Abstract
Type 1 diabetes is an autoimmune disease in which autoreactive T cells attack and destroy the insulin-producing pancreatic β cells. CD8+ T cells are essential for this β cell destruction, yet their specific antigenic targets are largely unknown. Here, we reveal that the autoantigen targeted by a prevalent population of pathogenic CD8+ T cells in nonobese diabetic mice is islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP). Through tetramer technology, IGRP-reactive T cells are readily detected in islets and peripheral blood directly ex vivo. The human IGRP gene maps to a diabetes susceptibility locus, suggesting that IGRP also may be an antigen for pathogenic T cells in human type 1 diabetes and, thus, a new, potential target for diagnostic and therapeutic approaches.
The nonobese diabetic (NOD) mouse is a widely studied model of human type 1 diabetes, an autoimmune disease characterized by inflammation of pancreatic islets (insulitis) followed by T cell-mediated destruction of insulin (INS)-producing β cells (1). Both CD4+ and CD8+ T cells are required for this pathogenic process (1); however, CD8+ T cells appear to be responsible for the initial β cell insult (1–3). Whereas the pathogenicity of B cells and autoantibodies is less clear, the autoantigens currently believed to contribute to autoimmune diabetes pathogenesis in NOD mice and humans all were originally identified based on the presence of specific autoantibodies rather than by T cell recognition (4–6). Little is known of the β cell antigens targeted by the pathogenic CD8+ T cells. Although one study identified an INS peptide as the antigenic target of the majority of islet-infiltrating CD8+ T cells in NOD mice (7), the prevalence of these INS-reactive CD8+ T cells was not confirmed in subsequent studies (8, 9).
A substantial proportion of β cell-autoreactive CD8+ T cells isolated from NOD islets express a shared T cell receptor α (TCRα) chain (Vα17-Jα42), suggesting recognition of a common β cell peptide (3, 10). These T cells do not recognize the antigenic INS peptide mentioned above (11, 12). The pathogenicity of this prevalent T cell population has been well established through studies of the 8.3 T cell clone (a representative T cell clone of the Vα17-Jα42-expressing T cell population) (13, 14). 8.3-Like T cells are present in the earliest islet infiltrates of NOD mice (3) and undergo avidity maturation as islet inflammation progresses to overt disease (8). At any given time, 8.3-like T cells can constitute up to 30–40% of the islet-associated CD8+ T cells (9). Strikingly, quantification of 8.3-like T cells in peripheral blood predicts diabetes development in individual NOD mice (9), unlike any other single immune indicator identified to date. Although the prevalence and pathogenicity of 8.3-like T cells has been clearly established, the identity of their ligand has remained elusive.
Materials and Methods
Mice. NOD/Lt mice were maintained by brother–sister mating. 8.3-TCRαβ-transgenic NOD mice, designated 8.3-NOD, have been described (14). All mice were maintained under specific pathogen-free conditions and used in accordance with institutional guidelines for animal welfare.
Class I MHC-Associated Peptides. H-2Kd molecules were immunoaffinity-purified from 1.4 × 1010 IFN-γ-treated NIT-1 pancreatic β cells (15) by using mAb SF1–1.1, and their associated peptides were extracted as described (16). Peptide extracts were fractionated by two rounds of reverse-phase HPLC as described (17).
Epitope Reconstitution Assays. Cytotoxic T lymphocytes (CTL) were generated by culturing splenocytes from 8.3-NOD mice with mitomycin C-treated NOD splenocytes pulsed with NRP-A7 peptide as described (18). CTL were used in 16-h 51Cr-release cytotoxicity assays to test for recognition of peptide-pulsed RMA-S/Kd target cells (provided by M. Bevan, University of Washington, Seattle) at an effector-to-target ratio of 40:1 as described (18). Synthetic peptides were used at concentrations as indicated in the figures, and 7 × 108, 6 × 108, and 2 × 109 NIT-1 cell equivalents of peptide were used for assays of first-, second-, and third-dimension HPLC fractions, respectively.
Peptide Analysis. Active second-dimension fraction 66 was loaded on a reverse-phase microcapillary column and analyzed by microelectrospray ionization on a home-assembled Fourier transform ion cyclotron resonance mass spectrometer, equipped with nanoflow liquid chromatography and an online effluent splitter (16, 17). Briefly, effluent from the microcapillary HPLC column was split such that 1/19th was directed into the Fourier transform ion cyclotron resonance mass spectrometer for electrospray ionization MS analysis, and the remaining 18/19ths were deposited directly into the wells of a 96-well plate for epitope reconstitution assays. In this way, each well could be correlated to a set of scans in the mass spectral data. Peptide masses eluting in the area of CTL activity and having an elution profile similar to the activity lysis profile were marked as antigen candidates. Good candidates had a deconvoluted (M+H)+ mass between 800 and 1,600 Da, the mass range characteristic of peptides eluted from class I MHC molecules.
Sequence Analysis and Synthesis of Candidate Antigens. Collision-activated dissociation mass spectra were recorded for selected peptide candidates by using a Thermo Finnigan LCQ ion trap mass spectrometer (Thermo Finnigan, San Jose, CA). Candidate masses were targeted throughout the chromatographic run. Candidate peptides (VYLKTNVFL and IYQKAFDLI) were synthesized by standard fluorenylmethoxycarbonyl chemistry by using a Gilson peptide synthesizer (model AMS422; Gilson) and purified to >95% by reverse-phase HPLC. Candidate antigen sequences were confirmed by comparing collision-activated dissociation spectra with those of synthetic peptides. Further sequence confirmation and an estimation of copy number per cell were determined by running an aliquot of the active fraction and comparing antigen ion abundance with an identical run with synthetic antigen spiked in at a known level. Synthetic and naturally processed peptide coelution further confirmed the identity of the antigen.
Synthetic Peptides. NRP-A7 (KYNKANAFL), NRP-V7 (KYNKANVFL), INS B15–23 (LYLVCGERG), INS-I9 (LYLVCGERI), TUM (KYQAVTTTL), and G6Pase (KYCLITIFL) peptides were synthesized by standard solid-phase methods by using fluorenylmethoxycarbonyl chemistry in an automated peptide synthesizer (model 433A; Applied Biosystems), and their identities were confirmed by MS.
Transient Transfection. COS-7 cells were transfected by using a DEAE-dextran protocol as described (19). DNA (10 ng/ml) for the class I MHC molecule (H-2Kd or H-2Db) expression constructs was used along with concentrations of islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) expression construct or vector alone as indicated in the Fig. 1G legend. Separate cultures were transfected with the H-2Kd construct alone and pulsed with varying concentrations of IGRP206–214 peptide. After coculture with 8.3 CTL, T cell response was measured as IFN-γ release by ELISA.
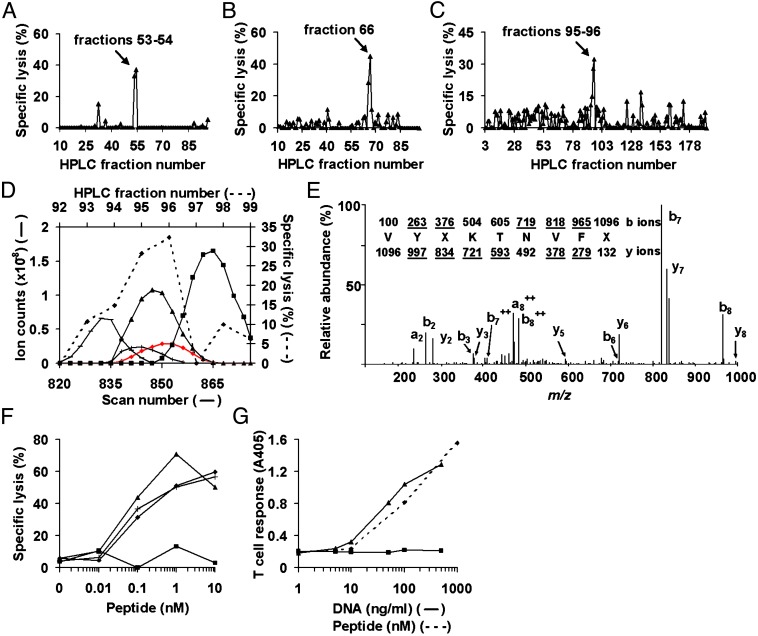
Fig. 1.
Identification of IGRP206–214 as the β cell peptide recognized by the pathogenic T cell clone 8.3. (A–C) Epitope reconstitution activity of first-dimension (A), second-dimension (B), and third-dimension (C) HPLC fractions of H-2Kd-eluted NIT-1 peptides. (D) Determination of candidate peptides by correlation of ion abundance curves (solid lines; plotted on the left and bottom axes) with epitope reconstitution activity of third-dimension HPLC fractions (broken line, ⋄; plotted on the right and top axes). Peptide m/z values are 548.845 (red diamonds), 513.285 (▴), 509.78 (+), 555.805 (-), and 555.835 (▪). (E) Collision-activated dissociation mass spectrum of candidate peptide (M + 2H)+2 ion with monoisotopic m/z of 548.845. X represents I or L. Ions observed in the spectrum are underlined; the b ions originate from the N terminus of the peptide, and the y ions originate from the C terminus. (F) Response of 8.3 CTL toward RMA-S/Kd target cells pulsed with varying concentrations of VYLKTNVFL (▴), IYQKAFDLI (▪), NRP-V7 (⋄), or NRP-A7 (+). (G) Response of 8.3 CTL toward COS-7 cells cotransfected (solid lines) with varying concentrations of an IGRP expression construct (▴), or empty vector (▪), together with 10 ng/ml H-2Kd expression construct, or toward separate cultures of COS-7 cells transfected with the H-2Kd construct alone and pulsed with varying concentrations of IGRP206–214 peptide (broken line, ⋄). T cell response was measured as IFN-γ release by ELISA and is presented as absorbance at 405 nm (A405).
TCR Transfectants. TCRs from the previously isolated NOD-derived, β cell-autoreactive, class I MHC-restricted T cell clones AI4, AI12.B1.1, AI12.B1.2, AI12.B1.3, AI15.A10, AI15.F5 (3), and 8.3 (14) were expressed in the TCR- T cell hybridoma 58α-β- (engineered to express the TCRζ chain and CD8αβ and provided by H.-C. Chang, Dana–Farber Cancer Institute, Boston) as described (12). TCR expression was verified by flow cytometric analysis of growing clones by using an antibody to CD3ε (145–2C11). RMA-S/Kd cells incubated overnight at 28°C were used to present exogenously added synthetic peptides at concentrations as indicated in the Fig. 2 legend. Peptide recognition by the TCR transfectants was measured by IL-2 production as described (12).
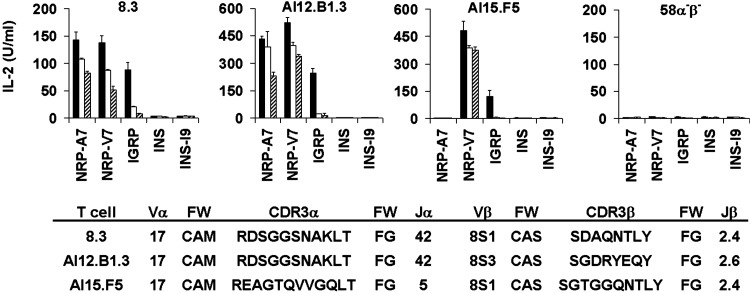
Fig. 2.
Multiple early insulitic T cell clones recognize IGRP206–214.58α-β- transfectants expressing the indicated TCRs were cultured with RMA-S/Kd cells pulsed with 1.0 (solid bars), 0.1 (open bars), or 0.01 μM (striped bars) of the indicated peptides, and IL-2 release was measured by ELISA. The partial TCRα and TCRβ chain sequences for 8.3 and for the early insulitic T cell clones AI12.B1.3 and AI15.F5 have been reported (3, 10, 13). The AI4, AI12.B1.1, AI12.B1.2, and AI15.A10 lines, all of which express non-Vα17 TCRα chains, did not respond to any of the peptides tested, although they were capable of signaling through the transfected TCR as evidenced by their release of IL-2 in response to plate-bound anti-CD3ε (data not shown). IGRP, IGRP206–214; INS, INS B15–23; FW, framework residues; CDR, complementarity-determining region.
H-2Kd Stabilization Assay. RMA-S/Kd cells, cultured overnight at 28°C, were pulsed with peptides in complete DMEM for 1 h at 28°C, incubated at 37°C for 3 h, washed, stained with anti-H-2Kd mAb SF1–1.1, counterstained with FITC-conjugated polyclonal goat anti-mouse antibody, and analyzed by flow cytometry. Data were calculated by subtracting mean fluorescence intensity of H-2Kd on nonpeptide-pulsed cells from that on peptide-pulsed cells.
Tetramer Staining and Flow Cytometry. Tetramers were prepared and islet-associated and peripheral blood-derived CD8+ T cells were isolated from NOD mice as described (8, 9).
Results and Discussion
Identification of the Antigenic Target of the 8.3-Like T Cell Population. The 8.3 T cell clone is restricted to the class I MHC molecule H-2Kd (13). To identify the pancreatic β cell antigen recognized by the diabetogenic 8.3-like CD8+ T cell population, we purified H-2Kd class I MHC molecules from the NOD-derived pancreatic β cell line NIT-1 by immunoaffinity chromatography. Peptides were eluted from the H-2Kd molecules and fractionated by reverse-phase HPLC. Fractions were tested for their ability to elicit 8.3 CTL-mediated lysis of peptide-pulsed target cells (Fig. 1A). Fractions composing the active peak were pooled and subjected to another round of HPLC under different conditions. A single peak of activity was observed (Fig. 1B). Second-dimension fraction 66 was rechromatographed, and a portion of the effluent was analyzed by electrospray ionization on a Fourier transform ion cyclotron resonance mass spectrometer equipped with nanoflow liquid chromatography and an online effluent splitter. For this splitter experiment, a portion of the effluent was deposited in 96-well plates for determination of epitope reconstitution activity, and the remainder was directed to the mass spectrometer. Testing of peptide fractions for recognition by 8.3 CTL yielded a single peak of activity (Fig. 1C). We identified candidate peptides by comparing the abundances of ions observed in the active and adjoining fractions with the lysis profile from the epitope reconstitution assay (Fig. 1D). More than 100 peptide candidates were present in the scan window; of these, ≈35 eluted entirely within the window. We ranked candidates based on the extent of alignment between their ion abundance curves and the lysis profile. Only the abundance curves for the best candidates and one very abundant, later-eluting peptide are shown (Fig. 1D). The millimass accuracy capability of the Fourier transform ion cyclotron resonance mass spectrometer allowed coeluting peptides differing by <0.1 mass unit to be easily distinguished, and the detection limit of the instrument (2–10 amol) made it possible to detect peptide candidates present at only a few copies per cell (20).
Based on alignment between abundance curves and the lysis profile, the best peptide candidate, although not the most abundant, was the one having a doubly charged monoisotopic m/z of 548.845 (Fig. 1D). Sequence analysis yielded VYXKTNVFX (Fig. 1E), where X represents either I or L, amino acids of identical mass that cannot be differentiated by the instrument. Similarly, we determined the sequence of the abundant, later-eluting peptide (m/z of 555.835+2) to be XYQKAFDXX (data not shown). Protein database searches yielded one perfect match each for VYXKTNVFX and XYQKAFDXX (VYLKTNVFL and IYQKAFDLI, respectively). We synthesized these peptides and tested them in epitope reconstitution assays. Our data reveal that VYLKTNVFL is the β cell peptide recognized by 8.3 (Fig. 1F). Activated 8.3 CTL respond to this peptide in an equivalent dose-dependent manner as to the previously described synthetic agonist NRP-A7 and superagonist NRP-V7 (11, 21), with half-maximal activity observed at a peptide concentration of ≈50 pM (Fig. 1F). Approximately 100 copies of VYLKTNVFL/H-2Kd are present per IFN-γ-treated NIT-1 cell.
A blast search of the entire National Center for Biotechnology Information nonredundant protein database resulted in only one exact hit for VYLKTNVFL corresponding to residues 206–214 of murine IGRP (22). To confirm that the source of the antigenic peptide was indeed IGRP, we transfected COS-7 cells with varying concentrations of an expression construct for IGRP, or vector alone, together with an H-2Kd expression construct and tested for recognition by 8.3 CTL. Cells transfected with IGRP, but not vector alone, stimulated 8.3 CTL to release IFN-γ in a dose-dependent manner upon coculture (Fig. 1G). This response required the expression of H-2Kd; transfection of the IGRP construct along with an H-2Db expression construct resulted in a T cell response profile similar to that of vector alone (data not shown). To our knowledge, IGRP has not been implicated previously as either a T or B cell antigen in NOD mice or in type 1 diabetes patients. Thus, IGRP is the natural target of the prevalent and pathogenic 8.3-like T cell population in NOD mice and represents a unique β cell autoantigen.
Multiple Early Insulitic T Cell Clones Recognize IGRP206–214. We previously isolated a set of six β cell-autoreactive CD8+ T cell clones of unknown antigenic specificity from early insulitic lesions of young NOD mice (designated AI4, AI12.B1.1, AI12.B1.2, AI12.B1.3, AI15.A10, and AI15.F5) (3). We had reported earlier that AI12.B1.3, which expresses a Vα17-Jα42 TCRα chain nearly identical to that of 8.3, recognizes NRP-A7 (12). Thus, we hypothesized it also would recognize IGRP206–214. To test whether this and any other clones in our panel were IGRP206–214-reactive, we assayed their ability to recognize IGRP206–214. AI12.B1.3 recognized IGRP206–214, as well as NRP-V7 and NRP-A7, but not the previously identified antigenic INS peptide (INS B15–23) or its I9 variant (INS-I9) (7, 23) (Fig. 2). Hence, the 8.3 clonotype is not unique; other β cell-autoreactive CD8+ T cells sharing the prevalent Vα17-Jα42 TCRα chain also recognize IGRP206–214. Importantly, AI15.F5, which expresses a Vα17-Jα5 TCRα chain and a similar TCRβ chain to 8.3, also responded to IGRP206–214 and NRP-V7, although it did not recognize NRP-A7 (Fig. 2). This finding demonstrates that reactivity to IGRP206–214 is not limited strictly to T cells expressing a Vα17-Jα42 TCRα chain. Further, the ability of AI15.F5 to recognize NRP-V7, but not NRP-A7, is consistent with the recent observation that NRP-V7/H-2Kd tetramers stain a larger population of islet T cells than do NRP-A7 tetramers (9). Thus, previous measurements of NRP-A7 reactivity (8) have underestimated the prevalence of the IGRP206–214-reactive T cell population.
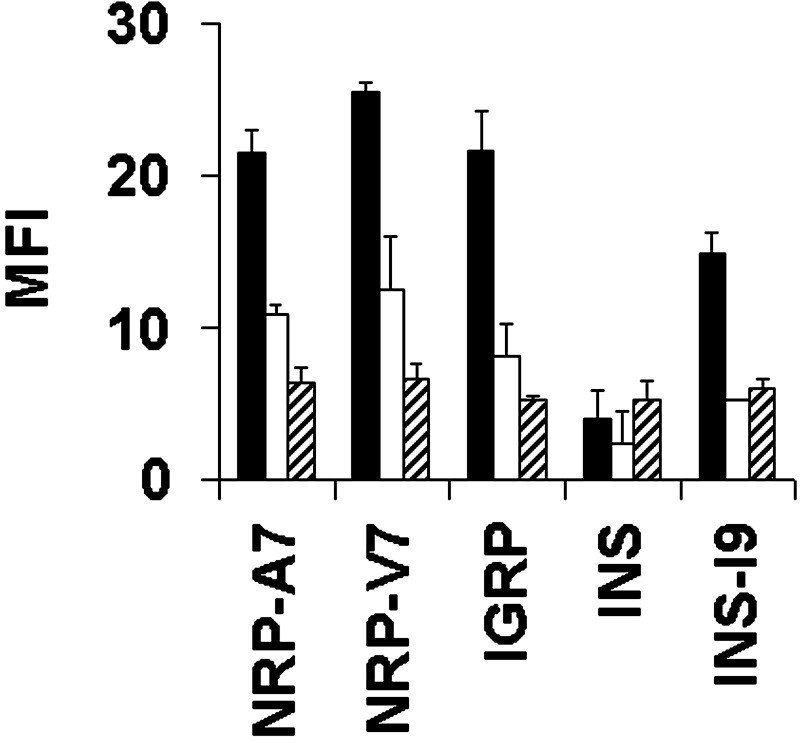
IGRP206–214 Does Not Exhibit Poor Binding to H-2Kd. The only other known natural ligand for an NOD-derived diabetogenic CD8+ T cell clone is INS B15–23 presented by H-2Kd (7). The INS peptide exhibits very poor binding to H-2Kd, and it has been suggested that this results in insufficient peptide presentation for T cell-negative selection in the thymus (23). To determine whether poor MHC binding is a characteristic of autoantigenic peptides in general, we tested the ability of IGRP206–214 to bind H-2Kd. In H-2Kd-stabilization assays, IGRP206–214 demonstrated good MHC binding, comparable to that of the synthetic ligands NRP-V7 and NRP-A7 and considerably better than that of INS B15–23, which was barely detectable even at the highest peptide concentration tested (Fig. 3). Like NRP-V7 and NRP-A7, IGRP206–214 contains the expected H-2Kd anchor residues, i.e., Y at position 2 and L at 9, whereas INS B15–23 has G at position 9, which makes its binding to H-2Kd unfavorable. When G at position 9 is replaced with I, the resulting INS-I9 peptide shows improved binding to H-2Kd (Fig. 3; ref. 23). Furthermore, when we analyzed the protein sequence of murine IGRP with several different algorithms designed to identify good MHC-binding peptides [SYFPEITHI (24), BIMAS (25), or RANKPEP (26)], IGRP206–214 consistently ranked among the best H-2Kd binders (third, first, or second, respectively). Taken together, these observations indicate that poor MHC class I binding is not a requirement for self-peptides recognized by autoreactive CD8+ T cells.
Fig. 3.
IGRP206–214 does not demonstrate poor peptide binding to H-2Kd. RMA-S/Kd cells were pulsed with 1.0 (solid bars), 0.1 (open bars), or 0.01 μM (striped bars) of the indicated peptides, stained with an anti-H-2Kd antibody, and analyzed by flow cytometry. IGRP, IGRP206–214; INS, INS B15–23; MFI, mean fluorescence intensity.
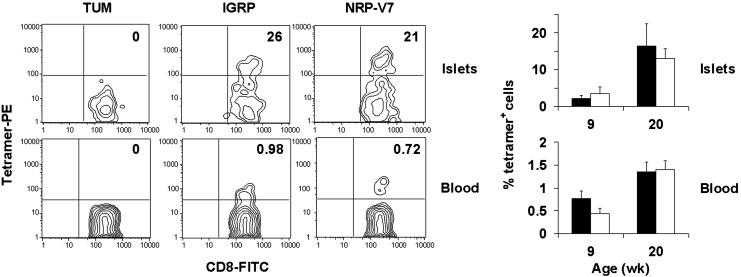
Detection of IGRP206–214-Reactive T Cells in Islets and Peripheral Blood. A recent study demonstrated that NRP-V7/H-2Kd tetramers could be used to detect and quantify islet- and peripheral blood-derived, NRP-V7-reactive CTL directly ex vivo (9). However, the use of natural self-peptides in tetramer studies to identify autoreactive T cells directly ex vivo has been reported only for melanocyte-antigen-specific T cells (27). To evaluate whether the natural IGRP peptide, which differs from the 8.3 superagonist NRP-V7 (KYNKANVFL) by only three residues, could be used similarly to quantify the prevalent β cell-autoreactive T cell population, we isolated T cells from both islets and peripheral blood of 9- and 20-week-old nondiabetic NOD mice and stained them with IGRP206–214, NRP-V7, or negative control peptide (TUM) tetramers. A sizable proportion of islet T cells from 9- or 20-week-old mice was IGRP-reactive, and a population of IGRP-reactive cells was clearly measurable in peripheral blood (Fig. 4). Importantly, for both islet and peripheral blood samples of 9- or 20-week-old mice, the size of the CD8+ T cell population that stained with tetramer was similar whether IGRP206–214 or NRP-V7 tetramers were used for detection. Thus, the natural peptide can be used to detect and quantify IGRP206–214-reactive islet and peripheral blood T cells directly ex vivo.
Fig. 4.
IGRP206–214-reactive T cells are readily detected in islets and peripheral blood of NOD mice directly ex vivo. Cells from islets or peripheral blood of 9- or 20-week-old nondiabetic NOD mice were stained with anti-CD8 antibody and the indicated peptide/H-2Kd tetramers. (Left) Representative tetramer staining patterns of samples from 20-week-old mice gated on the CD8+ population. Numbers indicate percentage of tetramer-positive cells within the CD8+ population. (Right) The percentage of IGRP (solid bars) or NRP-V7 (open bars) tetramer-positive cells within the CD8+ population for five individual mice per age group. TUM/H-2Kd tetramers stained 0% of cells in each group. IGRP, IGRP206–214.
The role of CD8+ T cells in autoimmune diseases such as type 1 diabetes is becoming more widely recognized (28); however, knowledge of the natural ligands of these pathogenic T cells in spontaneous autoimmune diseases is extremely limited. Here, we have identified IGRP as the source of the natural peptide recognized by a prevalent population of pathogenic CD8+ T cells in NOD mice. IGRP is an islet-specific protein expressed in pancreatic β cells and, to a lesser extent, in α cells and shares ≈50% identity with the catalytic subunit of the liver enzyme glucose-6-phosphatase (29). Importantly, IGRP206–214 differs from the homologous residues of murine glucose-6-phosphatase (KYCLITIFL) at six of nine positions. Accordingly, this liver glucose-6-phosphatase peptide is not recognized by 8.3 CTL (data not shown). Despite its homology to glucose-6-phosphatase, no catalytic activity has been demonstrated for IGRP, and its function is unknown (22, 29). From its sequence, it is predicted to be an ER-resident protein that spans the membrane nine times (22). The abundance of its RNA places IGRP among β cell genes expressed at moderate to high levels (22), and the protein can be readily detected in islets by immunohistochemistry (29). Intriguingly, the human IGRP gene (29) maps to a diabetes susceptibility locus on chromosome 2, IDDM7 (30), and several single nucleotide polymorphisms within the IGRP gene are known (www.ncbi.nlm.nih.gov/SNP).
The IGRP-reactive T cell population constitutes a substantial component of even the earliest NOD islet infiltrates (Fig. 2; ref. 3), and its pathogenicity has been clearly established (8, 9, 13, 14). Thus, the response to IGRP could be one of the first events leading to β cell destruction by CD8+ T cells in autoimmune diabetes. Now, with the identification of IGRP as the β cell antigen targeted by 8.3-like T cells, critical aspects of the development, activation, and expansion of this T cell population can be investigated further. This knowledge already has made possible the elucidation of the mechanisms responsible for the avidity maturation of these pathogenic 8.3-like T cells in NOD mice (B.H., A. F. M. Maree, P. Serra, J. Yamanouchi, A. Amrani, J. F. Elliott, P. Dickie, L. Edelstein-Keshet, T. P. D., and P.S., unpublished results). In addition, it is now possible to evaluate whether a similarly prevalent IGRP-reactive T cell population participates in the pathogenesis of human type 1 diabetes. Because several β cell autoantigens in NOD mice overlap those that have been identified in patients (2), and quantification of 8.3-like T cells in peripheral blood predicts diabetes in individual NOD mice (9), we predict that IGRP will demonstrate considerable importance in the development of the human disease.
Acknowledgments
We thank S.-C. Yip for technical assistance; S. A. Porcelli, J. R. Warner, and B. Diamond for critical comments on the manuscript; and A. G. Brickner and B. K. Lieberman for helpful discussion. This work was supported by grants from the National Institutes of Health (to T.P.D., D.F.H., S.G.N., and D.V.S.), the Juvenile Diabetes Research Foundation (to T.P.D., S.G.N., P.S., and D.V.S.), the Canadian Institutes of Health Research (to P.S.), and the Canadian Diabetes Association (to P.S.). S.M.L. was supported by a National Institutes of Health Medical Scientist Training Grant. T.P.D. is a member of the National Institutes of Health-supported Albert Einstein College of Medicine Diabetes Research and Training Center, and P.S. is a Senior Scholar of the Alberta Heritage Foundation for Medical Research.
Abbreviations: CTL, cytotoxic T lymphocytes; IGRP, islet-specific glucose-6-phosphatase catalytic subunit-related protein; INS, insulin; TCR, T cell receptor; NOD, nonobese diabetic.
References
- 1.Serreze, D. V. & Leiter, E. H. (2001) Curr. Dir. Autoimmun. 4 31-67. [DOI] [PubMed] [Google Scholar]
- 2.Tisch, R. & McDevitt, H. (1996) Cell 85 291-297. [DOI] [PubMed] [Google Scholar]
- 3.DiLorenzo, T. P., Graser, R. T., Ono, T., Christianson, G. J., Chapman, H. D., Roopenian, D. C., Nathenson, S. G. & Serreze, D. V. (1998) Proc. Natl. Acad. Sci. USA 95 12538-12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer, J. P., Asplin, C. M., Clemons, P., Lyen, K., Tatpati, O., Raghu, P. K. & Paquette, T. L. (1983) Science 222 1337-1339. [DOI] [PubMed] [Google Scholar]
- 5.Baekkeskov, S., Aanstoot, H. J., Christgau, S., Reetz, A., Solimena, M., Cascalho, M., Folli, F., Richter-Olesen, H., De Camilli, P. & Camilli, P. D. (1990) Nature 347 151-156. [DOI] [PubMed] [Google Scholar]
- 6.Rabin, D. U., Pleasic, S. M., Palmer-Crocker, R. & Shapiro, J. A. (1992) Diabetes 41 183-186. [DOI] [PubMed] [Google Scholar]
- 7.Wong, F. S., Karttunen, J., Dumont, C., Wen, L., Visintin, I., Pilip, I. M., Shastri, N., Pamer, E. G. & Janeway, C. A., Jr. (1999) Nat. Med. 5 1026-1031. [DOI] [PubMed] [Google Scholar]
- 8.Amrani, A., Verdaguer, J., Serra, P., Tafuro, S., Tan, R. & Santamaria, P. (2000) Nature 406 739-742. [DOI] [PubMed] [Google Scholar]
- 9.Trudeau, J. D., Kelly-Smith, C., Verchere, C. B., Elliott, J. F., Dutz, J. P., Finegood, D. T., Santamaria, P. & Tan, R. (2003) J. Clin. Invest. 111 217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santamaria, P., Utsugi, T., Park, B. J., Averill, N., Kawazu, S. & Yoon, J. W. (1995) J. Immunol. 154 2494-2503. [PubMed] [Google Scholar]
- 11.Anderson, B., Park, B. J., Verdaguer, J., Amrani, A. & Santamaria, P. (1999) Proc. Natl. Acad. Sci. USA 96 9311-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serreze, D. V., Johnson, E. A., Chapman, H. D., Graser, R. T., Marron, M. P., DiLorenzo, T. P., Silveira, P., Yoshimura, Y., Nathenson, S. G. & Joyce, S. (2001) Diabetes 50 1992-2000. [DOI] [PubMed] [Google Scholar]
- 13.Nagata, M., Santamaria, P., Kawamura, T., Utsugi, T. & Yoon, J. W. (1994) J. Immunol. 152 2042-2050. [PubMed] [Google Scholar]
- 14.Verdaguer, J., Schmidt, D., Amrani, A., Anderson, B., Averill, N. & Santamaria, P. (1997) J. Exp. Med. 186 1663-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamaguchi, K., Gaskins, H. R. & Leiter, E. H. (1991) Diabetes 40 842-849. [DOI] [PubMed] [Google Scholar]
- 16.Cox, A. L., Huczko, E. L., Engelhard, V. H., Shabanowitz, J. & Hunt, D. F. (1997) in MHC: A Practical Approach, eds. Fernandez, N. & Butcher, G. (Oxford Univ. Press, New York), Vol. 1, pp. 141-160. [Google Scholar]
- 17.Pierce, R. A., Field, E. D., den Haan, J. M., Caldwell, J. A., White, F. M., Marto, J. A., Wang, W., Frost, L. M., Blokland, E., Reinhardus, C., et al. (1999) J. Immunol. 163 6360-6364. [PubMed] [Google Scholar]
- 18.DiLorenzo, T. P., Lieberman, S. M., Takaki, T., Honda, S., Chapman, H. D., Santamaria, P., Serreze, D. V. & Nathenson, S. G. (2002) Clin. Immunol. 105 332-341. [DOI] [PubMed] [Google Scholar]
- 19.Karttunen, J., Sanderson, S. & Shastri, N. (1992) Proc. Natl. Acad. Sci. USA 89 6020-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, S. E., Shabanowitz, J., Hunt, D. F. & Marto, J. A. (2000) Anal. Chem. 72 4266-4274. [DOI] [PubMed] [Google Scholar]
- 21.Amrani, A., Serra, P., Yamanouchi, J., Trudeau, J. D., Tan, R., Elliott, J. F. & Santamaria, P. (2001) J. Immunol. 167 655-666. [DOI] [PubMed] [Google Scholar]
- 22.Arden, S. D., Zahn, T., Steegers, S., Webb, S., Bergman, B., O'Brien, R. M. & Hutton, J. C. (1999) Diabetes 48 531-542. [DOI] [PubMed] [Google Scholar]
- 23.Wong, F. S., Moustakas, A. K., Wen, L., Papadopoulos, G. K. & Janeway, C. A., Jr. (2002) Proc. Natl. Acad. Sci. USA 99 5551-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rammensee, H., Bachmann, J., Emmerich, N. P., Bachor, O. A. & Stevanovic, S. (1999) Immunogenetics 50 213-219. [DOI] [PubMed] [Google Scholar]
- 25.Parker, K. C., Bednarek, M. A. & Coligan, J. E. (1994) J. Immunol. 152 163-175. [PubMed] [Google Scholar]
- 26.Reche, P. A., Glutting, J. P. & Reinherz, E. L. (2002) Hum. Immunol. 63 701-709. [DOI] [PubMed] [Google Scholar]
- 27.Pittet, M. J., Zippelius, A., Valmori, D., Speiser, D. E., Cerottini, J. C. & Romero, P. (2002) Trends Immunol. 23 325-328. [DOI] [PubMed] [Google Scholar]
- 28.Liblau, R. S., Wong, F. S., Mars, L. T. & Santamaria, P. (2002) Immunity 17 1-6. [DOI] [PubMed] [Google Scholar]
- 29.Martin, C. C., Bischof, L. J., Bergman, B., Hornbuckle, L. A., Hilliker, C., Frigeri, C., Wahl, D., Svitek, C. A., Wong, R., Goldman, J. K., et al. (2001) J. Biol. Chem. 276 25197-25207. [DOI] [PubMed] [Google Scholar]
- 30.Pociot, F. & McDermott, M. F. (2002) Genes Immunol. 3 235-249. [DOI] [PubMed] [Google Scholar]