [DOI] [PubMed] [Google Scholar]
- 23.Muthalif, M. M., Benter, I. F., Uddin, M. R., Harper, J. L. & Malik, K. U. (1998) J. Pharmacol. Exp. Ther. 284 388-398. [PubMed] [Google Scholar]
- 24.Heringer-Walther, S., Batista, E. N., Walther, T., Khosla, M. C., Santos, R. A. S. & Campagnole-Santos, M. J. (2001) Hypertension 37 1309-1314. [DOI] [PubMed] [Google Scholar]
- 25.Brosnihan, K. B., Li, P. & Ferrario, C. M. (1996) Hypertension 27 523-528. [DOI] [PubMed] [Google Scholar]
- 26.Tran, Y. & Forster, C. (1997) J. Cardiovasc. Pharmacol. 30 676-682. [DOI] [PubMed] [Google Scholar]
- 27.Rooks, A. J., van-Geel, P. P., Pinto, Y. M., Buikema, H., Henning, R. H., deZeeuw, D. & van-Gilst, W. H. (1999) Hypertension 34 296-301. [DOI] [PubMed] [Google Scholar]
- 28.Ueda, S., Masumori-Maemoto, S., Ashino, K., Nagahara, T., Gotoh, E., Umemura, S. & Ishii, M. (2000) Hypertension 35 998-1001. [DOI] [PubMed] [Google Scholar]
- 29.Paula, R. D., Lima, C. V., Khosla, M. C. & Santos, R. A. S. (1995) Hypertension 26 1154-1156. [DOI] [PubMed] [Google Scholar]
- 30.Li, P., Chappell, M. C., Ferrario, C. M. & Brosnihan, K. B. (1997) Hypertension 29 394-400. [DOI] [PubMed] [Google Scholar]
- 31.Deddish, P. A., Marcic, B., Jackman, H. L., Wang, H. Z., Skidgel, A. R. & Erdos, E. G. (1998) Hypertension 31 912-917. [DOI] [PubMed] [Google Scholar]
- 32.Campagnole-Santos, M. J., Heringer, S. B., Batista, E. N., Khosla, M. C. & Santos, R. A. S. (1992) Am. J. Physiol. 263 R89-R94. [DOI] [PubMed] [Google Scholar]
- 33.Walther, T., Wessel, N., Kang, N., Malberg, M., Bader, M. & Voss, A. (1999) J. Clin. Bas. Cardiol. 2 281-282. [Google Scholar]
- 34.Walther, T., Wessel, N., Ning, L. K., Sander, A., Tschöpe, C., Malberg, H., Bader, M. & Voss, A. (2000) Braz. J. Med. Biol. Res., 33 1-9. [DOI] [PubMed] [Google Scholar]
- 35.Walther, T., Voigt, J. P., Fink, H. & Bader, M. (2000) Behav. Brain. Res. 107 105-109. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence, A. C., Evin, G., Kladis, A. & Campbell, D. J. (1990) J. Hypertens. 8 715-724. [DOI] [PubMed] [Google Scholar]