Abstract
The MLL (mixed-lineage leukemia) gene is involved in many chromosomal translocations associated with acute myeloid and lymphoid leukemia. We previously identified a transcriptional repression domain in MLL, which contains a region with homology to DNA methyltransferase. In chromosomal translocations, the MLL repression domain is retained in the leukemogenic fusion protein and is required for transforming activity of MLL fusion proteins. We explored the mechanism of action of the MLL repression domain. Histone deacetylase 1 interacts with the MLL repression domain, partially mediating its activity; binding of Cyp33 to the adjacent MLL-PHD domain potentiates this binding. Because the MLL repression domain activity was only partially relieved with the histone deacetylase inhibitor trichostatin A, we explored other protein interactions with this domain. Polycomb group proteins HPC2 and BMI-1 and the corepressor C-terminal-binding protein also bind the MLL repression domain. Expression of exogenous BMI-1 potentiates MLL repression domain activity. Functional antagonism between Mll and Bmi-1 has been shown genetically in murine knockout models for Mll and Bmi-1. Our new data suggest a model whereby recruitment of BMI-1 to the MLL protein may be able to modulate its function. Furthermore, repression mediated by histone deacetylases and that mediated by polycomb group proteins may act either independently or together for MLL function in vivo.
The MLL (mixed-lineage leukemia) gene located on chromosome band 11q23 is involved in many chromosomal translocations associated with acute leukemia (1–5). MLL is involved in translocations with >40 different genes, and breakpoints in MLL fall in an 8.3-kb breakpoint cluster region (refs. 6 and 7; see Fig. 6). The 430-kDa MLL protein is cleaved specifically into amino and carboxyl terminal peptides, which associate with each other (8–10). Domains of MLL include AT hooks, repression and activation domains, plant homeodomain (PHD) fingers, and a SET [Su(var)3–9, enhancer of zeste, and trithorax] domain, which was shown recently to have histone methyltransferase activity (10, 11). Recent murine models of MLL leukemia, including one from our lab, have confirmed that the aminoterminal portion of MLL fused in-frame to the partner gene is critical for leukemogenesis (12, 13). These fusions retain the AT hooks and the repression domain of MLL but lose the PHD, activation, and SET domains. The MLL repression domain initially was defined by using a reporter gene system (14) and was shown to be critical in the context of an MLL fusion for bone marrow transformation in vitro (15). Recently, this region of MLL also was shown to bind nonmethylated CpG DNA in vitro (16). Only by understanding how the MLL protein, including the repression domain in the amino-terminal portion of MLL, normally performs its regulatory functions can one infer how the MLL fusion proteins lead to hematopoietic cell transformation and leukemia development. In this respect, it will be important to characterize more extensively the interaction between the MLL repression domain and corepressors, and to assess the significance of these interactions.
It was shown recently that DNA methyltransferase 1 (DNMT1) binds to histone deacetylase 1 (HDAC1), and this activity maps immediately adjacent to the region of sequence similarity between DNMT1 and MLL (17). The region of similarity, the cysteine-rich CXXC domain, is highly conserved among a small group of proteins, including DNMT1, MLL, and methyl-CpG-binding protein 1 (MBD1/PCM1) (5, 14, 18–20). Two regions of MLL, the CXXC domain (RD1) and the adjacent region (RD2), behave independently as transcriptional repressors in a reporter gene system (14). Although it is unknown how HDAC1 mediates the repression function of DNMT1, several possibilities exist. HDACs are believed to repress transcription by recruiting repressor complexes (21) or by removing acetyl groups from core histone tails in chromatin; hypoacetylated chromatin is often associated with a transcriptionally inert state.
Recently, studies have shown that polycomb group (PcG) proteins and the C-terminal-binding protein (CtBP) are corepressors in gene regulation (22–28). In Drosophila and mouse, PcG proteins maintain the silencing of Hox gene expression (29), whereas trx or Mll are required to maintain expression of certain Hox genes (30, 31). The axial–skeletal transformations and altered Hox expression patterns of Mll- and Bmi-1-deficient mice are normalized when both Mll and Bmi-1 are deleted, demonstrating their antagonistic role in determining segmental identity (32). It also has been shown that some Hox genes, including c8 but not all Hox genes, are affected reciprocally by Mll and Bmi-1 (32).
We addressed the question of whether activity mediated by the MLL repression domain is conferred by HDACs or other corepressors. Here, we present evidence that the repression domain specifically interacts with HDACs 1 and 2, and repression activity of this MLL domain is relieved partially with trichostatin A (TSA), an inhibitor of HDACs. Furthermore, binding of HDAC1 to the MLL repression domain is increased by Cyp33 binding to the adjacent MLL-PHD finger domain. In addition, the cysteine-rich CXXC region of the MLL repression domain (RD1) interacts with two PcG proteins HPC2 and BMI-1 as well as with the corepressor CtBP. Although direct interaction between trithorax and polycomb group proteins has been hypothesized, we now demonstrate that such an interaction exists and likely has functional implications.
Materials and Methods
GST Pull-Down Assays, Coimmunoprecipitation, and Western Blot Analysis. HDACs either were endogenous or were expressed by transient transfection in 293T cells from pcDNA3-FLAG-HDAC1 (E. Verdin, University of California, San Francisco) or pCMV-HDAC3 (W. Yang, University of South Florida, Tampa) or by in vitro transcription/translation (IVTT) from PcS2-HDAC2 and PING14A-HDAC4 (T. Kouzarides, Cambridge University, Cambridge, U.K.). HPC2 was translated in vitro from pcDNA3-T7-HPC2 (A. Otte, University of Amsterdam, Amsterdam). IVTT was performed by using the TNT system (Promega). PMT7-tagged BMI-1 (A. Otte) was expressed in bacteria. GST and GST-fusion proteins were expressed in DH5α or BL21 and purified as described (14). Bound proteins were resolved by SDS/PAGE and autoradiographed, or immunoreactive bands were revealed by using an enhanced chemiluminescence kit (Amersham Biosciences). 293T cells were transiently transfected by calcium phosphate precipitation with DNA (20 μg) full-length pcDNA3-MLL-F (S. Korsmeyer, Harvard University, Cambridge, MA, and M. Seto, Aichi Cancer Center Research Institute, Nagoya, Japan), GAL4-CtBP, FLAG-CtBP (R. Baer, Columbia University, New York), pMT2SM-HA-BMI-1 (M. van Lohuizen, Netherlands Cancer Institute, Amsterdam), pcDNA3-MLL(RD+PHD)-F, or various pCMV-FLAG-MLL subdomains and cells were collected 48 h posttransfection. Cells were lysed in IPH buffer [50 mM Tris·HCl, pH 8.0/150 mM NaCl/5 mM EDTA/0.5% NP-40/10 μl/ml protease inhibitor mix (Sigma)], and a binding assay was performed as described (17). Antibodies were used according to the manufacturer's instructions. Antibodies used were: anti-GAL4 (Santa Cruz Biotechnology), anti-FLAG-M2 (Sigma), anti-T7 monoclonal (Novagen), anti-HDAC1 and -CtBP (Upstate Biotechnology), anti-HA (Sigma), anti-HDAC3 (P. Marks and R. Rifkind, Memorial Sloan–Kettering Cancer Center, New York), and anti-BMI-1 (Santa Cruz Biotechnology). Membranes were stripped (PBS with 7 μl/ml 2-mercaptoetanol and 2% SDS) at 50°C for 30 min of agitation, washed for 30 min in PBS, and then reequilibrated in blocking buffer.
Cell Culture, Transfections, and CAT Assay. 293T and HeLa cell lines were grown in DMEM with 10% FCS at 37°C and 5% CO2. CAT assays were performed as described (14).
Overexpression of Cyp33 and HOX RT-PCR. The plasmids pHA-Cyp33 and the deletion construct pHA-ΔCyp33, which lacks the conserved cyclophilin domain, have been described (33). Human erythroleukemia cell line K562 (5 × 106 cells) was transiently transfected, RNA was isolated, and the effect of cyclosporine was tested as described (33). TSA (100 nM) was added 5 h after transfection. RT-PCR was performed by using a Marathon cDNA kit (CLONTECH) with primers that have been described (33).
Results
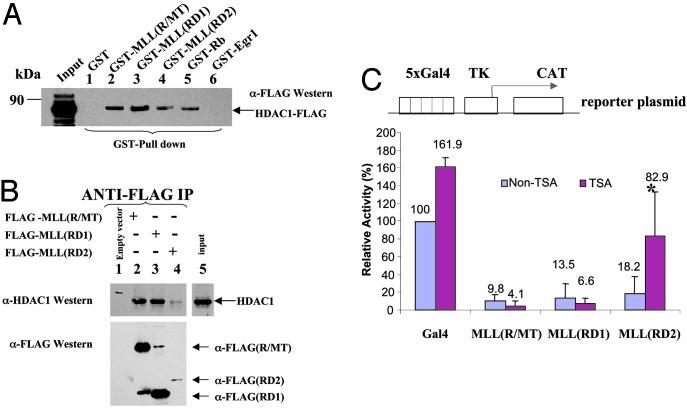
MLL Repression Domain Interacts with HDAC1 and -2. We previously defined the repression and activation domains in MLL by using a reporter gene assay (14), but the mechanism by which the repression activity is mediated is unknown. The MLL repression (R/MT) domain (amino acids 1101–1400) contains a region with homology to methyl DNA-binding proteins, including MBD1 and DNMT1 (17, 19). Interestingly, the DNMT repression activity, which maps to this region, is mediated partially through recruitment of HDAC1 (17). A GST pull-down assay initially was used to determine whether MLL(R/MT) interacts with HDACs in a similar manner. GST-fusion proteins of MLL (R/MT), Rb (protein known to interact with HDAC1, as a positive control; ref. 34), and Egr1 (as a negative control) or other proteins were expressed, and protein amounts were normalized by Coomassie blue staining (data not shown). Proteins were immobilized on GST-Sepharose and incubated with different HDACs expressed by transient transfection in 293T cells or by IVTT. After extensive washing, FLAG-tagged HDAC1 proteins bound to GST proteins were analyzed by SDS/ PAGE. FLAG-tagged HDAC1 was able to bind specifically to immobilized GST-MLL(R/MT) (amino acids 1101–1400; Fig. 1A, lane 2), to either GST-MLL RD1 (amino acids 1101–1250) or RD2 (amino acids 1251–1400) alone (Fig. 1A, lanes 3 and 4), and to positive control GST-Rb (Fig. 1A, lane 5), but did not bind to the immobilized GST alone or to GST-Egr1, another negative control (Fig. 1A, lanes 1 and 6). IVTT-expressed 35S-labeled HDAC2 was able to bind GST-MLL(R/MT) (data not shown), but neither the transiently transfected HDAC3 nor IVTT-expressed HDAC4 bound to GST-MLL(R/MT) (data not shown). Furthermore, coimmunoprecipitation experiments confirmed that endogenous HDAC1 interacts with MLL(R/MT), MLL(RD1), and MLL(RD2) (Fig. 1B, lanes 2–4). To determine whether HDAC1 enzymatic function was relevant to MLL repression domain function, reporter gene assays were performed in HeLa cells in the presence or absence of TSA, an inhibitor of HDAC activity. We found that repression mediated by the second half of the MLL repression domain (RD2) could be relieved partially by TSA, whereas that mediated by the CXXC domain (RD1) or by the full repression domain (R/MT) was not (Fig. 1C). The data indicate that the MLL repression mechanism is mediated at least partially through binding to HDACs. Coimmunoprecipitation was used to determine whether the full-length MLL protein, which contains both repression and activation domains as well as PHD and SET domains, interacts with HDAC1. Full-length FLAG-tagged MLL was incubated with nontagged HDAC1 and immunoprecipitated by anti-FLAG beads. After extensive washing, the immunoprecipitated protein bound to FLAG-MLL was analyzed by Western blotting with anti-HDAC1 antibody. HDAC1 was able to bind to full-length MLL (Fig. 2A, lane 4), and this interaction could be detected even with endogenous HDAC1 (Fig. 2A, lane 5).
Fig. 1.
The MLL repression domain interacts with HDAC1. (A) Equivalent amounts of bacterially expressed GST alone, negative control GST-Egr1 repression domain, GST-MLL(1101–1400)(R/MT), MLL(1101–1250)(RD1), and MLL(1251–1400)(RD2), or positive control GST-Rb were used to pull down FLAG-HDAC proteins expressed by transient transfection in 293T cells and then were detected with anti-FLAG antibody. Bound HDAC1, input (5%), and markers are indicated. (B) FLAG-MLL(R/MT), (RD1), and (RD2) expressed in 293T cells and immunoprecipitated with anti-FLAG beads. Bound HDAC1 (Upper) was detected with anti-HDAC1 antibody. After stripping, the membrane was rehybridized with anti-FLAG antibody. (C) CAT reporter gene assay. TSA partially relieves MLL(RD2) repression in a reporter gene assay. MLL domains expressed as Gal4 (amino acids 1–147) fusion proteins, G4-MLL(R/ MT), G4-MLLRD1, G4-MLLRD2, or Gal4 alone were transiently cotransfected with the reporter gene, Gal45tkCAT (18), in HeLa cells. After 24 h, cells were split into two dishes, and one was treated with HDAC inhibitor TSA (100 ng/ml). The results are the mean (+SD) of at least three independent transfections. *, Statistically significant difference.
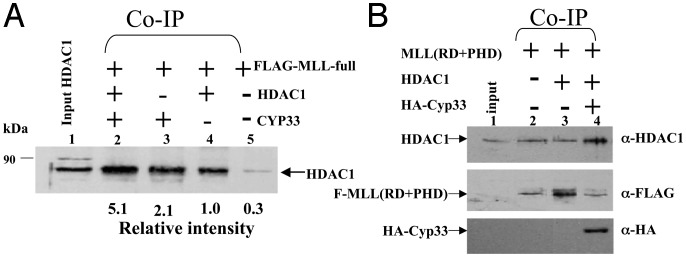
Fig. 2.
Full-length FLAG-MLL and FLAG-MLL(RD+PHD) interact with HDAC1 by coimmunoprecipitation. Equivalent amounts of full-length FLAG-MLL (A) or FLAG-MLL(RD+PHD) (B), plus HDAC1 and Cyp33 expressed by transient transfection in 293T cells, were incubated and immunoprecipitated with anti-FLAG beads. After electrophoresis and Western blot analysis, anti-HDAC1 antibody was used to detect HDAC1 bound to FLAG-MLL. Input (2.5%) and markers are indicated.
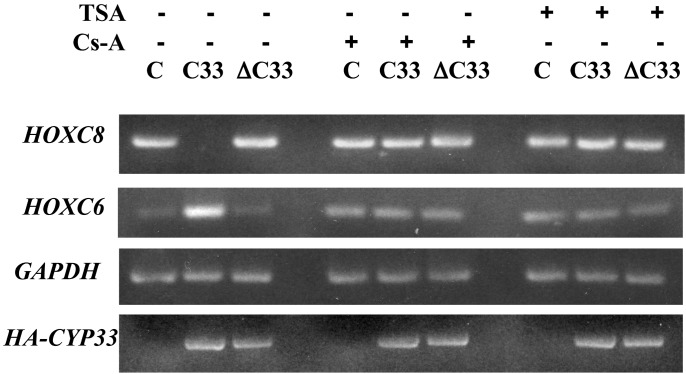
Cyp33 Increases the Binding of HDAC1 to the MLL Repression Domain. Cyp33 is a nuclear cyclophilin that interacts with the third PHD zinc finger domain of MLL (33). Cyp33 contains an RNA-recognition motif (RRM) and a conserved cyclophilin domain. It has recently been demonstrated that Cyp33 negatively affects transcription of the HOXC8 and HOXC9 genes (33), targets of MLL function. Because the MLL-PHD zinc finger domain is adjacent to the MLL repression domain, we wished to determine whether binding of Cyp33 to the PHD domain affected binding of HDAC1 to the MLL repression domain. FLAG-tagged MLL(RD+PHD) expressed by transient transfection was incubated in the presence or absence of HDAC1 and/or HA-tagged Cyp33 protein, also expressed by transient transfection. After immunoprecipitation with anti-FLAG beads, the proteins were detected with anti-HDAC1 antibody. Cyp33 increased the binding of HDAC1 to MLL(RD+PHD) compared with HDAC1 alone (Fig. 2B, compare lanes 4 and 3). Furthermore, we demonstrated the same effect of Cyp33 on HDAC1 binding to full-length MLL (Fig. 2 A), where Cyp33, in the presence of endogenous HDAC1, significantly increased binding (Fig. 2 A, compare lanes 3 and 5). Interestingly, additional expression of HDAC1 increased the binding even more (Fig. 2 A, compare lanes 2 and 4), suggesting that endogenous HDAC1 may have been limiting in this experiment. The results suggest that Cyp33 may be involved in regulation of MLL activity mediated through HDAC1. We recently have shown that the expression of HOXC8, a target of MLL function, is inhibited and HOXC6 expression is enhanced after expression of Cyp33 but not after expression of a truncated Cyp33 protein lacking the cyclophilin domain (ref. 33; Fig. 3). It is likely that HOXC6 expression is increased in these experiments because HOXC8 represses HOXC6 (33, 35). These effects are suppressed by cyclosporin A, which shows that they likely are mediated by the cis/trans prolyl-isomerase activity of Cyp33 and also by TSA, which in turn shows that they depend on the activity of histone deacetylases (Fig. 3).
Fig. 3.
TSA inhibits the ability of Cyp33 to regulate HOX gene expression. Expression of HOXC8 and HOXC6 by semiquantitative RT-PCR in K562 cells transfected with expression vector for HA-Cyp33 (C33), for a truncated form of HA-Cyp33 encoding only the RRM domain and the spacer (ΔC33), or with the vector alone (C). HOXC8 expression is inhibited and HOXC6 expression is enhanced after expression of Cyp33, but not after expression of the truncated protein. These effects are suppressed by cyclosporin A, showing that they may be mediated by the cis/trans prolyl isomerase activity of Cyp33, and also by TSA, showing that they depend on the activity of HDACs.
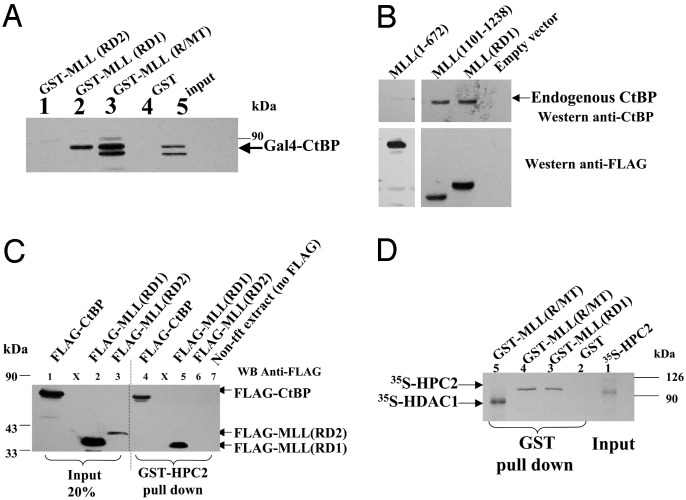
The Cysteine-Rich CXXC Region of MLL Interacts with CtBP and HPC2. Because repression mediated by the first half of the MLL repression domain (that containing the conserved CXXC motif) was not relieved by TSA (Fig. 1C), we performed experiments to determine whether other repressors could be mediating this activity. CtBP has been identified as a corepressor that can recruit PcG proteins (24, 36), whose repression activity is not inhibited by TSA (36). CtBP also can recruit HDACs, and, in that case, its repression activity is inhibited by TSA. A GST pull-down assay with various, immobilized GST-MLL RD proteins incubated with GAL4-tagged CtBP protein expressed by transient transfection demonstrated that CtBP binds to both MLL(R/MT) and MLL(RD1) but not to GST alone or to MLL(RD2) (Fig. 4A). Coimmunoprecipitation experiments confirmed the interaction of MLL(RD1) with endogenous CtBP (Fig. 4B), whereas MLL (1–672) that contains the AT hook domain was negative.
Fig. 4.
The MLL(R/MT) and RD1 domains interact with CtBP and HPC2. (A) Gal4-CtBP protein expressed in 293T cells was pulled down with equivalent amounts of bacterially expressed GST alone, GST-MLL(R/MT), GST-MLL(RD1), or GST-MLL(RD2). Anti-Gal4 antibody was used to detect bound CtBP. Input (5%) is indicated. (B) Anti-FLAG IP. Transfected FLAG-tagged MLL(RD1), MLL(1101–1238), and MLL (1–672) (as a negative control) or FLAG vector were immunoprecipitated from 293T extracts with anti-FLAG beads. Anti-CtBP antibody was used to detect endogenous bound CtBP. (C) HPC2 interacts with MLL(R/MT) and MLL(RD1). Bacterially expressed GST-HPC2 was used to pull down FLAG-MLL(RD1) and FLAG-MLL(RD2) expressed by transient transfection in 293T cells and detected with anti-FLAG antibody. FLAG-CtBP and 293T extract were positive and negative controls, respectively. Input proteins (20%) are indicated. (D) GST-MLL pull-down assay. IVTT-expressed 35S-labeled full-length HPC2 (or HDAC1 as a positive control) was incubated with equivalent amounts of bacterially expressed GST alone, GST-MLL(RD1), or GST-MLL (R/MT). Bound proteins were detected by autoradiography. Input (2.5%) is indicated.
Repression mediated by at least one type of PcG complex, PRC1, has been shown to be independent of HDAC activity (28). HPC2 is a member of this complex that has potent transcriptional repression activity (37) and has been shown to interact with CtBP (24). Because CtBP binds to the MLL repression domain, we wished to determine whether HPC2 also binds to the MLL repression domain. GST-HPC2 and various control GST-fusion proteins were expressed, immobilized proteins were incubated with FLAG-tagged MLL(RD1) or MLL(RD2), or FLAG-CtBP (as a positive control) expressed by transient transfection, and bound proteins were detected with anti-FLAG antibody. MLL(RD1) (Fig. 4C, lane5) was able to bind GST-HPC2 but not MLL(RD2) (Fig. 4C, lane 6) or GST alone (not shown). To determine whether MLL(RD1) might bind directly to HPC2, the binding assay was repeated by using IVTT-expressed 35S-labeled HPC2 (or HDAC1 as a positive control). Both GST-MLL(R/MT) and MLL(RD1) interacted with HPC2 but not GST alone (Fig. 4D). This suggests that MLL(RD1) might interact directly with HPC2.
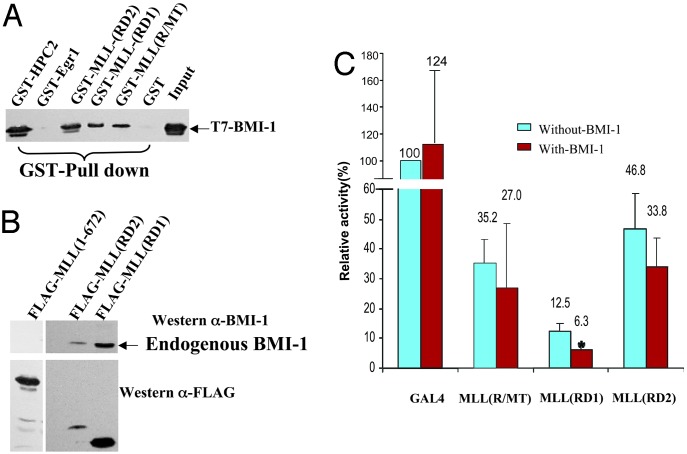
BMI-1, Another PcG Protein, Interacts with the MLL Repression Domain and Enhances MLL Repression Activity. Mll regulates expression of various Hox (Type I homeobox) target genes (31, 32). Hox gene regulation by Mll is thought to be mediated at the chromatin level and to be antagonized by Bmi-1, a mammalian homologue of Drosophila polycomb. Our data show that MLL(RD1) interacts with HPC2 and CtBP, but exogenously added HPC2 and CtBP do not potentiate the MLL(RD1) repression activity (data not shown). BMI-1 has been shown to interact with HPC2 (38) and to interact genetically with Mll (32). Therefore, we wished to test whether BMI-1 could interact with the MLL repression domain. GST-MLL repression domain proteins as well as various control GST proteins were incubated with bacterially expressed T7-tagged BMI-1 (Fig. 5A). GST-MLL(R/MT), GST-MLL(RD1), and GST-MLL(RD2) interacted with BMI-1 but not GST alone or GST-Egr1. GST-HPC2 was included as a positive control (Fig. 5A). Coimmunoprecipitation experiments demonstrated that FLAG-MLL(RD1) and FLAG-MLL(RD2) both can interact with endogenous BMI-1, whereas FLAG-MLL (1–672) does not (Fig. 5B). To determine whether BMI-1 binding was relevant to MLL repression domain function, reporter gene assays were performed in 293T cells. We found that repression mediated by the first half of the repression domain, MLL(RD1), could be enhanced significantly by exogenously expressed BMI-1. However, the second-half repression domain, MLL(RD2), and MLL(R/MT) repression activity was not enhanced by added BMI-1 (Fig. 5C).
Fig. 5.
BMI-1 interacts with the MLL repression domain and enhances MLL(RD1) repression. (A) T7-tagged BMI-1 protein expressed in bacteria was pulled down with equivalent amounts of bacterially expressed GST alone, negative control GST-Egr1 repression domain, GST-MLL(R/MT), GST-MLL(RD1), GST-MLL(RD2), or positive control GST-HPC2. Anti-T7 antibody was used to detect bound BMI-1. Input (2.5%) is indicated. (B) FLAG-MLL(RD1), FLAG-MLL(RD2), or FLAG-MLL(1–672) (as a negative control) expressed by transient transfection in 293T cells that express endogenous BMI-1 protein were immunoprecipitated by using anti-FLAG beads. Proteins were detected by using anti-BMI-1 or anti-FLAG antibody. (C) CAT reporter gene assay. 293T cells were transiently cotransfected with Gal45tk CAT reporter plasmid plus Gal4 alone, Gal4-MLL (R/MT), GAL4-MLL(RD1), or Gal4-MLL(RD2) effectors, with or without additional PMT2SM-HA-BMI-1. Results shown are the mean (+SD) of at least three independent transfections. *, Statistically significant difference.
Discussion
MLL is a large, multidomain protein, the product of a gene involved in chromosomal translocations associated with leukemia. The amino-terminal portion of MLL contains a region with transcriptional repression activity (14). Furthermore, this domain must be intact for an MLL fusion protein to be transforming (15). It is not known mechanistically how this domain may contribute to MLL function in vivo. Within the MLL transcriptional repression domain, a region with homology to other proteins, including DNMT1, is present. This homology includes what is termed the “CXXC” domain, a cysteine-rich region present in some proteins that recognize methylation status of DNA. It had been described that this region of DNMT1 functions as a transcriptional repression domain and binds HDAC1 (17). Our data demonstrate that the MLL repression domain binds class I HDACs, including HDAC1 and HDAC2. This interaction likely has functional relevance because treatment of cells with TSA, an inhibitor of HDAC activity, partially relieves repression mediated through the RD2 domain. This suggests that the function of this MLL domain may be mediated by a complex containing HDACs. Recently, a stable MLL complex was purified, and HDACs 1 and 2 were found in this complex (10). MLL repression domain activity was not completely dependent on HDAC activity, however, because repression mediated by the RD1 domain, which contains the CXXC motif, was not inhibited by TSA. This is similar to what was observed for DNMT1 function, where only repression mediated by the region adjacent to the CXXC domain, but not that mediated by the CXXC domain itself, depended on HDAC activity (17). This suggests that some additional factor(s) is involved in mediating the function of this MLL domain.
Repression activity mediated by the PRC1 complex of PcG proteins is independent of HDAC activity (25). Therefore, we explored the possibility that PcG proteins could be responsible for some of the MLL repression domain activity. We found that two PcG proteins, HPC2 and BMI-1, interact with MLL. It is possible that the interaction of HPC2 with MLL(RD1) is direct because bacterially expressed GST-MLL protein interacted with IVTT-expressed HPC2 (Fig. 4B). HPC2 and BMI-1 colocalize in nuclear domains and are present in a complex with RING1 and HPH1/2 (37, 38). It also has been shown that HPC2 interacts with the corepressor CtBP through a PXDLS motif, similar to the motif through which CtBP interacts with other proteins (24). We demonstrate here that CtBP coimmunoprecipitates with MLL(RD1), the region with the CXXC motif. It is likely that CtBP is recruited to this region of MLL indirectly through HPC2 because MLL(R/MT) does not contain a PXDLS motif. We do not yet know whether these proteins, HPC2, BMI-1, and CtBP, bind simultaneously and/or cooperatively to the MLL repression domain or whether binding of one can inhibit binding of others. Our data suggest that the HPC2/BMI-1/CtBP repression is dominant over HDAC repression in our reporter gene systems, but we do not know whether HDAC is part of the same complex(es) or of a competing complex in vivo. We do have preliminary data, however, that neither HPC2 nor CtBP compete with HDAC1 for binding to MLL(RD1) (data not shown).
It is intriguing that Mll has been shown to interact genetically with Bmi-1 to normalize partially some of the homeotic transformations and Hox gene-expression defects observed with either Mll mutant or Bmi-1 null mice (31, 32). Although the mechanism through which a trithorax group gene such as Mll can affect the function of a PcG gene such as Bmi-1 is unknown, it is possible that direct protein–protein interactions play a role. It has been shown recently that overexpressed MLL and overexpressed Bmi-1 partially colocalize in nuclear subdomains (32), suggesting that these two proteins interact in vivo. We have data that further support this by showing partial colocalization of endogenous BMI-1 with MLL proteins that contain all of the previously identified nuclear targeting sequences (39) of MLL (data not shown). It is of note that in Drosophila embryos, chromatin immunoprecipitation assays have been used to demonstrate that trx (homolog of MLL) and PSC (homolog of BMI-1) bind to distinct but adjacent DNA elements in the bxd enhancer of the Ultrabithorax gene (30), and a direct interaction has been hypothesized between a PcG complex containing PC, ASX, and SXC with TRX that would modulate activation vs. repression of target loci (40).
In cells with normal amounts of wild-type MLL protein, overall MLL activity depends on a balance of repression and activation, depending on the context of a particular downstream target gene at a particular time. The repression domain can recruit a polycomb and/or HDAC corepressor complex, whereas the activation domain can recruit CBP (41) and potentially other members of a coactivator complex (Fig. 6). The ultimate resultant function of MLL at a particular locus would depend on the specific transcription factors present and whether or not a gene was being actively transcribed. If the coactivator complex was dominant (in greater abundance or more stable at a particular locus), then it would be able to recruit a chromatin-remodeling complex, such as SWI/SNF, to that region, and an open chromatin configuration would be maintained by modification of nucleosome tails by HAT activity of complex members, including CBP and/or other recruited proteins with HAT activity. This could augment the positive effect of the MLL-SET domain's intrinsic H3K4 methyltransferase activity (10, 11). Alternatively, if the repression complex was dominant or more stable, it would recruit a polycomb complex and, either alone or in association with HDAC activity, would induce a repressed state of chromatin at target gene loci. At least two different polycomb complexes have been described: (i) the human HPC/HPH complex analogous to the Drosophila PRC1 complex contains HPC2 (human polycomb 2), BMI1 (the homolog of Drosophila PSC), HPH (human polyhomeotic), and RING1 and is resistant to HDAC inhibitors; and (ii) a complex composed of EED (homolog of Drosophila extra sex combs) and EZH2 (enhancer of zeste 2) that does not contain polycomb or BMI1 and mediates its repressive activity via interaction with HDAC proteins (27, 38, 42). Our evidence shows that components of both types of complexes can interact with the MLL repression domain. The presence of one type of polycomb complex bound to MLL vs. another may affect the ability of a remodeling complex to induce gene expression at the target locus. For example, it has been shown that if the polycomb-containing complex PRC1 binds first to a nucleosomal array in vitro, SWI/SNF cannot remodel chromatin (25). Our model would predict that in cells at different stages of development or differentiation, one or other of these complexes would be bound.
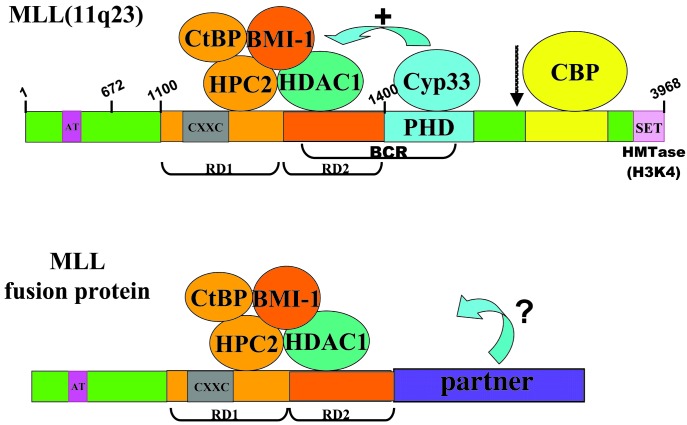
Fig. 6.
Model of repressor complexes associated with wild-type MLL vs. with an MLL fusion protein. The corepressor protein CtBP, the PcG proteins HPC2 and BMI-1, and HDAC1 (or HDAC2) bind to the MLL repression domain. Binding of HDAC1 is increased in the presence of the cyclophilin Cyp33. The coactivator CBP, which has acetyltransferase activity, can bind to the MLL activation domain. The MLL-SET domain has intrinsic histone H3, lysine 4 methyltransferase activity (HMTase H3K4). Corepressors and coactivators may both bind to wild-type MLL; the equilibrium of binding may be influenced by other factors that ultimately determine the function of MLL at a particular target gene locus. For leukemogenic MLL fusion proteins, the equilibrium of binding may be altered, resulting in the aberrant regulation of MLL target genes. Breakpoint cluster region (BCR) and MLL proteolytic cleavage site (arrow) are indicated. Numbering refers to MLL amino acids. The figure is not drawn to scale.
In the case of MLL fusion genes that cause leukemia, the balance of the repression and the activation activities would be altered or would be regulated abnormally. For example, the SET domain at the carboxyl terminus of MLL would not be present; therefore, it would not be able to methylate histone H3 lysine 4 at target gene loci (10, 11) or bind SNF5 (43), a member of the SWI/SNF chromatin-remodeling complex. This diminished ability to methylate chromatin and to recruit a chromatin-remodeling complex could have important implications in terms of downstream target gene regulation. In addition, the MLL activation domain would be replaced. Many of the MLL fusion partners have been shown to contain domains with transcriptional activation activity, but it is not yet known whether these would be able to recruit the same coactivator complexes and chromatin-remodeling complexes as recruited by normal MLL. One final domain of MLL that is not present in MLL fusion proteins is the PHD finger domain, which has been shown to bind to the nuclear cyclophilin Cyp33 (33). Overexpression of Cyp33 inhibits expression of HOXC8 and HOXC9, downstream targets of MLL function (33). Our data show that Cyp33 binding to MLL (when the PHD domain is present) increases the binding of HDAC1 to the MLL repression domain. Furthermore, we show that inhibition of HDAC activity with TSA suppresses the ability of Cyp33 to inhibit HOXC8 expression. One potential way to explain the effect of Cyp33 on the MLL-HDAC1 interaction has to do with the cis/trans prolyl-isomerase activity of its cyclophilin domain. One might expect that one of two alternative conformations of MLL, maintained by the prolyl-isomerase activity of Cyp33, may be needed for binding to HDAC1. In the context of the MLL fusion proteins, HDAC binding to the repression domain may not be as strong because Cyp33 would not be able to bind and effect the proper conformation of MLL. This also may affect binding of the PcG proteins, ultimately altering the balanced function of the protein. Although this model is hypothetical at this point, there are many aspects that can be tested experimentally.
Acknowledgments
HDAC constructs were kindly provided by T. Kouzarides, E. Verdin, W. M. Yang, and E. Seto; HPC2 constructs and pMT7-BMI-1 were kindly provided by A. Otte; full-length pcDNA3-MLL-F was kindly provided by S. Korsmeyer and M. Seto; GAL4-CtBP and FLAG-CtBP were kindly provided by R. Baer; pMT2SM-HA-BMI-1 was kindly provided by M. van Lohuizen; and anti-HDAC3 antibody was kindly provided by the Marks/Rifkind laboratory. This work was funded by National Institutes of Health (NIH)/National Cancer Institute (NCI) Grant CA78438 (to N.J.Z.-L.), the Dr. Ralph and Marian Falk Medical Research Trust (N.J.Z.-L.), and NIH/NCI Grant CA81269 (to M.O.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MLL, mixed-lineage leukemia; CtBP, C-terminal-binding protein; PHD, plant homeodomain; SET, Su(var)3–9, enhancer of zeste, and trithorax; DNMT, DNA methyltransferase; PcG, polycomb group; TSA, trichostatin A; HDAC, histone deacetylase; IVTT, in vitro transcription/translation.
References
- 1.Ziemin-van der Poel, S., McCabe, N. R., Gill, H. J., Espinosa, R., III, Patel, Y., Harden, A., Rubinelli, P., Smith, S. D., LeBeau, M. M., Rowley, J. D., et al. (1991) Proc. Natl. Acad. Sci. USA 88 10735-10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCabe, N. R., Burnett, R. C., Gill, H. J., Thirman, M. J., Mbangkollo, D., Kipiniak, M., van Melle, E., Ziemin-van der Poel, S., Rowley, J. D. & Diaz, M. O. (1992) Proc. Natl. Acad. Sci. USA 89 11794-11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djabali, M., Selleri, L., Parry, P., Bower, M., Young, B. D. & Evans, G. A. (1992) Nat. Genet. 2 113-118. [DOI] [PubMed] [Google Scholar]
- 4.Tkachuk, D. C., Kohler, S. & Cleary, M. L. (1992) Cell 71 691-700. [DOI] [PubMed] [Google Scholar]
- 5.Gu, Y., Nakamura, T., Alder, H., Prasad, R., Canaani, O., Cimino, G., Croce, C. M. & Canaani, E. (1992) Cell 71 701-708. [DOI] [PubMed] [Google Scholar]
- 6.Thirman, M. J., Gill, H. J., Burnett, R. C., Mbangkollo, D., McCabe, N. R., Kobayashi, H., Ziemin-van der Poel, S., Kaneko, Y., Morgan, R., Sandberg, A. A., et al. (1993) N. Engl. J. Med. 329 909-914. [DOI] [PubMed] [Google Scholar]
- 7.Rowley, J. D. (1999) Semin. Hematol. 36 59-72. [PubMed] [Google Scholar]
- 8.Yokoyama, A., Kitabayashi, I., Ayton, P. M., Cleary, M. L. & Ohki, M. (2002) Blood 100 3710-3718. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh, J. J., Ernst, P., Erdjument-Bromage, H., Tempst, P. & Korsmeyer, S. J. (2003) Mol. Cell. Biol. 23 186-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura, T., Mori, T., Tada, S., Krajewski, W., Rozovskaia, T., Wassell, R., Dubois, G., Mazo, A., Croce, C. M. & Canaani, E. (2002) Mol. Cell 10 1119-1128. [DOI] [PubMed] [Google Scholar]
- 11.Milne, T. A., Briggs, S. D., Brock, H. W., Martin, M. E., Gibbs, D., Allis, C. D. & Hess, J. L. (2002) Mol. Cell 10 1107-1117. [DOI] [PubMed] [Google Scholar]
- 12.Lavau, C., Szilvassy, S. J., Slany, R. & Cleary, M. L. (1997) EMBO J. 16 4226-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavau, C., Du, C., Thirman, M. & Zeleznik-Le, N. (2000) EMBO J. 19 4655-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeleznik-Le, N., Harden, A. M. & Rowley, J. D. (1994) Proc. Natl. Acad. Sci. USA 91 10610-10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slany, R. K., Lavau, C. & Cleary, M. L. (1998) Mol. Cell. Biol. 18 122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birke, M., Schreiner, S., Garcia-Cuellar, M. P., Mahr, K., Titgemeyer, F. & Slany, R. K. (2002) Nucleic Acids Res. 30 958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuks, F., Burgers, W. A., Brehm, A., Hughes-Davies, L. & Kouzarides, T. (2000) Nat. Genet. 24 88-91. [DOI] [PubMed] [Google Scholar]
- 18.Torchia, J., Glass, C. & Rosenfeld, M. G. (1998) Curr. Opin. Cell Biol. 10 373-383. [DOI] [PubMed] [Google Scholar]
- 19.Cross, S. H., Meehan, R. R., Nan, X. & Bird, A. (1997) Nat. Genet. 16 256-259. [DOI] [PubMed] [Google Scholar]
- 20.Kehle, J., Beuchle, D., Treuheit, S., Christen, B., Kennison, J. A., Bienz, M. & Muller, J. (1998) Science 282 1897-1900. [DOI] [PubMed] [Google Scholar]
- 21.Ahringer, J. (2000) Trends Genet. 16 351-356. [DOI] [PubMed] [Google Scholar]
- 22.van der Vlag, J., den Blaauwen, J. L., Sewalt, R. G., van Driel, R. & Otte, A. P. (2000) J. Biol. Chem. 275 697-704. [DOI] [PubMed] [Google Scholar]
- 23.van der Vlag, J. & Otte, A. P. (1999) Nat. Genet. 23 474-478. [DOI] [PubMed] [Google Scholar]
- 24.Sewalt, R. G., Gunster, M. J., van der Vlag, J., Satijn, D. P. & Otte, A. P. (1999) Mol. Cell. Biol. 19 777-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao, Z., Raible, F., Mollaaghababa, R., Guyon, J. R., Wu, C. T., Bender, W. & Kingston, R. E. (1999) Cell 98 37-46. [DOI] [PubMed] [Google Scholar]
- 26.Hendrich, B. & Bird, A. (1998) Mol. Cell. Biol. 18 6538-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sewalt, R. G., van der Vlag, J., Gunster, M. J., Hamer, K. M., den Blaauwen, J. L., Satijn, D. P., Hendrix, T., van Driel, R. & Otte, A. P. (1998) Mol. Cell. Biol. 18 3586-3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, Q., Yao, H., Vo, N. & Goodman, R. H. (2000) Proc. Natl. Acad. Sci. USA 97 14323-14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirrotta, V. (1997) Trends Genet. 13 314-318. [DOI] [PubMed] [Google Scholar]
- 30.Tillib, S., Petruk, S., Sedkov, Y., Kuzin, A., Fujioka, M., Goto, T. & Mazo, A. (1999) Mol. Cell. Biol. 19 5189-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu, B. D., Hess, J. L., Horning, S. E., Brown, G. A. & Korsmeyer, S. J. (1995) Nature 378 505-508. [DOI] [PubMed] [Google Scholar]
- 32.Hanson, R. D., Hess, J. L., Yu, B. D., Ernst, P., van Lohuizen, M., Berns, A., van der Lugt, N. M., Shashikant, C. S., Ruddle, F. H., Seto, M., et al. (1999) Proc. Natl. Acad. Sci. USA 96 14372-14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fair, K., Anderson, M., Bulanova, E., Mi, H., Tropschug, M. & Diaz, M. O. (2001) Mol. Cell. Biol. 21 3589-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo, R. X., Postigo, A. A. & Dean, D. C. (1998) Cell 92 463-473. [DOI] [PubMed] [Google Scholar]
- 35.Hooiveld, M. H., Morgan, R., in der Rieden, P., Houtzager, E., Pannese, M., Damen, K., Boncinelli, E. & Durston, A. J. (1999) Int. J. Dev. Biol. 43 665-674. [PubMed] [Google Scholar]
- 36.Koipally, J. & Georgopoulos, K. (2000) J. Biol. Chem. 275 19594-19602. [DOI] [PubMed] [Google Scholar]
- 37.Satijn, D. P., Olson, D. J., van der Vlag, J., Hamer, K. M., Lambrechts, C., Masselink, H., Gunster, M. J., Sewalt, R. G., van Driel, R. & Otte, A. P. (1997) Mol. Cell. Biol. 17 6076-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satijn, D. P., Gunster, M. J., van der Vlag, J., Hamer, K. M., Schul, W., Alkema, M. J., Saurin, A. J., Freemont, P. S., van Driel, R. & Otte, A. P. (1997) Mol. Cell. Biol. 17 4105-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yano, T., Nakamura, T., Blechman, J., Sorio, C., Dang, C. V., Geiger, B. & Canaani, E. (1997) Proc. Natl. Acad. Sci. USA 94 7286-7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milne, T. A., Sinclair, D. A. & Brock, H. W. (1999) Mol. Gen. Genet. 261 753-761. [DOI] [PubMed] [Google Scholar]
- 41.Ernst, P., Wang, J., Huang, M., Goodman, R. H. & Korsmeyer, S. J. (2001) Mol. Cell. Biol. 21 2249-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lessard, J., Schumacher, A., Thorsteinsdottir, U., van Lohuizen, M., Magnuson, T. & Sauvageau, G. (1999) Genes Dev. 13 2691-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozenblatt-Rosen, O., Rozovskaia, T., Burakov, D., Sedkov, Y., Tillib, S., Blechman, J., Nakamura, T., Croce, C. M., Mazo, A. & Canaani, E. (1998) Proc. Natl. Acad. Sci. USA 95 4152-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]