Abstract
The male-specific lethal (MSL) complex of Drosophila is responsible for the presence of a monoacetylated isoform of histone H4 (H4Ac16), found exclusively on the X chromosome of males. This particular covalent modification of histone H4 is correlated with a 2-fold enhancement of the transcription of most X-linked genes in Drosophila males, which is the basis of dosage compensation in this organism. Although widespread along the X chromosome, the MSL complex is not distributed uniformly, as can be seen by the indirect cytoimmunofluorescence staining of larval salivary-gland polytene chromosomes. This distribution pattern has been interpreted as a reflection of the tissue-specific transcriptional activity of the larval salivary gland and as an indication that the MSL complex associates with active chromatin. We have tested this hypothesis by comparing the chromosomal distribution of the complex in two different tissues. We performed this comparison by following the pattern of association of the complex at a specific site on salivary-gland chromosomes during larval development and determining whether an ectopic promoter located in a complex-devoid region of the X chromosome is able to attract the complex upon activation. Our results indicate that, in contrast to other chromatin-remodeling complexes that enhance transcription, the MSL complex targets active chromatin.
In eukaryotic cells, the architectural organization of chromatin is unfavorable to the process of transcription; it must be altered for the preinitiation complex to access promoter regions and for transcription to begin. The necessary alteration in the association of DNA and nucleosomes can be achieved by the covalent modification of the N-terminal tails of histones in a manner that alters their affinity for the DNA. Such modifications include acetylation, phosphorylation, methylation, ADP-ribosylation, and ubiquitination. Acetylation is the responsibility of multiprotein complexes that target histone acetyltransferases to their site of action in chromatin. A change in the association of DNA and nucleosomes also can be achieved by using the energy released by ATP hydrolysis. This type of chromatin remodeling is mediated by multiprotein complexes that contain an ATPase-enzymatic subunit (1–4).
In Drosophila, the male-specific lethal (MSL) complex is responsible for enhancing the rate of transcription of most X-linked genes in males, thereby achieving an equal level of gene products in males and females. Among its protein components, this complex includes both a histone acetyltransferase [males absent on the first (MOF)] and an adenosinetriphosphatase with demonstrated helicase activity [maleless (MLE)]; the latter feature distinguishes it from all other known chromatin complexes. Furthermore, the MSL complex contains at least one of two noncoding RNAs produced by the roX1 and roX2 (RNA on the X) genes (5–7). The function of this complex is unlikely to be the initiation of gene activity. This conclusion is derived from the observation that X-linked genes are activated normally in females where the complex is absent and in mutant males where the complex is inactive. In mutant males, X-linked gene products, although elaborated in normal temporal sequence and spatial distribution, are present at half the normal level; this diminished presence of X-linked gene products eventually leads to death. Current models suggest that the complex forms at the site of transcription of the roX genes that are located on the X chromosome, accesses a series of 30–40 entry sites on the X chromosome, and spreads to neighboring loci where it enhances gene transcription (8). To spread, the complex needs both the ATPase activity of MLE and the histone acetyltransferase activity of MOF; without this activity, fully formed but inactive complexes access only the entry sites (9). In this study, we investigated the contribution that targeted loci make to the spreading process.
Materials and Methods
Generation of Polytene Chromosomes in Ovarian Nurse Cells. Fly lines carrying the ovarian tumor (otu) alleles otu11 and otu7 were obtained from the Bloomington Drosophila Stock Center (Indiana Univ.) and from Lillie Searles (University of North Carolina, Chapel Hill). The chromosome carrying the MSL2 transgene P{H83M2} was provided by Mitzi Kuroda (Baylor College of Medicine, Houston). The genotype of individuals used in this study was otu11/otu7/y+Y; msl3 P{H83M2}/+. These females were generated by crossing otu11/FM7y+Y females with otu7/Y; msl3 P{H83M2}/TM6B males. Individuals of the genetic constitution otu11/otu7/y+Y are female-sterile and produce abnormal egg chambers that contain nurse cells without an oocyte (10). The presence of the y+Y chromosome results in improved cytology of these pseudo nurse cells. The addition of P{H83M2} to the genetic constitution of female individuals results in the production of the MSL2 protein and the formation of an ectopic MSL complex (11). Although expression of the msl2 transcript is controlled by the heat-shock promoter, uninduced levels of msl2 expression are sufficient to generate ectopic MSL complex.
Staging of Larvae. We used the gradual disappearance of the ng1 transcript and the appearance of the Sgs4 transcript in salivary glands, as development proceeded (12), to place third-instar larvae into groups: early, middle, and late. Larval salivary glands were dissected. One lobe was used for RT-PCR analysis; the other was prepared for immunostaining to detect MSL–protein association. Total RNA was isolated by using an RNeasy kit from Qiagen (Valencia, CA) and treated with DNase. RNA was reverse-transcribed by using random hexamers or rp49-specific primers. The levels of the Sgs4, ng1, and rp49 transcripts were assessed by PCR.
Enhancer–Promoter (EP) Constructs. Fly lines carrying P{EP} insertions (13) were obtained from the Bloomington Stock Center and the Szeged Drosophila Stock Centre (University of Szeged, Szeged, Hungary). A transgenic line carrying P{Act5C-GAL4.A}25 was provided by Steven Henikoff (Fred Hutchison Cancer Center, Seattle). Males of the genotype y w1118/Y; P{Act5C-GAL4.A}25/Bc were crossed to females homozygous for the EP element under study. The resulting male siblings were either EP/Y; P{Act5C-GAL4.A}25 or EP/Y; Bc.
Immunostaining of Polytene Chromosomes. Polytene chromosomes from the ovaries of otu mutants or from the salivary glands of third-instar larvae were prepared by using the same standard procedure. Ovaries or glands were dissected in phosphate-buffered saline (1× PBS) and fixed in an acetic acid/water/lactic acid (3:2:1) solution for 2 min. The tissue was then moved to a siliconized coverslip, squashed on a slide, and stored in liquid nitrogen. Slides were prepared as described (9). Both rabbit and mouse antisera against MSL1 were used; anti-GAL4 serum was purchased from Babco (Richmond, CA).
Results
The Association of the MSL Complex Exhibits Tissue Specificity. In salivary-gland chromosomes of wild-type third-instar male larvae, the MSL complex is present at numerous distinct sites (14–16). It can be inferred from this observation that the distribution of the complex reflects the distribution of hyper-transcribed genes on the male X chromosome. If this inference were the case, one would expect the distribution pattern of the complex to differ between tissues because of differences in gene expression. Although nuclei with polytene chromosomes are found in many larval and some adult tissues, only the chromosomes in the salivary glands are suitable for cytological analysis. An exception to this general rule is provided by certain combinations of otu mutant alleles (10). Therefore, to compare the distribution of the MSL complex in different tissues, we induced its formation in otu mutant females. This comparison can be accomplished by using a transgene that allows the expression of the MSL2 protein (11). We analyzed the distribution of the complex on the distal third of the X chromosome in salivary glands and in ovarian nurse cells of female larvae. Our observations revealed that, in addition to most sites that are constant between the two tissues, there are subtle tissue-specific differences in MSL-complex distribution along the chromosome (Fig. 1).
Fig. 1.
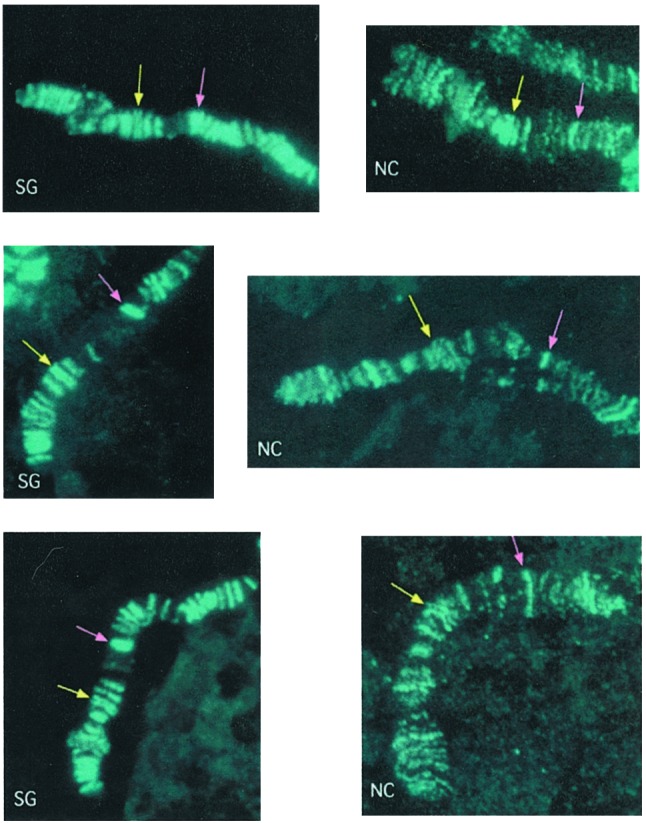
Distribution of the MSL complex on the X chromosomes of two different tissues. Formation of the MSL complex was induced in females by means of the MSL2 transgene, P{H83M2}. Polytene chromosome preparations from nuclei of larval salivary glands (SG) or nurse cells (NC) of otu11/otu7/y+Y; msl3 P{H83M2}/+ females were stained for the presence of the MSL complex with anti-MSL1 serum (aqua). The yellow arrows point to a double band of complex that serves as a cytological point of reference in both SG and NC chromosomes. The pink arrows point to another double band in SG chromosomes that is replaced by a single band in NC. Immediately distal to this band there is a significantly puffed region in NC chromosomes, wherein two bands of MSL complex can be seen.
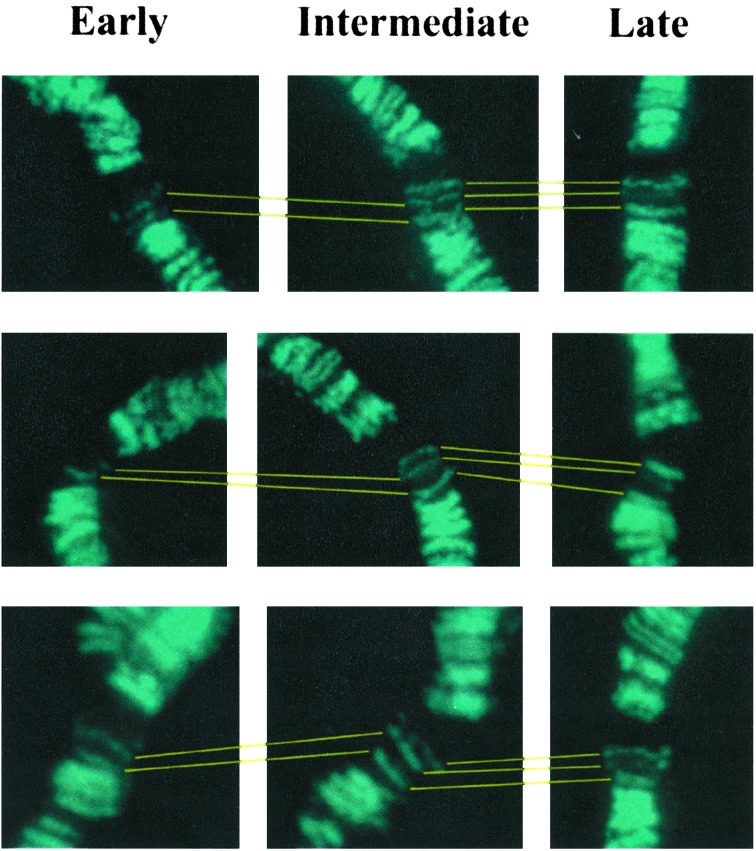
MSL-Complex Association Changes as a Function of Developmental Time. If MSL-complex association with the X chromosome shows a direct correlation with gene expression, then it should be possible to follow the course of MSL association as the program of gene expression in a particular tissue changes over time. In previous studies, we noticed some variability in the pattern of association of the complex with the X chromosome and in the intensity of this association at specific loci in any given stock. To investigate whether this variability is a function of developmental time, we used the pattern of expression of salivary-gland-specific genes (16) to establish precisely the relative age of a series of third-instar larvae of an Oregon-R wild-type strain. The changes in relative intensity of the complex at some sites of region 3C of progressively older larvae are illustrated in Fig. 2.
Fig. 2.
Changes in the level of MSL complex at an X-chromosome site as a function of developmental time. Male larvae of an Oregon-R wild-type strain are classified as early, intermediate, or late on the basis of the relative levels of specific gene products. Salivary-gland polytene chromosomes were stained for the presence of the MSL complex with anti-MSL1 serum (aqua). In early larvae, region 3C of the cytological map contains two MSL bands; a third band is present in intermediate larvae, and the relative intensity of all three bands has changed in late larvae.
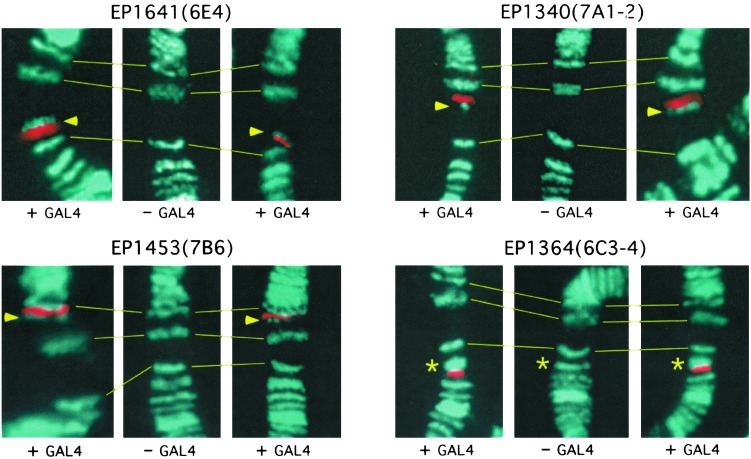
The Induced Activation of Ectopic Transcription Can Result in Association of the MSL Complex. As a more precisely controlled test of our hypothesis, we analyzed a series of established stocks carrying single insertions of a misexpression construct (EP element) that had been used to generate a large distribution of developmental dominant mutations (13). The construct contains 2 GAGA binding sites followed by 14 GAL4 binding sites that act as a GAL4-dependent enhancer of an Hsp70 promoter, poised to transcribe a genomic sequence at the site of insertion. We selected lines with insertions in regions of the X chromosome where the complex is absent in salivary glands, introduced a GAL4-expressing transgene under the control of the actin 5C (Act5C) gene promoter, and determined whether the MSL complex would target the activated construct. Polytene chromosomes were obtained from sibling third-instar male larvae that carried or lacked the GAL4 transgene; the presence of the GAL4 protein was determined by immunofluorescence. We analyzed 10 strains with single EP elements inserted at sites on the X chromosome where no MSL band was present in controls and, therefore, where the occurrence of a new band would be noted more easily. The ubiquitous presence of the GAL4 protein and its association with the 14×(UAS)GAL4 of the EP element resulted in the recruitment of the MSL complex to the site of insertion in 7 of 10 cases (Table 1 and Fig. 3). No MSL association was ever seen at the site of EP-element insertion in the absence of GAL4 expression. In each of the seven positive cases, the new MSL band was present in all of the nuclei where the region of the EP insert could be seen, although in some cases the intensity of the band varied among nuclei. We analyzed also several strains of X-linked EP-element insertions that, upon activation, revealed themselves to be within MSL bands present in controls (or so close to them that the occurrence of a new band could not be determined with accuracy). In some of these cases, induction by GAL4 seemed to result in the modification of an existing band. Given the difficulty in assessing these effects, the EP elements in question are omitted from Table 1, although an example of putative band modification is included in Fig. 3.
Table 1. Cytological position and effect of the EP elements analyzed.
| EP line | Insertion site | Result | No. of Iarvae* |
|---|---|---|---|
| EP356 | 1B6-7 | New MSL band (weak) | 15 |
| EP1395 | 3C9-10 | New MSL band (weak) | 6 |
| EP1376 | 3D6 | New MSL band | 12 |
| EP1198 | 3F1-2 | New MSL band | 9 |
| EP1641 | 6E4 | New MSL band | 6 |
| EP1340 | 7A1-2 | New MSL band | 6 |
| EP1453 | 7B6 | New MSL band (weak) | 9 |
| EP973 | 3C11-12 | No MSL band | 12 |
| EP1558 | 3E5 | No MSL band | 6 |
| EP1315 | 3F4-5 | No MSL band | 6 |
| EP1184 | 24F2 | No MSL band | 3 |
| EP407 | 42A10 | No MSL band | 3 |
| EP2165 | 45F3 | No MSL band | 3 |
| EP531 | 57A6 | No MSL band | 3 |
| EP1065 | 60E1 | No MSL band | 3 |
| EP305 | 61B3 | No MSL band | 3 |
| EP3082 | 78A1 | MSL band (faint, variable) | 3 |
| EP1015 | 89E6 | MSL band (faint, variable) | 3 |
| EP409 | 93A2 | No MSL band | 3 |
| EP3127 | 96B1 | MSL band (faint, variable) | 3 |
A minimum of 20 nuclei were examined per Iarva
Fig. 3.
Activated EP constructs recruit the MSL complex. EP/Y; Bc/+ males were compared with their EP/Y; P{Act5C-GAL4.A}25/+ brothers of similar developmental stage. Salivary gland polytene chromosomes were stained with anti-MSL1 serum (aqua) and anti-GAL4 serum (red). The three panels of each line contain chromosomes from a control (- GAL4) and two different GAL4-expressing larvae (+ GAL4). Binding of the GAL4 activator resulted in the appearance of a new MSL complex band in lines carrying EP1641, EP1340, and EP1453 (arrowheads), and in an increase of an existing band in EP1364 (asterisks). The horizontal lines mark three MSL bands present in both experimental and control larvae and are provided as cytological reference points.
As a control, we examined 10 lines where the EP elements had been inserted in different autosomal positions. Two of these lines appeared to recruit the MSL complex at the site of GAL4 binding. At these sites, the level of binding is significantly less than at any of the positive EP elements on the X chromosome. This reduced level is evidenced by the fact that to be able to see the autosomal association, the immunofluorescence photomicrographs must be so overexposed that they blur the normal distribution pattern of the complex on the X chromosome completely (Fig. 4). Not surprisingly, the association of the complex with autosomal sites can be seen in only a few nuclei per gland because of its significantly low level.
Fig. 4.

Example of MSL complex binding to an autosomal EP insert. Salivary gland polytene chromosomes of males from the EP3127 line were stained with anti-MSL1 serum (aqua) and anti-GAL4 serum (red). Activation of the EP construct resulted in the presence of a very reduced level of MSL complex colocalized with the GAL4 at its site of binding. (a) Site of insertion of the EP element at position 96B1–2 on chromosome III [counterstained with 4′,6-diamidino-2-phenylindole (DAPI), blue]. (b) Presence of a MSL band that overlaps the GAL4 band. (c) A segment of the X chromosome in the same nucleus photographed at the same exposure as the autosomal MSL band.
In the X-linked EP lines that recruited a new MSL band upon activation, the immunofluorescence signals generated by the GAL4 and MSL antisera did not overlap (Fig. 3). In all cases where the EP site of insertion had been sequenced, the detected MSL protein was in a position, relative to the GAL4 protein, that would be predicted if transcription were directed from the hsp70 promoter. These last two observations lead us to conclude that the MSL complex is localized on the transcribed portion of the region activated by the EP element promoter.
Discussion
To understand fully the mechanism of transcriptional enhancement mediated by the MSL complex, it is necessary to understand the conditions and parameters that result in its association with chromatin. This association includes not only attributes of the complex proper, but also specific aspects of the targeted regions. Long-standing cytological analyses of salivary-gland polytene chromosomes have indicated that the MSL complex does not spread indiscriminately along the X chromosome. To determine whether the distribution of the complex reflected the transcriptional pattern of X-linked genes characteristic of a particular tissue, we have compared the distribution of the complex in salivary glands and ovarian nurse cells. The use of females was mandated by the fact that, in males, only the polytene chromosomes of larval salivary glands are amenable to cytological analysis. In contrast, the use of the otu mutation in females leads to ovarian nurse cells with analyzable polytene chromosomes (10). In females, the induced MSL complex is active, as evidenced by its association with both X chromosomes; this association results in the overexpression of X-linked genes with eventual severe consequences on viability (11). Therefore, although there are some differences in the distribution of the native complex in males and the induced complex in females, we believe that the use of females is justified to determine whether there are tissue-specific differences concordant with the hypothesis that the complex distribution reflects particular transcription patterns. The majority of the MSL sites in salivary gland and nurse cell chromosomes are similar. This similarity presumably reflects the expression of housekeeping genes active in both tissues. Nevertheless, subtle differences, such as the one illustrated in Fig. 1, provide prima facie evidence for tissue-specific distribution.
We also documented changes in the level of complex present in a specific region of the salivary-gland X chromosome as a function of developmental progression (Fig. 2). These results support the assertion that the pattern of complex association in a tissue is a reflection of the transcriptional activity of the tissue, at a particular time in development.
The affinity of the complex for activated genes is borne out by its association with several X-linked EP elements upon their transcriptional activation (Fig. 3). In these cases, the complex is found to be adjacent to, but not directly associated with, promoter regions, as defined by the localization of the GAL4 protein. At this level of resolution, it is not possible to correlate the relative positions of the GAL4 and MSL bands with the size of expected transcription units. Nevertheless, our observations are consistent with the previous results of chromatin-immunoprecipitation assays, demonstrating that the specific form of histone acetylation carried out by the complex is found along extended domains that include dosage-compensated units (17). Failure of the MSL complex to form a new band in association with some of the X-linked EP elements may be due to the failure of a particular EP insertion to create a bona fide transcription unit, either because of some mutation in the EP cassette or because of some unfavorable feature of the site of insertion. It may also reflect the fact that some X-linked genes such as Lsp1α are not dosage-compensated (18). When this gene is relocated to a different region of the X chromosome, it becomes subject to the transcriptional enhancement characteristic of compensation; this change indicates that in its normal location it is sheltered from association with the complex (19). An EP element inserted in such a location may also be prevented from recruiting the MSL complex. The same result would be expected if an EP element were inserted into a region similar to the one where the gene runt (run), which is dosage-compensated but not by the MSL complex (20), is located: ≈200 kbp of this region appear to be devoid of complex in most if not all tissues of developing embryos (17). The presence of the MSL complex on some autosomal EP elements, albeit variable and at a minimal level, may reflect the fact that there are ≈20–30 sites along the autosomes where low levels of complex are normally found (15–16, 21). Perhaps the binding of GAL4 to some EP elements mimics in some fashion the features of these autosomal sites that normally attract the complex for reasons that are not understood.
It is also possible that the MSL complex is recruited directly or indirectly by GAL4 to promoters from where it accesses and spreads along transcribed regions because of its affinity for some transcription-associated histone modification of nucleosomes in these regions. An analogous situation has been reported regarding the NuA4 complex of yeast, which contains the homologues of Drosophila MOF and MSL3 and acetylates histone H4 preferentially. After its targeting to a 5×(UAS)GAL4-binding site in a reconstituted nucleosomal array by the recombinant activator GAL4-VP16, this complex acetylated histone H4 over a chromatin domain of >3 kbp (22). In our experiments, the absence of visible colocalization of the MSL complex and GAL4 at the sites of GAL4 binding may reflect the weak affinity of the activator and the complex for each other relative to the strong affinity of the complex for its substrate (i.e., for the nucleosomes of transcribing chromatin). As soon as it is recruited, the complex translocates from the promoter to the transcription unit, where it accumulates and gives rise to a new immunofluorescent band.
If GAL4 recruits the complex to X-linked EP lines, it would be expected to recruit it to autosomal EP inserts as well. Its failure to recruit the complex beyond a minimal level (Fig. 4) could be explained by the fact that to target an autosomal site, GAL4 must compete for complex binding with the whole X chromosome where the complex is formed and where most of it is sequestered because of the presence of 30–40 high-affinity entry sites and the proximity of active transcription units to these sites. The competition for available complex between the X chromosome and an entry site relocated to an autosome has been documented (23). In contrast, EP sites embedded in the X chromosome benefit from the presence of a high local concentration of complex.
Surprisingly, in those instances where the complex is seen to associate with an autosomal EP line, it appears to colocalize with GAL4 rather than to be clearly adjacent to it, as in the case of positive X-linked EP lines. If the spreading of the complex along active transcription units depends on its ability to acetylate histone H4 at lysine-16, the autosomal EP elements may not acquire sufficient complex to allow its translocation from the promoter region. This failure of the complex to translocate results in its accumulation at the site of GAL4 binding. Some evidence in support of this assertion is provided by our observation that a complex with a mof1 mutation that is unable to access any of its normal sites of action (i.e., to spread along the X chromosome) nevertheless retains sufficient residual activity to acetylate histone H4 at the entry sites where it is found (W. Gu and J.C.L., unpublished data). These considerations imply that the complex is present at the site of autosomal GAL4 binding in levels insufficient to access the transcriptional unit that has been activated. The same situation may prevail in the case of X-linked EP lines where no new MSL band has appeared. In these cases, however, the much higher background of MSL immunostaining (present on the X chromosome because of the normal, ubiquitous association of the complex) may have prevented the detection of a minimal level of complex colocalizing at the site of GAL4 binding.
The observations that are reported in this article indicate that the MSL complex recognizes and associates with open, active chromatin. This feature of the complex and its proposed effect on transcription at the level of elongation (17) are reminiscent of the association of the yeast and human Elongator complexes with activated RNA polymerase II (24–26). Elongator complexes acetylate histone H3 and, to a lesser extent, histone H4, but it is not clear whether this enzymatic activity is responsible for their specific role in facilitating transcription, or whether it is responsible for maintaining a global level of acetylation throughout activated regions of the genome (27). The association of the MSL complex for active transcriptional domains provides an explanation for the distribution of the complex when it is induced to form on, or is recruited to, an autosome by the relocation of roX sequences (8, 23). The pattern of spreading of the complex from such ectopic sites is discontinuous and, in this respect, resembles the pattern characteristic of the normal X chromosome. Consistent with the discontinuous pattern exhibited by the complex along polytene chromosomes, its association with activated X-linked EP elements does not appear to require its uninterrupted “flow” from adjacent regions where it is normally present. Rather, the dynamic association of complexes in these regions allows free complexes to access an isolated active gene located in relative proximity.
Acknowledgments
We thank Krista Fehr and Ebony Courtney for excellent technical assistance and Edwin Smith, Arri Eisen, and Weigang Gu for helpful discussions of our results. This work was supported by National Institutes of Health Grant GM15691.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: MSL, male-specific lethal.
References
- 1.Hassan, A. H., Neely, K. E., Vignali, M., Reece, J. C. & Workman, J. L. (2001) Front. Biosci. 6 D1054-D1064. [DOI] [PubMed] [Google Scholar]
- 2.Roth, S. Y., Denu, J. M. & Allis, C. D. (2001) Annu. Rev. Biochem. 70 81-120. [DOI] [PubMed] [Google Scholar]
- 3.Becker, P. B. & Horz, W. (2002) Annu. Rev. Biochem. 71 247-283. [DOI] [PubMed] [Google Scholar]
- 4.Peterson, C. L. (2002) Curr. Biol. 12 R245-R247. [DOI] [PubMed] [Google Scholar]
- 5.Lucchesi, J. C. (1998) Curr. Opin. Genet. Dev. 8 179-184. [DOI] [PubMed] [Google Scholar]
- 6.Meller, V. H. (2000) Trends Cell Biol. 10 54-59. [DOI] [PubMed] [Google Scholar]
- 7.Park, Y. & Kuroda, M. I. (2001) Science 293 1083-1085. [DOI] [PubMed] [Google Scholar]
- 8.Kelley, R. L., Meller, V. H., Gordadze, P. R., Roman, G., Davis, R. L. & Kuroda, M. I. (1999) Cell 98 513-522. [DOI] [PubMed] [Google Scholar]
- 9.Gu, W., Wei, X., Pannuti, A. & Lucchesi, J. C. (2000) EMBO J. 19 5202-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King, R. C., Riley, S. F., Cassidy, J. D., White, P. E. & Paik, Y. K. (1981) Science 212 441-443. [DOI] [PubMed] [Google Scholar]
- 11.Kelley, R. L., Solovyeva, I., Lyman, L. M., Richman, R., Solovyev, V. & Kuroda, M. I. (1995) Cell 81 867-877. [DOI] [PubMed] [Google Scholar]
- 12.Furia, M., D'Avino, P. P., Crispi, S., Artiaco, D. & Polito, L. C. (1993) J. Mol. Biol. 231 531-538. [DOI] [PubMed] [Google Scholar]
- 13.Rørth, P. (1996) Proc. Natl. Acad. Sci. USA 93 12418-12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda, M. I., Kernan, M. J., Kreber, R., Ganetzky, B. & Baker, B. S. (1991) Cell 66 935-947. [DOI] [PubMed] [Google Scholar]
- 15.Palmer, M. J., Mergner, V. A., Richman, R., Manning, J. E., Kuroda, M. I. & Lucchesi, J. C. (1993) Genetics 134 545-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorman, M., Franke, A. & Baker, B. S. (1995) Development (Cambridge, U.K.) 121 463-475. [DOI] [PubMed] [Google Scholar]
- 17.Smith, E. R., Allis, C. D. & Lucchesi, J. C. (2001) J. Biol. Chem. 276, 31483-31486. [DOI] [PubMed] [Google Scholar]
- 18.Roberts, D. B. & Evans-Roberts, S. (1979) Nature 280 691-692. [DOI] [PubMed] [Google Scholar]
- 19.Gosh, S., Chatterjee, R. N., Bunick, D., Manning, J. E. & Lucchesi, J. C. (1989) EMBO J. 8 1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gergen, J. P. (1987) Genetics 117 477-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bashaw, G. J. & Baker, B. S. (1995) Development (Cambridge, U.K.) 121 3245-3258. [DOI] [PubMed] [Google Scholar]
- 22.Vignali, M., Steger, D. J., Neely, K. E. & Workman, J. L. (2000) EMBO J. 19 2629-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, Y., Kelley, R. L., Oh, H., Kuroda, M. I. & Meller, V. H. (2002) Science 298 1620-1623. [DOI] [PubMed] [Google Scholar]
- 24.Otero, G., Fellows, J., Li, Y., de Bizemont, T., Dirac, A. M., Gustafsson, C. M., Erdjument-Bromage, H., Tempst, P. & Svejstrup, J. Q. (1999) Mol. Cell 3 109-118. [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. H., Lane, W. S. & Reinberg, D. (2002) Proc. Natl. Acad. Sci. USA 99 1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkes, N., Otero, G., Winkler, G. S., Marshall, N., Dahmus, M. E., Krappman, D., Scheidereit, C., Thomas, C. L., Schiavo, G., Erdjument-Bromage, H., et al. (2002) J. Biol. Chem. 277 3047-3052. [DOI] [PubMed] [Google Scholar]
- 27.Winkler, G. S., Kristjuhan, A., Erdjument-Bromage, H., Tempst, P. & Svejstrup, J. Q. (2002) Proc. Natl. Acad. Sci. USA 99 3517-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]