Abstract
Most forest birds include arthropods in their diet, sometimes specializing on arthropods that consume plant foliage. Experimental tests of whether bird predation on arthropods can reduce plant damage, however, are few and restricted to relatively low-diversity systems. Here, we describe an experimental test in a diverse tropical forest of whether birds indirectly defend foliage from arthropod herbivores. We also compare how the indirect effects of bird predation vary with different levels of foliage productivity in the canopy vs. the understory. For three Neotropical tree species, we observed that birds decreased local arthropod densities on canopy branches and reduced consequent damage to leaves. In contrast, we observed no evidence of bird–arthropod limitation on conspecific saplings in the less productive understory of the same forest. Our results support theory that predicts trophic cascades where productivity is high and suggest that birds play an important role in Neotropical communities by means of their indirect defense of some canopy tree species.
For decades, ecologists have debated the circumstances under which a predator limits its prey's consumption of organisms in lower trophic levels (i.e., a predator-driven trophic cascade) (1–4). One body of theory predicts such cascades will occur in terrestrial systems with high plant productivity (5, 6). Opposing theory predicts that cascades will not occur in terrestrial systems and only in low-diversity aquatic or marine systems (7). Proponents of the latter theory suggest that higher diversity in terrestrial systems leads to diffuse food webs, rendering trophic levels nonexistent (7). Field experiments demonstrate that insectivorous birds can limit arthropod abundances and decrease damage to plants, but these tests have been conducted in settings with relatively low tree species diversity such as temperate forests (8–11) or agricultural systems (12, 13). Along with high tree species diversity, tropical forests support a high diversity and biomass of leaf-chewing arthropods (14) as well as a high biomass of birds that consume them (15). In a lowland forest of Panama, we used canopy crane access (16) to test the hypotheses that (i) birds limit arthropod densities and consequent herbivore damage, and (ii) the effects of bird predation are strongest where foliage production rates are high.
For one year, we observed how the local density and taxonomic composition of the arthropod community responded to the absence of bird predation and also assessed changes in herbivore damage. We estimated and compared these quantities on control branches/saplings where birds had access to foliage and on branches/saplings in experimental exclosures where foliage was inaccessible to birds. A within-site comparison of canopy branches and conspecific understory/edge saplings allowed us to investigate the effects of bird predation across a 3-fold vertical gradient of foliage production.
Methods
A canopy crane provided forest canopy access in a dry, semideciduous lowland tropical forest in Panama (16). Mean annual rainfall is 1,850 mm, with most rain occurring in the wet season (May through December). The experiment simultaneously included three tree species, Anacardium excelsum (Bertero and Balb.) Skeels Anacardiaceae, Cecropia longipes Pittier, and Cecropia peltata Linnaeus Moraceae, which are common in the area under the crane. In the canopy, we installed exclosures on 22 randomly chosen exclosure branches (7 or 8 per tree species) and paired them to 22 control branches on the same trees. On the same three tree species in the understory, we installed exclosures on 19 saplings (4–7 per species), pairing exclosure and control saplings by height and proximity to the forest edge. Most saplings grew only near forest edges or in gaps. Mean sapling height at the start of the experiment was 1.16 m for exclosures and 1.17 m for controls. Overall, the experiment included 44 canopy branches on 18 individual trees and 38 saplings.
Exclosures were constructed with untreated wooden dowels wired together and covered with agricultural netting (mesh opening = 2 × 2 cm). Each exclosure surrounded a volume of ≈1 m3, which enclosed an average of 1.3 (±0.7) m2 of leaf area in the canopy and 0.35 (±0.15) m2 of leaf area in the understory. Procedural control (sham) exclosures, where materials were in the canopy but were open on the sides to bird foraging, were constructed and monitored throughout the year. The exclosure materials did not attract arthropods, did not damage leaves or branches, and did not significantly reduce light (17).
We attribute the changes in arthropod density and damage primarily to the 31 species of birds that we observed consuming arthropods in the canopy (17). Some insectivorous bats glean arthropods from understory and canopy foliage, however, and their numbers and impact are currently under study in a nearby forest (E. Kalko, personal communication). The exclosures allowed access to Anolis spp. lizards.
Nondestructive censuses of arthropod density were completed at 3-week intervals between April 2000 and March 2001. Arthropod censuses were performed by visual inspection of all leaf surfaces on experimental branches. For each arthropod encountered, we noted the order, guild, and position on leaf surface (top or bottom) and the presence or absence of camouflage or spines. Although we recognize that birds may perceive color differently than humans, camouflage was assumed when arthropods were the same shade and color as the surface on which they were observed. All Cecropia spp. branches and saplings in the experiment were hosts to Azteca spp. ant colonies; however, colonial arthropod species could not be counted accurately and were not included in arthropod counts. All arthropod densities are reported as the number of arthropods per m2 of leaf area. Leaf area was estimated by counting leaves, establishing a mean leaf length for each sapling/branch, and using the mean in species-specific allometric equations to sum over the number of leaves present.
Nondestructive censuses of leaf damage were completed at 6-week intervals between April 2000 and March 2001. Leaf damage was determined by using a plastic grid. We measured damage only for exclosure and control leaves that flushed after the experiment began. Rates of new leaf production (leaf area at time 2 - leaf area at time 1/number of days) were measured on control saplings and canopy branches between July 10 and August 17. Rates of leaf production were calculated on a per exclosure basis (≈1 m3). Means are reported with one SE.
Arthropod density and leaf damage measurements were log transformed and subjected to repeated-measures analysis using a mixed model test in SAS (2000). This test accounts for both fixed and random factors in the model and calculates variance components by using restricted maximum-likelihood estimates (18). The fixed effects in the model were treatment, census period, host tree species, and all two-way interactions. All three-way interactions were nonsignificant and were removed from the models. The random effect in the model was the host tree nested within host species. We analyzed additional models that combined canopy and understory response variables and found significant treatment by strata effects, as would be expected from Table 1. Arthropod data relating to the position on leaf surfaces and the presence/absence of morphological characteristics were analyzed by using Pearson's χ2 tests with exact P values. For arthropod order and guild comparisons, branch averages of arthropod density (averaged over all time periods) were compared in paired permutation tests using Monte Carlo resampling to estimate P values (19).
Table 1. Results of repeated-measures mixed models for arthropod density and leaf damage in the canopy and understory.
|
Canopy branches
|
Understory/edge saplings
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Arthropod density
|
% leaf damage
|
Arthropod density
|
% leaf damage
|
|||||||||
| Fixed effects | df | F | P | df | F | P | df | F | P | df | F | P |
| Bird exclosure treatment | 1, 81 | 4.4 | 0.04 | 1, 54 | 5.6 | 0.02 | 1, 76 | 0.0 | 0.97 | 1, 31 | 0.1 | 0.77 |
| Time | 7, 201 | 7.7 | <0.001 | 6, 174 | 20.5 | <0.001 | 7, 156 | 1.3 | 0.25 | 5, 127 | 7.6 | <0.001 |
| Tree species | 2, 16 | 7.3 | 0.006 | 2, 16 | 2.4 | 0.13 | 2, 41 | 9.8 | <0.001 | 2, 25 | 10.5 | <0.001 |
| Tree species × time | 14, 198 | 3.6 | <0.001 | 12, 175 | 6.5 | <0.001 | 12, 125 | 0.8 | 0.62 | 8, 124 | 5.6 | <0.001 |
The models include arthropod densities from the wet season only (May through December). Dry season densities did not vary significantly with bird exclusion. All models included time by treatment and tree species by treatment effects, which were nonsignificant in all cases. Boldface P values are significant
Results
We documented a 3-fold vertical difference in foliage production from the canopy to the understory. Average rates of foliage production were 144 (±18) cm2/day on canopy branches and 54 (±15) cm2/day on saplings during the wet season. All three tree species flushed new leaves in May, at the onset of the wet season.
We observed that bird predation significantly decreased local densities of arthropods on canopy branches (Tables 1 and 2). This effect was seasonal and was significant only during the wet season (May through December), when chewing arthropods increased by 90% relative to the dry season. Thus, the effects of bird predation were greatest when overall arthropod densities and leaf production were generally high.
Table 2. Wet season arthropod densities by order and guild.
|
Density,†
no./m2 (SE)
|
|||
|---|---|---|---|
| Arthropods | On accessible branches | On inaccessible branches | % change‡ |
| By order | |||
| Arachnida | 1.00 (0.17) | 1.93 (0.37) | 92*** |
| Blattaria | 0.11 (0.03) | 0.22 (0.05) | 98* |
| Coleoptera | 0.58 (0.12) | 0.80 (0.27) | 37 |
| Hemiptera | 3.85 (0.82) | 3.16 (0.56) | -18 |
| Lepidoptera§ | 0.28 (0.06) | 0.78 (0.24) | 181* |
| Orthoptera | 0.24 (0.05) | 0.29 (0.09) | 18 |
| By guild | |||
| Chewing | 0.85 (0.18) | 1.74 (0.36) | 103* |
| Predatory | 1.14 (0.24) | 2.06 (0.43) | 80*** |
| Phloem-feeding | 3.98 (0.83) | 3.16 (0.66) | -20 |
Mean (SE) number of arthropods per m2 of leaf area
Calculated as [(density inaccessible - density accessible)/density accessible] × 100
, P < 0.05
, P < 0.001 for paired permutation tests
Of the total Lepidoptera, 81% (211/257) were leaf-chewing larvae and 19% were adults, with similar age proportions on accessible and inaccessible branches
In addition to greater overall arthropod densities on inaccessible branches, we observed differences in the taxonomic and morphological composition of canopy arthropods. Chewing and predatory arthropods showed the greatest increase on inaccessible branches (Table 2 and Fig. 1A). Local densities of Arachnida, Blattaria, and Lepidoptera were significantly greater on inaccessible branches (Table 2). Moreover, we observed shifts in the frequency of arthropods with morphologies and behaviors that suggest defense from visually oriented predators. We observed a higher frequency of camouflaged arthropods (e.g., same color as leaf surface, χ2 = 3.22, df 1, P = 0.036) and a higher frequency of arthropods resting on the bottom vs. the top of leaf surfaces on accessible branches than on inaccessible branches (χ2 = 5.15, df 1, P = 0.012). These patterns imply that birds more frequently consumed visually detectable arthropods. Additionally, we observed a higher frequency of spiny vs. smooth caterpillars on accessible vs. inaccessible branches (χ2 = 14.32, df 1, P = 0.0001), suggesting bird preference for smooth caterpillars (9).
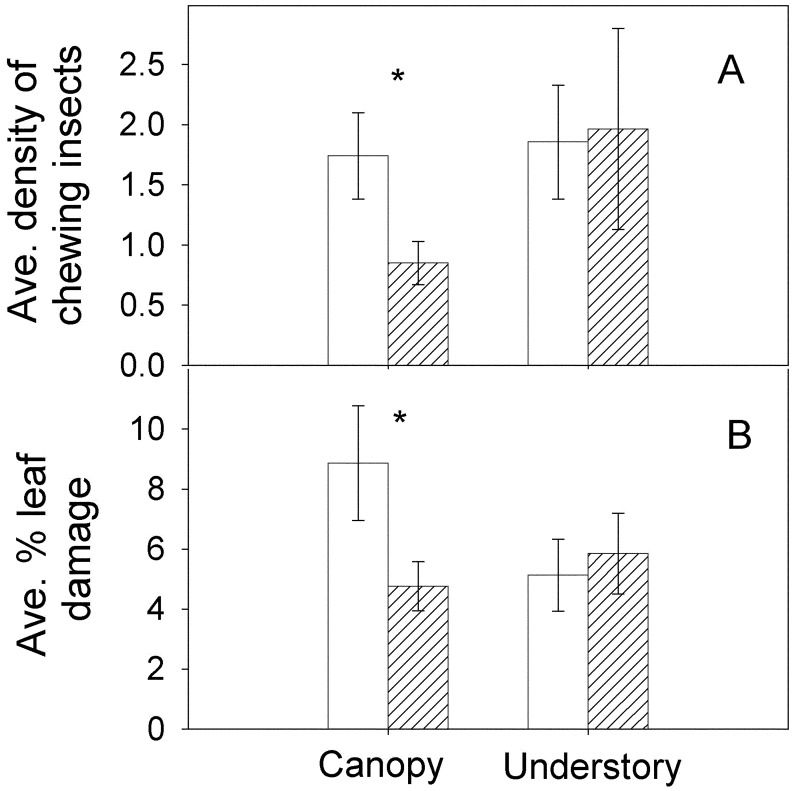
Fig. 1.
Chewing-arthropod density and leaf damage for canopy branches and understory/edge saplings, where foliage was inaccessible (open bars) and accessible (hatched bars) to bird foraging. (A) Chewing-arthropod density (number per m2 of leaf area) averaged over the wet season (May through December). Chewing-arthropod densities were higher in the understory than in the canopy, but because canopy branches had approximately three times as much leaf area as saplings, the actual abundance of arthropods was higher in the canopy. On accessible foliage, canopy/understory densities (mean ± 1 SE) were 2.06 ± 0.43/3.06 ± 0.57 for predatory arthropods and 3.16 ± 0.66/2.54 ± 0.77 for phloem-feeding arthropods. (B) Percent leaf damage at the end of the wet season (December through January). Error bars indicate 1 SE. *, P < 0.05.
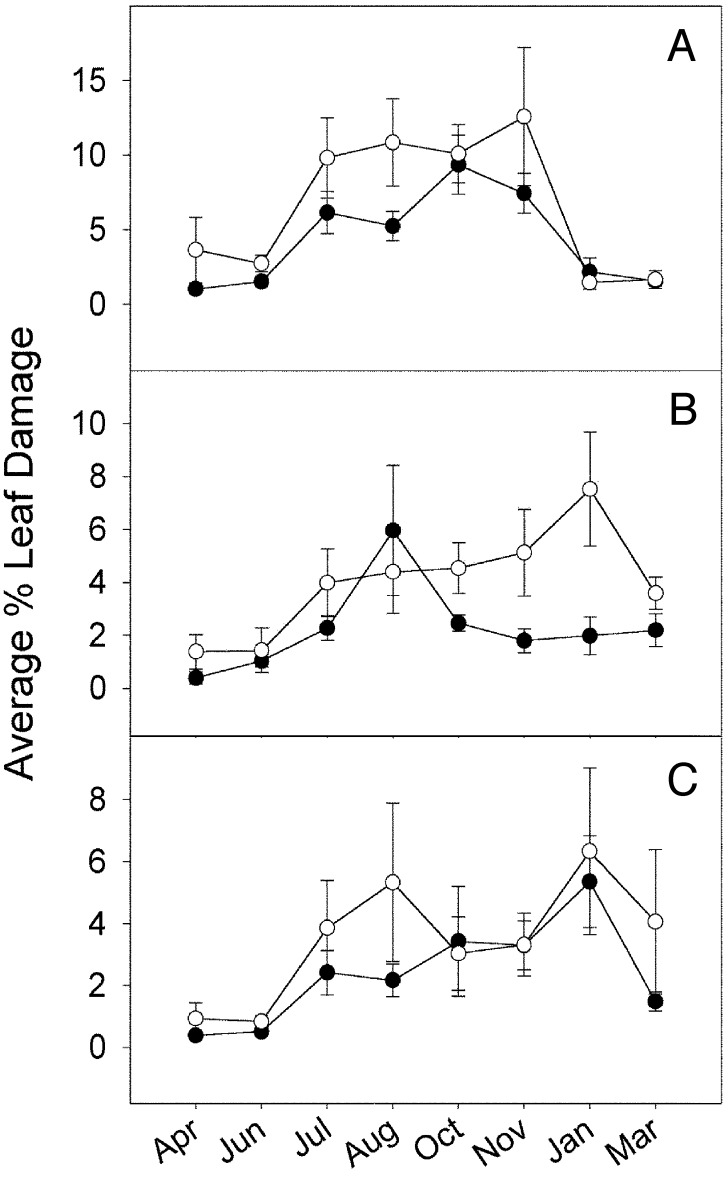
The impact of bird predation on local arthropod densities was sufficient to have indirect influence on herbivore damage to the canopy branches. Increased densities of chewing arthropods were associated with greater leaf damage (Table 1 and Fig. 1). Most herbivore damage occurred during the wet season, and damage levels peaked at the end of the wet season (Fig. 2). At the peak, average damage levels increased by 86% where foliage was inaccessible to birds (from 4.7% to 8.9% of total leaf area consumed, Fig. 1B).
Fig. 2.
Mean herbivore damage increased on canopy branches that were inaccessible (○) compared with accessible branches (•). Error bars indicate 1 SE. Tree species were A. excelsum (A), C. longipes (B), and C. peltata (C). Peak herbivory levels occurred earlier for A. excelsum because the leaf-growing season ends in December, when all wet season leaves are dropped. Reduction in mean herbivory levels at the end of the experiment coincided with leaf abscission.
In contrast to experimental effects observed in the canopy, excluding birds from understory/edge saplings did not affect arthropod density (Fig. 1 A) or composition. We observed no significant differences in leaf damage on saplings that were inaccessible and accessible to bird foraging (Table 1 and Fig. 1B). Arthropods were more abundant on canopy branches than on saplings because of higher leaf densities in the canopy (Fig. 1 A). Thus, birds reduced arthropods and damage on canopy branches but not for conspecific saplings in the understory/edge of the same forest.
Discussion
Trophic theory suggested that predators should limit herbivores in areas of high plant productivity (5, 6). Accordingly, we observed that the effects of bird predation varied with plant productivity, both spatially and temporally. In vertical space, canopy branches produced approximately three times as much leaf area per day as understory saplings, and overall arthropod numbers were higher on canopy branches. Moreover, abundances of foliage-gleaning birds were higher in the canopy than in the understory at this site (ref. 17; J. Brawn, unpublished data). Temporally, the significant effects of bird predation coincided with high canopy leaf production and arthropod abundance during the wet season. Interestingly, these temporal effects of bird predation corresponded to the nesting season of resident birds but did not coincide with the presence of migrant birds during the dry season.
Theoretical arguments have been posed against the likelihood of trophic cascades occurring in high-diversity food webs (7). By this theory, omnivory and compensation by intraguild predators should produce diffuse food webs rather than strong predator-driven trophic cascades. In our experiment, 58% of the canopy-foraging bird species we observed were omnivorous, including both fruit and arthropods in their diets (17). Intraguild predation occurred in the canopy food web as well, because both birds and spiders consume herbivorous arthropods. We observed a doubling of spider densities on inaccessible branches (Table 2), but spider predation on caterpillars, if it occurred, did not compensate enough to mute the indirect effect of bird predation (Table 1). An additional form of compensation may have occurred if birds consumed the Azteca spp. ants that live inside of Cecropia spp. branches. Some, but not all, of these ant species defend the foliage from herbivores (20). Although we were not able to accurately assess Azteca ant abundances, we suspect that birds probably did not consume enough ants, if any at all, to compensate for the antiherbivore activity of the large ant colonies in canopy trees. Rather, differences in herbivore damage between the two Cecropia spp. (Fig. 2) are potentially caused by C. peltata having more aggressive ants than C. longipes, a possibility that needs further investigation. Despite omnivory and potential compensation, then, the effects of bird predation were strong enough to produce indirect effects on trees. Our bird study joins recent empirical demonstrations of strong predator effects in other terrestrial tropical food webs, with arthropod (21), reptilian (22, 23), and mammalian (24) predators.
Several studies in temperate forests have shown that bird predation can impact arthropod prey (25, 26), but few have established that this limitation leads to changes in plant damage. Moreover, these studies are generally limited to understory shrubs or saplings (9, 11). Our results extend the demonstration of indirect bird defense of plants to a Neotropical forest canopy and suggest that the relative importance of bird predation may differ in temperate and tropical forest understories. Seasonality and vertical distribution of arthropod prey may be factors in this tropical/temperate difference. For example, arthropod abundances are much higher near the ground than in the canopy of a temperate, hardwood forest (27), a vertical trend that is reversed in tropical forests (14, 28). Further experiments that compare vertical differences in arthropod abundance and bird predation in temperate regions are necessary for direct comparison.
The experimental effects reported here probably underestimate the impacts of bird foraging on leaf damage for several reasons. First, we created small predator-free spaces, and migration of arthropods in and out of the exclosures prevented a large buildup of herbivores. Second, high levels of damage and high densities of arthropods may have encouraged migration out of the exclosures and discouraged adult Lepidoptera from ovipositing on these damaged leaves. Furthermore, our measures of foliage damage likely underestimate the detrimental effects of herbivory on trees. For example, our estimates did not account for leaves that were abscised after arthropod damage, which could underestimate leaf loss by up to 50% (29). Also, recent work has shown that simply measuring holes in leaves, as in this study, may underestimate by a factor of 6 the actual damage to photosynthetic tissue caused by chewing insects (30).
Finally, our findings have potential conservation implications. A recent study has reported dramatic changes in the flora and fauna of fragmented Neotropical communities where predators are few or absent (24). Although it is unlikely that birds will be completely extirpated from forests, several migratory and resident bird populations have declined as forests are lost and fragmented (31, 32). Further declines and local extirpations are expected to be major, with projected trends in habitat loss (31). Our study reinforces other suggestions that conservation efforts should emphasize retaining the integrity of predator communities and trophic processes (24).
Acknowledgments
We thank E. A. Herre, E. G. Leigh, Jr., G. O. Batzli, J. Dalling, K. Paige, D. Levey, and an anonymous reviewer for comments on the manuscript; S. J. Wright and the Smithsonian Tropical Research Institute for logistical support; M. Libsch, I. Ochoa, and M. Samaniego for field assistance; and the U.S. Environmental Protection Agency (Science to Achieve Results Fellowship U-91581201-2), the Smithsonian Institution Fellowship Program, the American Ornithologists' Union, the American Natural History Museum, and the University of Illinois at Urbana–Champaign for financial support.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Paine, R. T. (1980) J. Anim. Ecol. 49 666-685. [Google Scholar]
- 2.Persson, L. (1999) Oikos 85 385-397. [Google Scholar]
- 3.Schmitz, O. J., Hamback, P. A. & Beckerman, A. P. (2000) Am. Nat. 155 141-153. [DOI] [PubMed] [Google Scholar]
- 4.Halaj, J. & Wise, D. H. (2001) Am. Nat. 157 262-281. [DOI] [PubMed] [Google Scholar]
- 5.Fretwell, S. D. (1977) Perspect. Biol. Med. 20 169-185. [Google Scholar]
- 6.Oksanen, L., Fretwell, S., Arruda, J. & Niemela, P. (1981) Am. Nat. 118 240-261. [Google Scholar]
- 7.Polis, G. A. & Strong, D. R. (1996) Am. Nat. 147 813-846. [Google Scholar]
- 8.Atlegrim, O. (1989) Oecologia 79 136-139. [DOI] [PubMed] [Google Scholar]
- 9.Marquis, R. J. & Whelan, C. J. (1994) Ecology 75 2007-2014. [Google Scholar]
- 10.Murakami, M. & Nakano, S. (2000) Proc. R. Soc. London Ser. B 267 1597-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strong, A. M., Sherry, T. W. & Holmes, R. T. (2000) Oecologia 125 370-379. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg, R., Bichier, P., Angon, A. C., MacVean, C., Perez, R. & Cano, E. (2000) Ecology 81 1750-1755. [Google Scholar]
- 13.Mols, C. & Visser, M. (2002) J. Appl. Ecol. 39 888-899. [Google Scholar]
- 14.Erwin, T. L. (1982) Coleopt. Bull. 36 74-75. [Google Scholar]
- 15.Leigh, E. G. & Windsor, D. M. (1982) in The Ecology of a Tropical Forest: Seasonal Rhythms and Long-Term Changes, eds. Leigh, E. G., Rand, A. S. & Windsor, D. M. (Smithsonian Inst., Washington, DC), pp. 111-122.
- 16.Parker, G., Smith, A. P. & Hogan, K. (1992) BioScience 42 664-670. [Google Scholar]
- 17.Van Bael, S. (2003) Ph.D. dissertation (Univ. of Illinois, Urbana).
- 18.Littell, R. C., Milliken, G. A., Stroup, W. W. & Wolfinger, R. D. (1996) SAS System for Mixed Models (SAS, Cary, NC).
- 19.Gajjar, Y., Mehta, C., Patel, N. & Senchaudhusi, P. (1998) stat-xact 4 (Cytel, Cambridge, MA).
- 20.Longino, J. T. (1991) in Ant–Plant Interactions, eds. Huxley, C. R. & Cutler, D. F. (Oxford Univ. Press, Oxford), pp. 271-288.
- 21.Letourneau, D. K. & Dyer, L. A. (1998) Ecology 79 1678-1687. [Google Scholar]
- 22.Spiller, D. A. & Schoener, T. W. (1994) Ecology 75 182-196. [Google Scholar]
- 23.Dial, R. & Roughgarden, J. (1995) Ecology 76 1821-1834. [Google Scholar]
- 24.Terborgh, J., Lopez, L., Nuñez, P., Rao, M., Shahabuddin, G., Orihuela, G., Riveros, M., Ascanio, R., Adler, G. H., Lambert, T. D. & Balbas, L. (2001) Science 294 1923-1926. [DOI] [PubMed] [Google Scholar]
- 25.Otvos, I. S. (1979) in The Role of Insectivorous Birds in Forest Ecosystems, ed. Dickson, J. (Academic, New York), pp. 341-374.
- 26.Holmes, R. T. (1990) Studies Avian Biol. 13 6-13. [Google Scholar]
- 27.Preisser, E., Smith, D. & Lowman, M. (1998) Selbyana 19 141-146. [Google Scholar]
- 28.Smythe, N. (1982) in The Ecology of a Tropical Forest, eds. Leigh, E. G., Rand, A. S. & Windsor, D. M. (Smithsonian Inst., Washington, DC), pp. 309-318.
- 29.Lowman, M. D. (1984) Biotropica 16 264-268. [Google Scholar]
- 30.Zangerl, A. R., Hamilton, J. G., Miller, T. J., Crofts, A. R., Oxborough, K., Berenbaum, M. R. & de Lucia, E. H. (2002) Proc. Natl. Acad. Sci. USA 99 1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stotz, D. F., Fitzpatrick, J. W., Moskovits, D. K. & Parker, T. A., eds. (1996) Neotropical Birds: Ecology and Conservation (Univ. of Chicago Press, Chicago).
- 32.Robinson, W. D. (2001) Anim. Biodiversity Conserv. 24 51-65. [Google Scholar]