Abstract
Tumor necrosis factor α (TNF-α) is an important mediator of programmed cell death, and TNF-α blockade significantly improves disease severity in several inflammatory conditions, including Crohn's disease (CD), one of the idiopathic inflammatory bowel diseases. However, the precise mechanism(s) of action of anti-TNF-α therapy in CD remains poorly understood. SAMP1/YitFc mice develop a spontaneous ileitis with similarities to human CD in regard to histological features as well as response to conventional treatments. In this report, we tested the novel hypothesis that the beneficial effects of anti-TNF-α therapy in CD are mediated by a mechanism that involves down-regulation of intestinal epithelial cell (IEC) apoptosis. Similar to the efficacy of monoclonal anti-TNF-α antibodies in human CD, a single injection of a chimeric anti-murine TNF-α antibody into SAMP1/YitFc mice resulted in a marked suppression of intestinal inflammation and epithelial cell damage compared with mice injected with an isotype control antibody. These effects were associated with a significant reduction in apoptosis of freshly isolated IEC as assessed by propidium iodide staining and DNA laddering. In contrast, an increase in lamina propria mononuclear cell apoptosis was observed in anti-TNF-α-treated mice compared with control. These results were confirmed in vivo by using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling-assay. In addition, neutralization of TNF-α reduced membrane bound FAS/CD95 expression in IEC from SAMP1/YitFc mice compared with control antibody. These data demonstrate a novel mechanism of action of anti-TNF-α therapy that involves homeostatic regulation of mucosal cell apoptosis, which results in the net decrease of chronic inflammation typically found in CD.
Crohn's disease (CD) is a chronic inflammatory bowel disease of unknown etiology. CD is characterized by patchy and transmural inflammation of the bowel wall, with heavy infiltration of acute and chronic inflammatory cells. During the active phase of CD, various proinflammatory cytokines are released within the gut mucosal compartment (1). Among them, tumor necrosis factor α (TNF-α) appears to play a pivotal role in the pathogenesis of chronic intestinal inflammation (2, 3). The most compelling evidence supporting the central role of TNF-α in this process comes from clinical studies reporting a dramatic improvement in CD patients treated with a human–mouse chimeric TNF-α-neutralizing antibody (infliximab) (4). Indeed, a single injection of infliximab has been shown to induce a clinical response in 70% of patients with steroid-refractory CD (4).
Several potential mechanisms by which anti-TNF-α treatment exerts its beneficial effects have been proposed. These include the ability of monoclonal antibodies against TNF-α to decrease the expression of activation markers on circulating lymphocytes (5), as well as the ability to down-regulate T helper 1 producing T cells in the lamina propria resulting in a net overall decrease in TNF-α expression (6). However, the precise mechanism(s) of action of anti-TNF-α therapy in CD remain poorly understood. There is increasing evidence that apoptosis, or programmed cell death, represents an important event during gut inflammatory responses, allowing strict control of clonal expansion of immune cells in response to a broad spectrum of antigenic stimuli, as well as clearance of invading pathogenic organisms (7, 8). As a result, cells that are no longer needed, or are potentially autoreactive, are eliminated through this process. Recent data show that, in patients with CD, gut mucosal T lymphocytes are resistant to multiple apoptotic stimuli (9, 10). In addition, TNF-α has been shown to play an essential role in regulating intestinal epithelial cell (IEC) apoptosis and/or survival during chronic inflammation (2, 11). Fas/CD95/APO-1 is a death receptor of the TNF-α receptor family and engagement of its ligand (Fas-L) initiates a series of intracellular events leading to programmed cell death (8). TNF-α has been shown to share common intracellular pathways of apoptosis with Fas and has the ability to modulate Fas expression itself (12, 13).
The SAMP1/YitFc mouse is a unique murine model of intestinal inflammation, which spontaneously develops chronic ileitis with virtually 100% penetrance by week 30 (14). Ileitis in SAMP1/YitFc mice closely resembles CD, with discontinuous, transmural inflammation, and is characterized by prominent muscular hypertrophy with the occasional formation of granulomas. Recently, our group has reported the development of perianal disease in SAMP1/YitFc mice as early as 4 weeks (15). In this model, ileitis is mediated by lymphocytes that infiltrate the lamina propria, show an activated immunologic phenotype, as well as possess the ability to induce ileitis on adoptive transfer (14). In addition, lymphocytes from mesenteric lymph nodes secrete high levels of IFNγ and TNF-α on stimulation, indicating that, as in CD, intestinal inflammation in this model is T helper 1-mediated (14).
In the present study, we investigated the efficacy and potential mechanism(s) of action of anti-TNF-α therapy in the SAMP1/YitFc murine model of CD-like ileitis. We report herein that administration of anti-TNF-α antibodies significantly decreased active and chronic intestinal inflammation observed in the SAMP1/YitFc strain. Furthermore, our results show that anti-TNF-α treatment prevented IEC apoptosis that was associated with a significant decrease in membrane bound Fas/CD95 expression. At the same time, anti-TNF-α treatment simultaneously enhanced programmed cell death in lamina propria mononuclear cells (LPMCs). Taken together, these data indicate that anti-TNF-α therapy may restore the homeostatic balance of mucosal cell apoptosis within the gut, and demonstrate a novel mechanism of action of anti-TNF-α therapy mediated through inhibition of IEC apoptosis.
Materials and Methods
Animals and Treatment Protocol. A chimeric rat/mouse monoclonal anti-TNF-α antibody (clone cV1q, a kind gift from David Shealy, Centocor, Malvern, PA) was administered as a single i.p. injection (0.01, 0.1, and 1.0 mg per mouse) to 40-week-old SAMP1/YitFc mice. The SAMP1/YitFc model of ileitis has been described (14, 15). Control animals were age-matched SAMP1/YitFc mice treated with a single i.p. injection of an irrelevant isotype antibody (clone MT412, kindly provided by David Shealy). All animals were killed 7 days after treatment, based on preliminary studies in which maximum efficacy of anti-TNF-α treatment was observed (data not shown).
Histological Assessment of Ileitis. Ilea from each experimental group were harvested and rinsed with PBS. Hematoxylin/eosin-stained sections of Swiss-rolled tissues were histologically evaluated by a single pathologist in a blinded fashion. Villous distortion, active and chronic inflammation, as well as the total inflammatory indeces were assessed as described (16).
Epithelial Cell Isolation and Purification. Freshly isolated small bowel segments (control, n = 13; 0.1 mg of anti-TNF-α, n = 12; 1.0 mg of anti-TNF-α, n = 11) were minced into 1-cm pieces and incubated in a solution containing DTT (Sigma) and EDTA (Life Technologies, Grand Island, NY), using a modification of the method described by Whitehead et al. (17). The specimens were then washed with 1× Hanks' balanced salt solution (HBSS) containing 15 mM Hepes, decanted, renewed with 10 ml of the 1× HBSS/Hepes solution, and shaken vigorously for 15 s. Intestinal villi and crypts were collected and the shaking process was repeated three times. Resulting epithelial cells were subsequently washed three times with 10 ml of HBSS/Hepes solution and immediately evaluated for apoptosis and Fas expression.
LPMC Isolation and Purification. LPMC were isolated from freshly procured terminal ileal specimens as described (18). In brief, gut specimens were incubated at 37°C for 2 × 15 min in HBSS containing EDTA (0.37 mg/ml) and DTT (0.145 mg/ml). Tissue samples were further digested in RPMI medium 1640 containing collagenase D (200 units/ml, Boehringer Mannheim) and DNase I (0.01 mg/ml) at 37°C in a shaking incubator. The LPMC released from the tissues were resuspended in 100% Percoll (Sigma), layered under a 40% Percoll (Sigma) gradient, and centrifuged at 543 × g max for 30 min. The mononuclear cell-enriched population, accumulating at the 40/100% interface, was collected, and cells were washed twice in calcium- and magnesium-free HBSS and resuspended in complete medium.
Flow Cytometric Analysis. The percentage of Fas-positive IEC and LPMC was assessed by flow cytometric analysis of cells stained with a phycoerythrin-conjugated anti-Fas monoclonal antibody (clone Jo2, BD PharMingen), kept in the dark for 30 min at 4°C and subsequently washed two times with PBS. Fas-stained IEC were then analyzed by FACSCalibur (Becton Dickinson).
Detection of Apoptosis in Freshly Isolated IEC and LPMC. Freshly isolated mucosal cells were washed twice in PBS, resuspended in a hypotonic fluorochrome solution [50 μg/ml propidium iodide (PI) (Sigma) in 0.1% sodium citrate plus 0.1% Triton X-100], and incubated for 2 h, allowing the PI to penetrate into the nucleus and bind within the DNA breaks. Cells were then analyzed by flow cytometry using the FACSCalibur. The percentage of apoptotic cells was determined by evaluating hypodiploid nuclei after proper gating on DNA content as described in previous studies (19).
Analysis of Intestinal Mucosal Cell Apoptosis in Full Thickness Ileal Specimens. Paraffin-embedded sections of terminal ileum specimens were analyzed with TASC (R & D Systems), according to manufacturer's instructions to visualize apoptotic cells with terminal deoxynucleotidyltranferase-mediated dUTP-biotin nick end labeling (TUNEL). In brief, after permeabilization, tissue sections were incubated in a humidified chamber with a TdT–dNTP reaction mixture for 1 h at 37°C. Sections were then washed in double-distilled H2O twice for two min, labeled with streptavidin/horseradish peroxidase for 10 min and subsequently with TACS blue label solution for 3 min at room temperature. Blue-labeled apoptotic cells were analyzed under light microscopy (Olympus).
DNA Laddering. Fragments of DNA were isolated from single cell suspensions and visualized after electrophoresis on an ethidium bromide-stained 1.5% Tris-borate agarose gel according to manufacturer's instructions (R & D Systems). Briefly, 1 × 106 IEC were washed in 5 ml of ice-cold PBS twice, and lysed with lysis solution. DNA was recovered from the aqueous layer, washed twice in 1 ml of 70% ethanol, and resuspended in 100 μl of DNase-free water. The concentration (μg/μl) of DNA was detected by using a densitometry set for reading at a wavelength of 260 nm by using a water blank. One microgram of DNA was loaded in a 1.5% gel and stained with ethidium bromide. DNA laddering was then visualized by using an UV transilluminator.
Statistical Analysis. Histological indices, percent of PI-positive cells, and percent of Fas-positive cells, between treated and control animals, were compared by using a two sided Student's t test. Data are expressed as mean ± standard error. An α level of 0.05 was considered significant.
Results
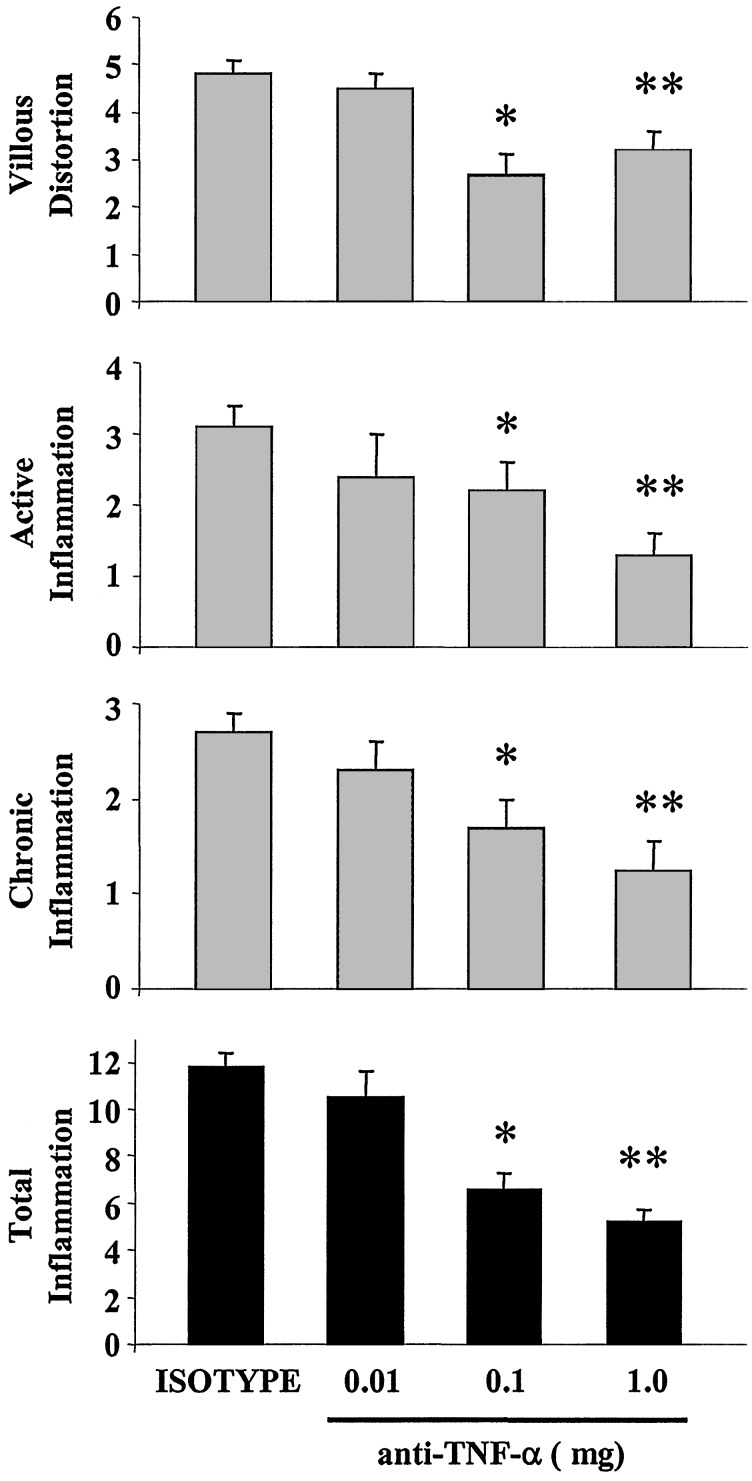
Anti-TNF-α Treatment Ameliorates the Severity of Ileitis. A single administration of a neutralizing antibody against murine TNF-α significantly diminished the severity of intestinal inflammation in 40-week-old SAMP1/YitFc mice treated with either 0.1 mg or 1.0 mg of anti-TNF-α compared with isotype-control treated mice (Figs. 1 and 2). No dose-response effect was observed, and there were no statistical differences between the 0.1- and 1.0-mg anti-TNF-α treatments. The active inflammatory index was reduced from 3.1 ± 0.3 in isotype treated mice to 2.2 ± 0.6 (P < 0.03) and 1.3 ± 0.3 (P < 0.01) in the 0.1- and 1.0-mg group of anti-TNF-α treated mice, respectively (Fig. 1). The chronic inflammatory index was also reduced from 2.7 ± 0.2 in isotype control mice compared with 1.7 ± 0.3 (P < 0.02) and 1.25 ± 0.3 (P < 0.01) for the 0.1- and 1.0-mg group of anti-TNF-α, respectively (Fig. 1). In addition, the villous distortion index was significantly reduced from 4.8 ± 0.3 in the isotype-treated group to 2.7 ± 0.4 (P < 0.01) and 3.2 ± 0.4 (P < 0.01) in the 0.1- and 1.0-mg group of anti-TNF-α treated mice, respectively (Fig. 1). Overall, the total inflammatory index was significantly reduced from 11.8 ± 0.6 in isotype-treated mice to 6.6 ± 0.7 in 0.1-mg-treated mice (P < 0.01), and to 7.2 ± 0.7 in 1.0-mg-treated mice (P < 0.02) (Fig. 1). Representative histological sections from the different experimental groups are shown in Fig. 2.
Fig. 1.
Anti-TNF-α treatment ameliorates the severity of ileitis in SAMP1/YitFc mice. Indices for villous distortion, active inflammation, and chronic inflammation were assessed histologically. Total inflammation (Bottom) was calculated by adding the individual indices. Treatment with either 0.1 or 1.0 mg of anti-mouse TNF-α neutralizing antibody significantly diminished the severity of ileitis. Data represent mean ± SEM (*, P < 0.02; **, P < 0.01; n = 20, 6, 17, and 14 mice for the isotype, 0.01, 0.1, and 1.0 mg anti-TNF-α-treated groups, respectively).
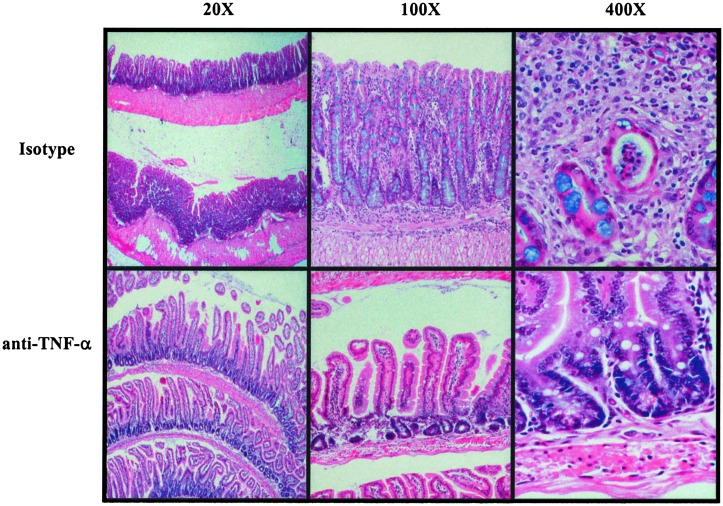
Fig. 2.
Histological changes in the terminal ileum of 40-week-old SAMP1/YitFc mice after isotype or anti-TNF-α treatment. Mice received a single i.p. injection of isotype control or 0.1 mg of an anti-TNF-α monoclonal antibody. Histological analysis of ileitis was performed 7 days after treatment. Hematoxylin/eosin staining of paraffin embedded terminal ilea from SAMP1/YitFc mice treated with isotype antibody shows severe villous blunting and prominent muscular hypertrophy (×20, Upper Left) and heavy inflammatory infiltration of the lamina propria (×100, Upper Center) with the occasional formation of crypt abscesses (×400, Upper Right). Administration of anti-TNF-α monoclonal antibody resulted in reconstitution of the villous architecture, regression of muscular hypertrophy, and a significant decrease in the number of inflammatory cells infiltrating the lamina propria (×20–400, Lower).
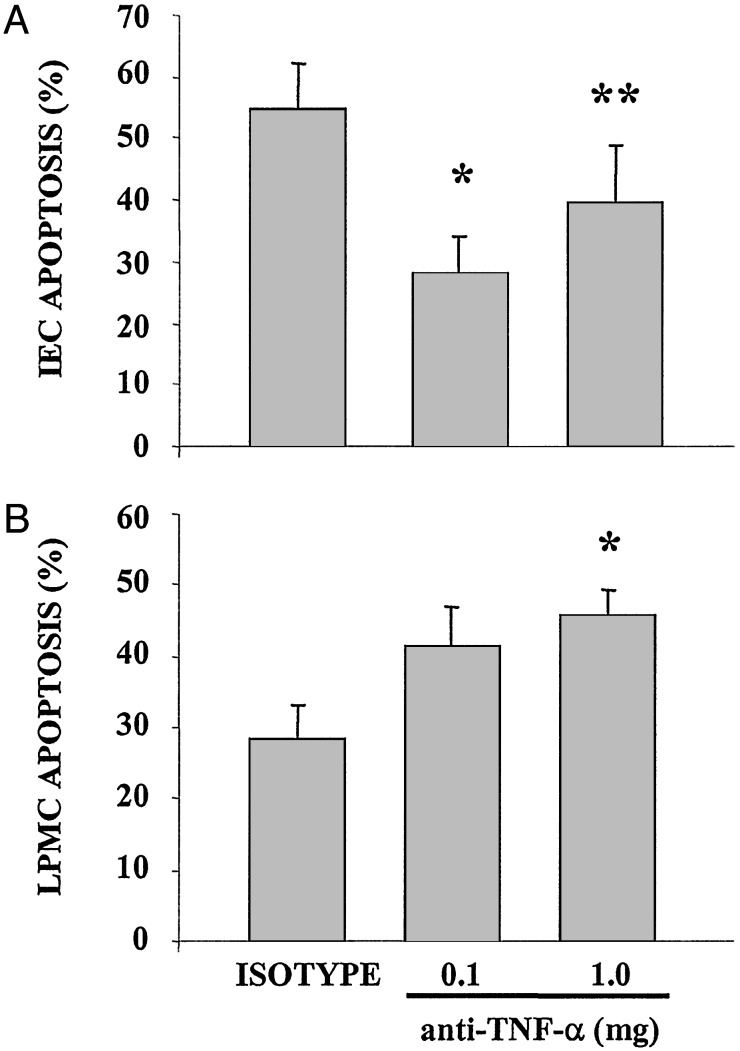
Anti-TNF-α Treatment Modulates Gut Mucosal Cell Apoptosis. Treatment with anti-TNF-α monoclonal antibody therapy significantly decreased the percentage of apoptotic cells in freshly isolated IEC of 40-week-old SAMP1/YitFc mice from 56 ± 4.1% in isotype control-treated mice (n = 5) to 28 ± 3.0% in 0.1 mg anti-TNF-α-treated (n = 6) (P < 0.01) and 39 ± 4.2% in 1.0 mg anti-TNF-α-treated mice (n = 6) (P < 0.02) (Fig. 3A). By comparison, anti-TNF-α monoclonal antibody treatment increased LPMC apoptosis in SAMP1/YitFc mice compared with isotype treated mice from 28 ± 4.9% in isotype control-treated mice to 41.5 ± 5.4% in 0.1 mg anti-TNF-α-treated mice (not significant) and 46 ± 3.7% in 1.0 mg anti-TNF-α-treated mice (P < 0.02) (Fig. 3B). The inhibition of IEC apoptosis was also confirmed by using DNA laddering in freshly isolated IEC as shown in Fig. 4. In addition, TUNEL assay of terminal ileal tissue sections obtained from anti-TNF-α- and isotype-treated mice reveal increased TUNEL staining not only in LPMC but also in cells with morphology consistent with intraepithelial lymphocytes (IEL) (Fig. 5).
Fig. 3.
Effect of anti-TNF-α treatment on gut mucosal cell apoptosis in SAMP1/YitFc mice. Freshly isolated IEC (A) and LPMC (B) were permeabilized and stained with PI, as described in Materials and Methods. The percentage of cells with hypodiploid nuclei was evaluated by flow cytometric analysis after gating for live cells. Blockade of TNF-α significantly decreased apoptosis in IEC (A), whereas opposing effects were observed in LPMC, in which apoptosis was increased in anti-TNF-α-treated vs. isotype-treated mice (B). Data represent mean ± SEM (*, P < 0.01; **, P < 0.02) from three independent experiments.
Fig. 4.
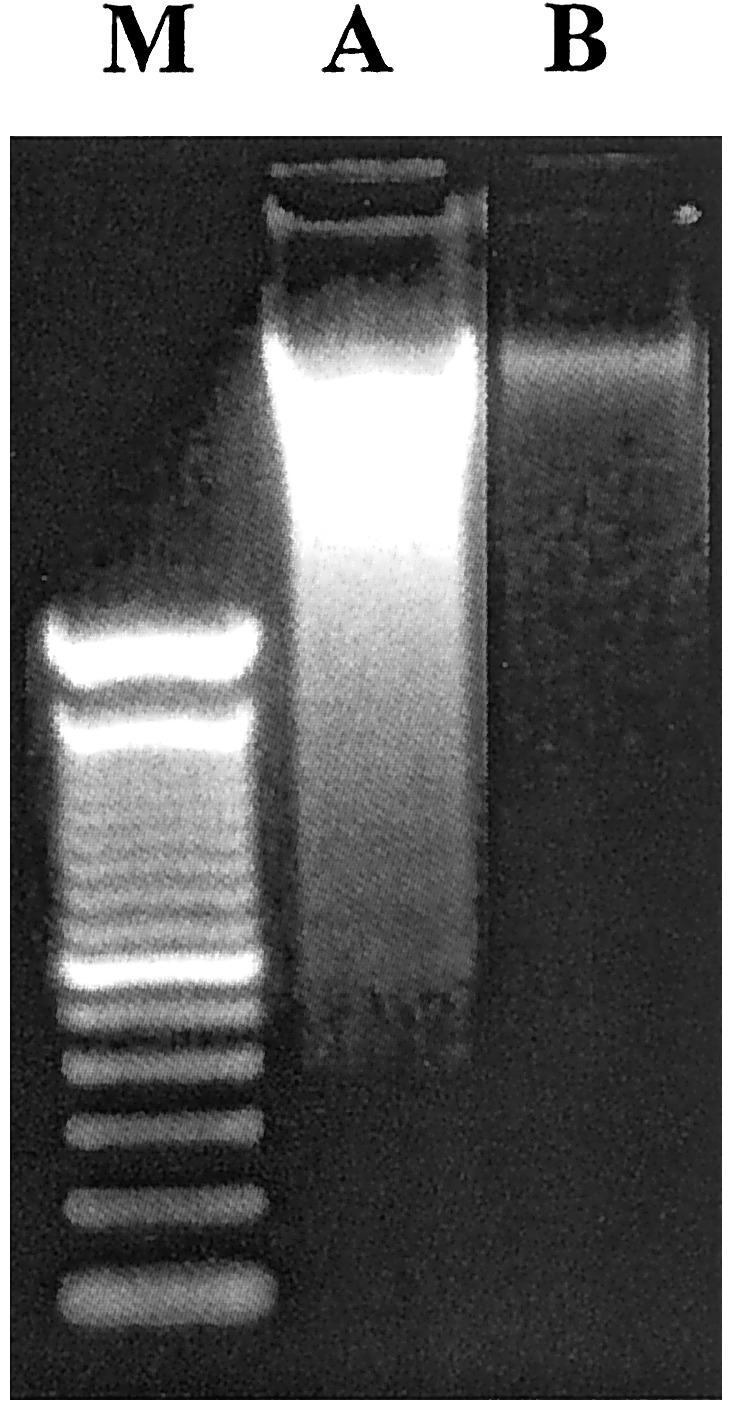
Detection of apoptosis in IEC by DNA laddering. IEC were freshly isolated from three mice per experimental condition and pooled. Fragments of DNA were isolated from single cell suspensions and visualized by ethidium bromide, as described in Materials and Methods. Lanes: M, 100-bp marker; A, isotype-treated SAMP1/YitFc mice; B, 0.1 mg anti-TNF-α-treated SAMP1/YitFc mice. Treatment with anti-TNF-α antibody decreased apoptosis in IEC, as shown by a decrease in DNA fragmentation. Results are representative of three independent experiments.
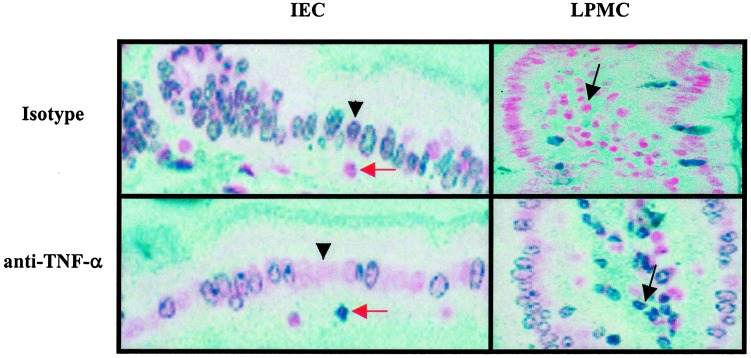
Fig. 5.
In vivo detection of ileal mucosal cells apoptosis by TUNEL assay. Cryosections of ilea from SAMP1/YitFc mice were subjected to blue-labeled TUNEL (see Materials and Methods) of apoptotic cells. Results are representative of three independent experiments. In each experiment, three slides per mouse were stained (original magnification =×200). Black arrowheads indicate decreased staining of IEC in anti-TNF-α-treated mice vs. isotype-treated mice (Left). Increased staining of LPMC in anti-TNF-α-treated mice, as compared with isotype-treated mice, is indicated with black arrows (Right). Red arrows indicate cells with morphology consistent to IEL.
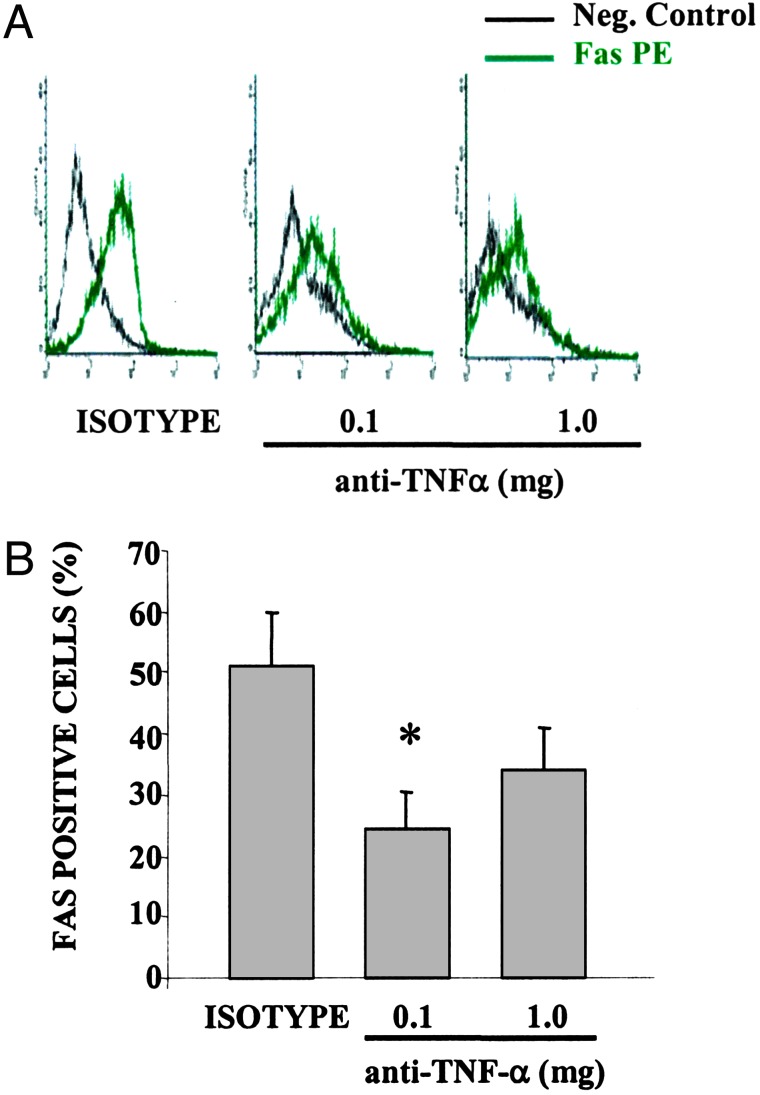
Anti-TNF-α Therapy Down-Regulates Fas Expression in IEC. We next measured the expression of Fas on both IEC and LPMC after anti-TNF-α treatment. As shown in Fig. 6, Fas expression in freshly isolated IEC was reduced in mice treated with 0.1 mg and 1.0 mg of anti-TNF-α compared with isotype control (24.5 ± 5.9% and 34.2 ± 6.6% vs. 51 ± 8.7%, P < 0.05 and not significant, respectively). Conversely, the percentage of Fas-positive cells was unchanged in freshly isolated LPMC obtained from anti-TNF-α-treated mice compared with control mice (data not shown).
Fig. 6.
Decreased Fas expression on IEC after anti-TNF-α treatment in SAMP1/YitFc mice. Freshly isolated IEC were stained with a phycoerythrin-conjugated monoclonal antibody against Fas. Treatment with anti-TNF-α reduced the expression of Fas on IEC, as shown by a decrease in the intensity of fluorescence (A), as well as a lower percentage of Fas-positive cells (B). Data are expressed as the percentage of positive cells ± SEM of three independent experiments with four to six mice per experimental group (*, P < 0.05).
Discussion
Despite the recognized efficacy of anti-TNF-α monoclonal antibody treatment in patients with CD (4), studies in animal models of intestinal inflammation have generated controversial results. In fact, in some models, TNF-α neutralization or deletion improves the severity of colitis (20, 21), whereas in others, disease severity is actually increased (22). This discrepancy may be a reflection of the differences between the particular animal model studied and the human condition. Therefore, we focused initially on investigating the effects of anti-TNF-α therapy in the SAMP1/YitFc model of ileitis. This mouse characteristically develops a spontaneous ileitis closely resembling human CD, which first appears as early as 4 weeks of age and progressively becomes more severe (15). A single administration of a neutralizing antibody against murine TNF-α significantly diminished the severity of intestinal inflammation in 40-week-old SAMP1/YitFc mice treated with either 0.1 mg or 1.0 mg of anti-TNF-α antibody compared with isotype-control-treated mice. Taken together, the results of our study are consistent with the reported effects of infliximab, a chimeric monoclonal antibody against human TNF-α, in patients with CD (4). In fact, similar to our study, no dose-response was observed in patients with severe refractory CD as the 5 mg/kg dose appeared to be more efficacious than the higher doses (10 and 20 mg/kg) in the treatment of human CD (4). Interestingly, the anti-murine TNF-α antibody used in our experiments has similar characteristics to infliximab, a chimeric mouse–human antibody, in that it is a chimeric rat–mouse monoclonal antibody. The similarities between the response of anti-TNF treatment in our mice compared with those obtained in patients with CD may suggest that the mechanism of action of TNF blockade in this mouse model is likely to be similar to that of the human disease.
One of the major histological features of the intestinal lesions in CD, as well as in SAMP1/YitFc mice, is the loss of villous architecture due to intestinal epithelial cell damage, heavy infiltration of T lymphocytes into the lamina propria, as well as occasional increased numbers of IEL. Histological analysis of SAMP1/YitFc mice treated with anti-TNF-α monoclonal antibody showed a significant reduction in villous distortion as well as a marked reduction in the number of lymphocytes infiltrating the lamina propria. Because apoptosis has been proposed as a key mechanism of cell turnover in the gut mucosa (7–10), we next tested the hypothesis that the beneficial effects of anti-TNF-α treatment in CD are mediated by regulation of gut mucosal cell apoptosis. Our results show that anti-TNF-α monoclonal antibody treatment of 40-week-old SAMP1/YitFc mice decreased the percentage of apoptotic cells in freshly isolated IEC. This observation was associated with an opposite effect in freshly isolated LPMC, for which we observed an increase in the percentage of apoptotic cells. Differences in apoptosis were also confirmed by using DNA laddering in freshly isolated gut mucosal cells as well as TUNEL assay in tissue sections obtained from the terminal ileum. Interestingly, we observed increased TUNEL staining not only in LPMC, but also in cells with morphology consistent with IEL. Thus, these results suggest that anti-TNF-α treatment, in addition to protecting IEC from TNF-α-mediated apoptosis, may also decrease gut inflammation by eliminating potential pathogenic T cells within the intestinal mucosa. Recent studies have, in fact, implicated a resistance of gut mucosal T cells to apoptosis as a key mechanism for the perpetuation of chronic intestinal inflammation in CD (9, 10). In addition, our results are consistent with recent studies in humans showing that anti-TNF-α antibody treatment has the ability to induce apoptosis in circulating monocytes as well as in activated mucosal T lymphocytes from CD patients (23, 24).
Our data demonstrating that anti-TNF-α treatment regulates gut mucosal cell apoptosis may provide immunological evidence to potentially explain two important clinical effects commonly found in infliximab-treated CD patients. First, the ability of infliximab to promote complete mucosal healing with restitution of normal gut epithelial architecture in infliximab-treated CD patients (4). These effects may clearly be related to the ability of anti-TNF-α treatment to protect against TNF-α-induced apoptosis of IEC. Second, the prolonged remission (several weeks, up to 1 year) obtained with a single infusion of anti-TNF-α monoclonal antibody in a subgroup of patients with refractory CD (25, 26), which may be due to the proapoptotic effects of anti-TNF-α treatment on gut mononuclear cells. Interestingly, our observation of increased apoptosis of IEL after anti-TNF-α treatment may suggest a pathogenic role of this T cell population during chronic intestinal inflammation.
One of the most intriguing findings of the present report is the ability of anti-TNF treatment to decrease IEC apoptosis while simultaneously increasing LPMC apoptosis. One possibility to explain this phenomenon is that the decrease in IEC apoptosis is a consequence of the effects on LPMC, which results in the inhibition of pathogenic T cells and their secreted proinflammatory cytokines. These results are in agreement with previous studies demonstrating the ability of cytokines produced by activated T cells, such as IFNγ and TNF-α, to synergistically induce apoptosis in IEC (26). Alternatively, the possibility exists that monoclonal antibodies against TNF-α may affect gut mucosal cell apoptosis independently, by interacting with transmembrane TNF-α and/or TNF-α receptors expressed on the surface of IEC and LPMC. Because Fas antigen, a member of the TNF-α receptor superfamily, is known to share common pathways with TNF-α in the process of programmed cell death (12) and its expression is inducible by TNF-α itself (13), we next measured the expression of Fas on both IEC and LPMC after anti-TNF-α treatment. Our results show that Fas expression in freshly isolated IEC was reduced in mice treated with 0.1 mg and 1.0 mg of anti-TNF-α compared with isotype control. These data suggest that anti-TNF-α treatment may reduce IEC apoptosis by a mechanism that involves a reduction in TNF-α-mediated Fas antigen expression. Conversely, Fas expression was unchanged in freshly isolated LPMC obtained from anti-TNF-α treated mice compared with control mice (data not shown). Thus, our results suggest that differently from IEC, Fas antigen expression may not be involved in apoptosis mediated by anti-TNF-α in LPMC. This observation is consistent with results obtained in patients with CD in which resistance to apoptosis was not linked to a differential expression of Fas antigen on freshly isolated lamina propria T cells (9). Recently, it was reported that the ability of infliximab to induce apoptosis in circulating monocytes from patients with CD was associated with the activation of an intracellular caspase-dependent pathway (23). Further studies are necessary to understand the precise intracellular mechanism(s) by which anti-TNF-α treatment may induce apoptosis in LPMC of CD patients.
In summary, our findings suggest a novel mechanism for the anti-inflammatory effects of anti-TNF-α therapy in experimental ileitis, which involves protection of IEC apoptosis and induction of programmed cell death in LPMC. We speculate that these results may be applicable to the human condition by providing a potential immunologic mechanism for the dramatic clinical effects of anti-TNF-α therapy, which may be related to the resetting of immunologic homeostasis in the gut by regulating programmed cell death in the gut mucosa. Thus, our studies provide the rationale for developing novel therapeutic modalities for the treatment of CD, and other chronic mucosal inflammatory diseases, based on deleting pathogenic T cells, while at the same time maintaining the integrity of the epithelial cell barrier.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 DK55812, R37 DK41191, and PO1 DK70555 (to F.C.), and RO1 DK56762 (to T.T.P). G.B. is a recipient of a Crohn's and Colitis Foundation of America Fellowship Award. J.R.-N. is a recipient of a Minority Medical Faculty Development fellowship from the Robert Wood Johnson Foundation.
Abbreviations: CD, Crohn's disease; TNF-α, tumor necrosis factor α; IEC, intestinal epithelial cell; IEL, intraepithelial lymphocytes; TUNEL, terminal deoxynucleotidyltranferase-mediated dUTP-biotin nick end labeling; PI, propidium iodide; LPMC, lamina propria mononuclear cells.
References
- 1.Fiocchi, C. (1998) Gastroenterology 115 182-205. [DOI] [PubMed] [Google Scholar]
- 2.Papadakis, K. A. & Targan, S. R. (2000) Gastroenterology 119 1148-1157. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis, D., Pasparakis, M., Pizarro, T. T., Cominelli, F. & Kollias, G. (1999) Immunity 10 387-398. [DOI] [PubMed] [Google Scholar]
- 4.Targan, S. R., Hanauer, S. B., van Deventer, S. J., Mayer, L., Present, D. H., Braakman, T., DeWoody, K. L., Schaible, T. F. & Rutgeerts, P. J. (1997) N. Engl. J. Med. 337 1029-1035. [DOI] [PubMed] [Google Scholar]
- 5.Baert, F. J., D'Haens, G. R., Peeters, M., Hiele, M. I., Schaible, T. F., Shealy, D., Geboes, K. & Rutgeerts, P. J. (1999) Gastroenterology 116 22-28. [DOI] [PubMed] [Google Scholar]
- 6.Plevy, S. E., Landers, C. J., Prehn, J., Carramanzana, N. M., Deem, R. L., Shealy, D. & Targan, S. R. (1997) J. Immunol. 159 6276-6282. [PubMed] [Google Scholar]
- 7.Levine, A. D. & Fiocchi, C. (2001) Semin. Immunol. 13 195-199. [DOI] [PubMed] [Google Scholar]
- 8.Lenardo, M., Chan, K. M., Hornung, F., McFarland, H., Siegel, R., Wang, J. & Zheng, L. T. (1999) Annu. Rev. Immunol. 17 221-253. [DOI] [PubMed] [Google Scholar]
- 9.Boirivant, M., Marini, M., Di Felice, G., Pronio, A. M., Montesani, C., Tersigni, R. & Strober, W. (1999) Gastroenterology 116 557-565. [DOI] [PubMed] [Google Scholar]
- 10.Ina, K., Itoh, J., Fukushima, K., Kusugami, K., Yamaguchi, T., Kyokane, K., Imada, A., Binion, D. G., Musso, A., West, G. A., et al. (1999) J. Immunol. 163 1081-1090. [PubMed] [Google Scholar]
- 11.Kaiser, G. C. & Polk, D. B. (1997) Gastroenterology 112 1231-1240. [DOI] [PubMed] [Google Scholar]
- 12.Bang, S., Jeong, E. J., Kim, I. K, Jung, Y. K. & Kim, K. S. (2000) J. Biol. Chem. 275 36217-36222. [DOI] [PubMed] [Google Scholar]
- 13.Choi, C., Park, J. Y., Lee, J., Lim, J. H., Shin, E. C., Ahn, Y. S., Kim, C. H., Kim, S. J., Kim, J. D., Choi, I. S. & Choi, I. H. (1999) J. Immunol. 162 1889-1895. [PubMed] [Google Scholar]
- 14.Kosiewicz, M. M., Nast, C. C., Krishnan, A., Rivera-Nieves, J., Moskaluk, C. A., Matsumoto, S., Kozaiwa, K. & Cominelli, F. (2001) J. Clin. Invest. 107 695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Nieves, J., Bamias, G., Vidrich, A., Marini, M., Pizarro, T. T., McDuffie, M. J., Cohn, S., Moskaluk, C. A. & Cominelli, F. (2003) Gastroenterology 124 972-982. [DOI] [PubMed] [Google Scholar]
- 16.Burns, R. C., Rivera-Nieves, J., Moskaluk, C. A., Matsumoto, S., Cominelli, F. & Ley, K. (2001) Gastroenterology 121 1428-1436. [DOI] [PubMed] [Google Scholar]
- 17.Whitehead, R. H., Demmler, K., Rockman, S. P. & Watson, N. K. (1999) Gastroenterology 117 858-865. [DOI] [PubMed] [Google Scholar]
- 18.Van der Heijden, P. J. & Stok, W. (1987) J. Immunol. Methods 103 161-167. [DOI] [PubMed] [Google Scholar]
- 19.Zamai, L., Falcieri, E., Zauli, G., Cataldi, A. & Vitale, M. (1993) Cytometry 14 891-897. [DOI] [PubMed] [Google Scholar]
- 20.Neurath, M. F., Fuss, I., Pasparakis, M., Alexopoulou, L., Haralambous, S., Meyer zum Buschenfelde, K. H., Strober, W. & Kollias, G. (1997) Eur. J. Immunol. 27 1743-1750. [DOI] [PubMed] [Google Scholar]
- 21.Murthy, S., Cooper, H. S., Yoshitake, H., Meyer, C., Meyer, C. J. & Murthy, N. S. (1999) Aliment. Pharmacol. Ther. 13 251-260. [DOI] [PubMed] [Google Scholar]
- 22.Olson, A. D., DelBuono, E. A., Bitar, K. N. & Remick, D. G. (1995) J. Pediatr. Gastroenterol. Nutr. 21 410-418. [DOI] [PubMed] [Google Scholar]
- 23.Lugering, A., Schmidt, M., Lugering, N., Pauels, H. G., Domschke, W. & Kucharzik, T. (2000) Gastroenterology 118 1145-1157. [DOI] [PubMed] [Google Scholar]
- 24.Ten Hove, T., van Montftrans, C., Peppelenbosch, M. P. & van Deventer, S. J. H. (2002) Gut 50 206-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutgeerts, P., D'Haens, G., Targan, S., Vasiliauskas, E., Hanauer, S. B., Present, D. H., Mayer, L., Van Hogezand, R. A., Braakman, T., DeWoody, K. L., et al. (1999) Gastroenterology 117 761-769. [DOI] [PubMed] [Google Scholar]
- 26.Hanauer, S. B., Feagan, B. G., Lichtenstein, G. R., Mayer, L. F., Schreiber, S., Colombel, J. F., Rachmilewitz, D., Wolf, D. C., Olson, A., Bao, W. & Rutgeerts, P. (2002) Lancet 4 1541-1549. [DOI] [PubMed] [Google Scholar]
- 27.Deem, R. L., Shanahan, F. & Targan, S. R. (1991) Clin. Exp. Immunol. 83 79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]