Abstract
The influence of transforming growth factor β (TGF-β) signaling on Neu-induced mammary tumorigenesis and metastasis was examined with transgenic mouse models. We generated mice expressing an activated TGF-β type I receptor or dominant negative TGF-β type II receptor under control of the mouse mammary tumor virus promoter. When crossed with mice expressing activated forms of the Neu receptor tyrosine kinase that selectively couple to the Grb2 or Shc signaling pathways the activated type I receptor increased the latency of mammary tumor formation but also enhanced the frequency of extravascular lung metastasis. Conversely, expression of the dominant negative type II receptor decreased the latency of Neu-induced mammary tumor formation while significantly reducing the incidence of extravascular lung metastases. These observations argue that TGF-β can promote the formation of lung metastases while impairing Neu-induced tumor growth and suggest that extravasation of breast cancer cells from pulmonary vessels is a point of action of TGF-β in the metastatic process.
Members of the transforming growth factor β (TGF-β) family of cytokines suppress the growth of multiple epithelial cell lineages (1). Components of the TGF-β signaling pathway are disrupted in certain human tumors, arguing for a tumor-suppressive role in these cancers (2). Moreover, resistance of many breast cancer-derived cell lines to growth inhibition by TGF-β infrequently results from inactivating mutations of the TGF-β receptors or their substrates, the Smad transcription factors (2). The ability of TGF-β to induce an epithelial-to-mesenchymal transition in transformed mammary epithelial cells contributes greatly to the invasive phenotype (3, 4). Furthermore, late-stage human breast tumors often display increased TGF-β expression (5, 6) that is thought to exert angiogenic and immunosuppressive effects in the tumor microenvironment (7, 8). These observations argue that primary tumor cells can reprogram their response to TGF-β, converting this cytokine into a tumor invasion and immunosuppression factor (7).
Less is known, however, about the role of TGF-β in metastasis. TGF-β has been shown to promote osteolytic metastasis by delivery of breast cancer cells to the bloodstream of mice (9). The ability of tumor cells to invade and metastasize relies on the acquisition of concrete functions and an ability to influence and respond to their environment (10). Because of its multifunctional nature, TGF-β might influence several processes during the metastatic cascade.
Mammary tumorigenesis and subsequent metastasis has been modeled through the use of transgenic mice. Expression of a WT Neu receptor tyrosine kinase (11) or oncogenic versions of this receptor (12–14) in the mammary epithelium of transgenic mice leads to the development of metastatic mammary tumors. These observations support a causal role for the Neu receptor tyrosine kinase during mammary tumorigenesis and confirm numerous studies correlating overexpression of ErbB-2, the human orthologue of Neu, with a poor clinical prognosis in breast cancer patients (15).
To dissect the importance of individual signaling pathways in Neu-induced mammary tumorigenesis, transgenic mice expressing oncogenic versions of Neu that couple to the Grb-2 [Neu(YB)] or Shc [Neu(YD)] adaptor proteins have been characterized (16). Mouse mammary tumor virus (MMTV)/Neu(YB) mice develop focal mammary tumors that frequently metastasize to the lungs whereas MMTV/Neu(YD) animals develop multifocal mammary tumors with low incidence of pulmonary metastases (16). We have used these transgenic strains in conjunction with mice expressing an activated TGF-β type I receptor (TβRI) or dominant negative TGF-β type II receptor (TβRII) in the mammary gland to investigate the effects of TGF-β signaling on Neu-induced mammary tumorigenesis and metastasis. While suppressing Neu-induced mammary tumor growth, TGF-β signaling increased the subsequent formation of lung metastases by enhancing the extravasation of breast cancer cells into the lung parenchyma.
Materials and Methods
Plasmid Construction. The MMTV/TβRI(AAD) and MMTV/TβRII(ΔCyt) expression constructs contain the human TβRI and TβRII cDNAs in pMMTV-simian virus 40 (17). Riboprobes are directed against nucleotides 459–1044 of TβRI (NM_004612), nucleotides 307–932 of TβRII (NM_003242), and nucleotides 493–783 of mouse β-actin (X03765).
Primary Cell Cultures and Growth Inhibition Assays. Mammary epithelial cell cultures were isolated as described (18). Mammary glands were harvested from day 9–12 pregnant FVB/N mice, and cells were cultured in F12 media containing 10% FBS, 5 μg/ml insulin, 1 μg/ml hydrocortisone, 3 μg/ml prolactin, 50 μg/ml gentamycin, and penicillin/streptomycin. Primary mammary tumor cultures were maintained in the same media lacking hydrocortisone and prolactin and supplemented with 20 ng/ml epidermal growth factor. 125I-deoxyuridine incorporation assays were performed in 10% FBS as described (19).
RNase Protection Analysis. RNA was isolated and used for RNase protection assays as described (12). The TβRI and β-actin riboprobes were linearized with NotI or BamHI, respectively, and transcribed with T7 RNA polymerase, whereas the TβRII riboprobe was digested with EcoRI and synthesized by using T3 RNA polymerase.
Generation and Identification of Transgenic Mice. The MMTV/TβRI(AAD) and MMTV/TβRII(ΔCyt) injection fragments were prepared as described (12). Microinjections were performed by the Transgenic Mouse Core Facility (Memorial Sloan-Kettering Cancer Center). Transgenic progeny were identified by Southern blot analysis using a fragment corresponding to the simian virus 40 polyadenylation cassette (17) as described (20). Mammary tumor formation was monitored in nulliparous mice by weekly physical palpation.
Histological and Immunohistochemical Analysis. Samples from developing mammary glands were obtained from 8- to 12-week-old female mice. Lactating samples were obtained from females nursing litters for 7–10 days, and litter sizes were normalized to four pups to equalize the pressure for milk production. Whole-mount analyses were performed by using the left thoracic mammary gland (21). Detection of apoptotic cell death was performed by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay as described (22, 23). To measure mammary epithelial cell proliferation, female mice were injected i.p. with 50 μg/g of body weight of BrdUrd (Roche Molecular Biochemicals) at 2 h and again at 1 h before death. Immunohistochemistry with an anti-BrdUrd antibody (Roche Molecular Biochemicals) was performed by using the Vector M.O.M. Immunodetection kit (Vector Laboratories) following the manufacturer's instructions.
Mammary tumors and lung tissue were harvested from mice bearing tumors for 60 days, and histological services were provided by HistoServ (Gaithersburg, MD). Lung metastases were identified by microscopic analysis of lung sections. Intravascular metastases are defined as a group of tumor cells that are either free floating or lodged in a pulmonary vessel, without invasion through the vessel. Extravascular metastases are defined as tumor cells that colonize the lung by growth into the parenchyma. In mammary tumors and lung metastases, proliferating cells were detected by immunohistochemistry using an anti-phospho-histone H3 antibody (Upstate Biotechnology, Lake Placid, NY) on an automated Discovery Staining Module (Ventana Medical Systems, Tucson, AZ).
Morphometric Analysis. Apoptotic and proliferation indices were quantified by using METAMORPH software (version 4.5r5). The number of positively stained cells was manually counted in 15 high-power fields (×400) at each stage of mammary gland development or five high-power fields for mammary tumors and lung metastases from each genotype. The METAMORPH software was used to define the total area occupied by epithelial cells in each field. The number of mammary cells in each field was then estimated by dividing the total epithelial area by the area of a single cell (defined by the average area of 10–15 individual mammary cells within the field).
Results
Modulation of TGF-β Signaling in the Mammary Gland. To assess the role of TGF-β in the regulation of mammary tumorigenesis and metastasis in vivo, we first targeted the expression of modified TGF-β receptors to the mammary gland to constitutively activate or impair TGF-β signaling in mammary epithelial cells and mated these mice to transgenic strains expressing Neu receptors that induce metastatic mammary tumor formation by coupling to distinct signal transduction pathways.
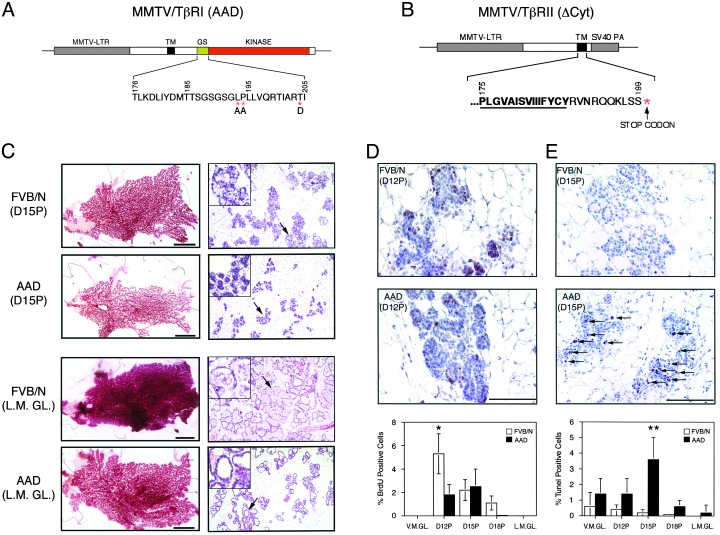
We expressed an activated TβRI or dominant negative TβRII under the transcriptional control of the MMTV-LTR promoter/enhancer (Fig. 1 A and B). The transgene-encoded type I receptor harbors three missense mutations: T204D that constitutively activates the TβRI kinase (24) and L193A/P194A that prevent binding of the TβRI inhibitor, FKBP-12 (25–27) (Fig. 1 A). To create a dominant negative TβRII a stop codon was introduced just downstream of the transmembrane domain, rendering it incapable of phosphorylating and activating the type I receptor (28) (Fig. 1B). Expression of TβRI(AAD) and TβRII(ΔCyt) was confined primarily to the mammary gland or salivary gland in female mice (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org) and was detectable during all stages of mammary gland development (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 1.
Decreased proliferation and enhanced cell death diminish mammary epithelial cell density in TβRI(AAD) transgenic mice. Shown are schematic diagrams of the MMTV/TβRI(AAD) transgene indicating the position of activating mutations (*) within the GS domain (A) and the MMTV/TβRII(ΔCyt) transgene highlighting insertion of a stop codon downstream of the transmembrane domain (B), depicted by bold and underlined type. (C) Whole-mount and histological analyses were conducted on control (FVB/N) and transgenic (AAD) mice from day-15 pregnant (D15P) and lactating mammary glands (L.M.GL.). (Left) Scale bars represent 0.5 cm. (Right) Arrows indicate the region magnified in the Inset. Representative sections illustrating the appearance of BrdUrd-positive cells in day-12 pregnant (D12P) mice (D) and apoptotic cells (arrows) in day-15 pregnant (D15P) mammary glands of control (FVB/N) and transgenic (AAD) mice (E). Scale bars represent 100 μm. The percentage of BrdUrd and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling-positive cells in FVB/N (open bars) and AAD (filled bars) mammary glands is shown during different stages of mammary gland development (D and E Bottom).
Decreased Proliferation and Enhanced Apoptosis Contribute to a Lactaton-Deficient Phenotype in TβRI(AAD) Mice. Although TβRII(ΔCyt) expression had no discernable phenotype through different stages of mammary gland development in 8- to 12-week-old females (data not shown), TβRI(AAD) expression resulted in a lactation-deficient phenotype. We routinely observed the death of two to three pups from TβRI(AAD) litters within days after birth, and the remaining pups reared by TβRI(AAD) dams displayed reduced body weights compared with pups nursed from nontransgenic dams. Moreover, we observed reduced levels of WAP expression throughout pregnancy in the TβRI(AAD) mice (Fig. 8, which is published as supporting information on the PNAS web site). Examination of mammary gland whole mounts from virgin females (Fig. 9, which is published as supporting information on the PNAS web site) or those taken at early time points during pregnancy did not reveal significant differences between TβRI(AAD) animals and controls. However, a reduction in the amount of mammary epithelium became apparent late during pregnancy (day 15), with a dramatic reduction evident in the lactating glands of TβRI(AAD) females compared with nontransgenic controls (Fig. 1C Left), which was confirmed by histological analyses (Fig. 1C Right). In nontransgenic females, epithelial cell proliferation and differentiation produced lobulo-alveolar structures that completely filled the fat pad with fully dilated lumens containing secretory material at lactation (Fig. 1C, FVB/N, L.M.GL.). In contrast, the lactating glands of transgenic animals were composed of discrete epithelial islands that failed to fill the fat pad and were composed of dilated lumens of which many were devoid of secretory material (Fig. 1C, AAD, L.M.GL.).
Mammary epithelial proliferation in vivo was assessed by BrdUrd incorporation into DNA, revealing that 3-fold more mammary cells were proliferating in nontransgenic mice at day 12 during pregnancy compared with TβRI(AAD) females (Fig. 1D). In addition, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling analysis revealed a marked wave of apoptosis occurred at day 15 during pregnancy in TβRI(AAD) mice that was not evident in the FVB/N control animals (Fig. 1E). These data suggest that the decreased lobulo-alveolar density in late pregnant and lactating mammary glands of TβRI(AAD) transgenic mice results from decreased proliferation and increased apoptosis of mammary epithelial cells.
Suppression of Neu-Coupled Grb2 and Shc-Induced Mammary Tumorigenesis by TGF-β Signaling. To assess the involvement of the TGF-β pathway in both mammary tumor formation and subsequent lung metastasis, MMTV/TβRI(AAD) and MMTV/TβRII(ΔCyt) mice were bred with transgenic mice expressing oncogenic variants of the Neu receptor tyrosine kinase. Two transgenic strains, MMTV/Neu(YB) and MMTV/Neu(YD), express Neu receptors harboring a deletion within their extracellular domains, which oncogenically activates the receptors (12, 16). Moreover, autophosphorylation sites within the cytoplasmic tails of these receptors contain tyrosine to phenylalanine substitutions that impair their ability to activate the full spectrum of downstream signaling pathways normally engaged by Neu. Single tyrosine residues were reintroduced within the Neu cytoplasmic tail to individually restore the Grb2 (YB) and Shc (YD) signaling pathways (16, 29) (Fig. 2A).
Fig. 2.
TGF-β signaling delays Neu-induced mammary tumorigenesis. (A) The Neu receptors harbor an oncogenic cysteine deletion within the extracellular domain (star) and tyrosine to phenylalanine substitutions of autophosphorylation sites within the cytoplasmic tail. A single phenylalanine was reverted back to a tyrosine to restore either the Shc (YD) or Grb2 (YB) binding site. (B) Kinetics of mammary tumor formation in Neu(YD) versus YD/AAD mice (P = 0.0001, Student's t test). (C) Mammary tumor onset in Neu(YB) versus YB/AAD (P = 0.0002, Student's t test) and Neu(YB) versus YB/ΔCyt (P = 0.04, Student's t test) transgenic mice. Age of onset is the time that a palpable mammary tumor first appears. T50 denotes the age at which 50% of the mice first possess a tumor, and n is the number of mice examined.
Importantly, both Neu(YB) and Neu(YD) transgenic mice exhibit mammary tumor phenotypes and metastatic potentials that differ in severity. Neu(YD) mice develop multifocal mammary tumors with a relatively low frequency of animals (19%) possessing lung metastases. In contrast, the latency of Neu(YB)-induced mammary tumors is increased and the tumor burden is reduced, yet 67% of these animals develop lung metastases (16). The different rates of primary tumor growth and metastatic abilities displayed by these transgenic strains provide good model systems for examining whether activation or attenuation of the TGF-β pathway can alter the formation of Neu-induced primary mammary tumors or subsequent lung metastases.
Constitutive activation of the TGF-β pathway in either a Neu(YB) or Neu(YD) genetic background delayed the onset of mammary tumors relative to the respective Neu monogenic controls (Fig. 2 B and C). In contrast, impairment of endogenous TGF-β signaling accelerated the kinetics of Neu(YB) mammary tumor formation compared with expression of Neu(YB) alone (Fig. 2C). Moreover, the observed differences in tumor kinetics do not reflect altered neu transgene expression (Fig. 10, which is published as supporting information on the PNAS web site) nor are they associated with different histological appearance of the tumors (data not shown) in bigenic strains compared with the respective monogenic controls. Therefore, TGF-β signaling functions to impair Neu-induced primary breast tumor formation in the mammary glands of these transgenic mice.
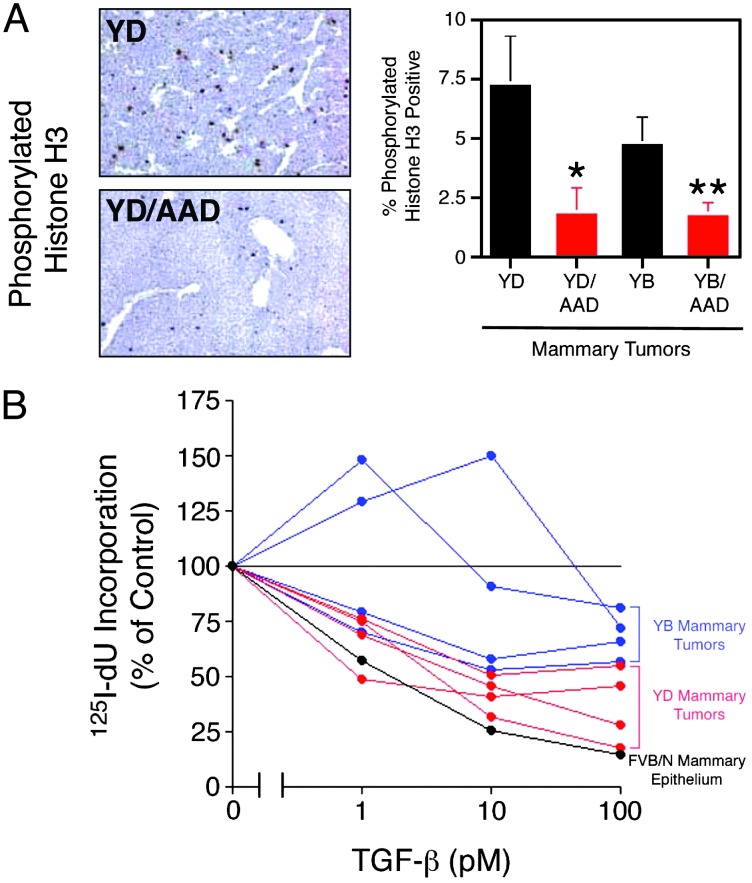
To determine whether inhibition of mammary tumorigenesis in these strains reflects TGF-β cytostatic effects, proliferation of tumor cells was determined in situ by immunohistochemical staining for phosphorylated histone H3, a mitotic cell marker (30). We observed a 4-fold reduction in the percentage of tumor cells undergoing mitosis in the YD/AAD bigenics versus Neu(YD) controls (Fig. 3A). TβRI(AAD) expression also resulted in an ≈3-fold reduction in the percentage of proliferating cells in a Neu(YB) genetic background relative to monogenic controls (Fig. 3A). However, TβRII(ΔCyt) did not significantly alter the mitotic potential of Neu(YB) mammary tumors (data not shown).
Fig. 3.
Neu(YD) and Neu(YB) mammary tumor growth is inhibited by TGF-β. (A) Immunostaining of phosphorylated histone H3 reveals a significant reduction in the percentage of mitotic cells in YD/AAD versus Neu(YD) (*, P = 0.04, Student's t test) and YB/AAD versus Neu(YB) (**, P = 0.01, Student's t test) mammary tumors. (B) Primary mammary tumor cultures from four individual Neu(YD) (red) and Neu(YB) (blue) mice were treated with increasing concentrations of TGF-β in the presence of 125I-deoxyuridine, and the percentage incorporation into DNA relative to control cells without TGF-β stimulation is shown (data are representative of two independent experiments performed with triplicate cultures). Primary mammary epithelial cells isolated from mid-pregnant, nontransgenic mammary glands were included as a control (FVB/N, black). In each case, standard deviations did not exceed 18% of the average value.
Mammary tumors were also explanted from four independent Neu(YB) and Neu(YD) mice and analyzed for their growth inhibitory response to TGF-β in vitro. Individual Neu(YB) and Neu(YD) mammary tumors were growth arrested by TGF-β in a dose-dependent manner (Fig. 3B). Mammary tumor cultures from Neu(YD) transgenic mice were more sensitive to TGF-β-mediated growth arrest compared with Neu(YB)-expressing mammary tumors (Fig. 3B). The ability of TβRII(ΔCyt) to accelerate mammary tumor formation in vivo may result from the impairment of endogenous TGF-β signaling that exists in these tumors. Indeed, both Neu(YB) and Neu(YD) mammary tumors express transcripts for all three TGF-β isoforms (Fig. 11, which is published as supporting information on the PNAS web site). Therefore, Neu-induced mammary tumor cells express a functional TGF-β pathway, rendering them sensitive to TGF-β-mediated growth suppression and impaired mammary tumor formation.
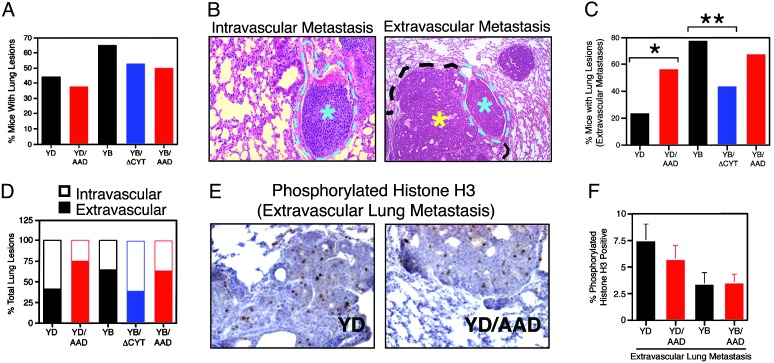
TGF-β Promotes Lung Metastasis of Neu-Induced Mammary Tumors. Neu(YB)- and Neu(YD)-expressing mammary tumors differ in their metastatic potential to the lung, with Neu(YB) transgenic mice exhibiting the more aggressive phenotype (16). We observed that 65% of Neu(YB) and 44% of Neu(YD) transgenic animals, that were 2 months tumor bearing, possessed tumor foci in the lungs (Fig. 4A). The incidence of total lung lesions was not significantly altered in any of the bigenic strains compared with that observed in the Neu monogenic controls (Fig. 4A).
Fig. 4.
TGF-β signaling enhances the formation of extravascular lung metastases. (A) Percentage of mice with lung lesions; Neu(YD), n = 27; YD/AAD, n = 24; Neu(YB), n = 20; YB/ΔCyt, n = 15; and YB/AAD, n = 12. (B) Lesions were classified as intravascular (light blue asterisk) if they remained confined within a blood vessel (outlined by light blue dotted line) or extravascular (yellow asterisk) if the tumor cells had breached the vessel wall and were growing into the lung parenchyma. (C) The percentage of mice harboring lung lesions that contained extravascular metastases: Neu(YD) versus YD/AAD (*, mid P = 0.05, Fisher's exact test) and Neu(YB) versus YB/ΔCyt (**, mid P = 0.004; Fisher's exact test). (D) The percentage of total lung lesions was segregated into intravascular or extravascular metastases by histological examination (a minimum of 80 lung lesions was scored from each genotype). (E) Representative sections illustrating phosphorylated histone H3-positive cells in Neu(YD) versus YD/AAD extravasated lung lesions. (F) Quantitation of the percentage proliferating cells in extravascular lung lesions from the indicated genotypes.
Upon closer examination, the observed lung lesions fell into two categories: intravascular metastases representing tumor cells that remain fully contained within a pulmonary vessel and extravascular metastases that represent a group of tumor cells that have extravasated from the pulmonary vessel and colonized the lung parenchyma (Fig. 4B). When the mice with lung lesions were further analyzed, we observed a significant increase in the percentage of YD/AAD transgenic mice with extravascular lung metastases relative to Neu(YD) controls (Fig. 4C). Whereas expression of TβRI(AAD) did not alter the incidence of extravascular metastases in YB/AAD mice (Fig. 4C), expression of TβRII(ΔCyt) significantly decreased the percentage of extravascular metastases in a Neu(YB) genetic background (Fig. 4C). When the total number of lung lesions was quantified, we observed that coexpression of TβRI(AAD) and Neu(YD) increased the frequency of extravascular metastases compared with Neu(YD) controls but did not significantly alter the percentage of extravascular lung metastases in a Neu(YB) genetic background (Fig. 4D). However, inhibition of endogenous TGF-β signaling significantly impaired the ability of Neu(YB)-expressing mammary tumors to metastasize to the lung (Fig. 4D).
Interestingly, when proliferation within extravascular lung metastases was assessed by anti-phospho-histone H3 immunohistochemistry, no significant changes were observed between Neu(YD) and YD/AAD transgenic strains (Fig. 4 E and F). Likewise, the percentage of proliferating cells was very similar in extravascular lung lesions arising in Neu(YB), YB/AAD and YB/ΔCyt transgenic mice (Fig. 4F, data not shown). Therefore, despite suppressive effects within the primary tumor (Fig. 3A), TGF-β signaling facilitates the formation of extravascular pulmonary metastases that are not growth inhibited in the lung parenchyma (Fig. 4F).
Discussion
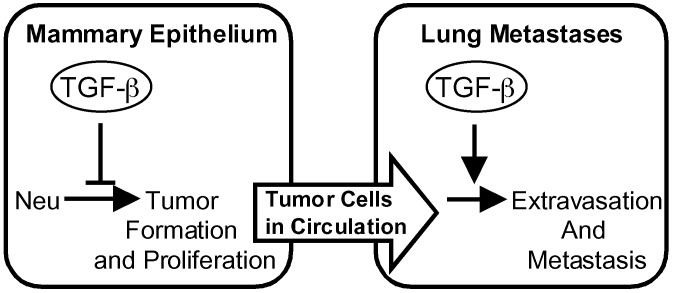
TGF-β signaling controls the growth of the normal mammary gland during various stages of development (31). Although forced expression of an activated TβRI resulted in underdeveloped mammary glands during pregnancy and a lactation-deficient phenotype, the expression of this construct or a dominant negative TβRII did not result in discernable phenotypic changes in the mammary glands of virgin mice during the time frame analyzed. These phenotypes are less severe than those described for transgenic mice expressing TGF-β or a dominant negative TβRII in the mammary epithelium (32–35). Nonetheless, our results show that this level of TGF-β1 signaling can impede mammary tumor formation by oncogenic versions of Neu. The constitutively activated TβRI delayed the onset of mammary tumors induced by Neu receptors that couple to either the Grb2(YB) or Shc(YD) adaptor molecules. Despite these tumor suppressive effects, TGF-β signaling ultimately facilitates tumor progression by enhancing the ability of Neu-induced tumors to form extravascular pulmonary metastases (Fig. 5).
Fig. 5.
TGF-β enhances lung metastases despite suppressive effects on primary tumor formation. The onset of Neu-induced mammary tumors is delayed by expression of an activated TβRI and accelerated by a dominant negative TβRII. However, activation of TGF-β signaling enhances extravasation of Neu-induced mammary tumor cells into lung tissue, which can be impaired by inhibition of the TGF-β pathway.
Previous studies have suggested that loss of TGF-β function can accelerate tumorigenesis in diverse model systems. Expression of a dominant negative type II receptor can accelerate the onset of carcinogen-induced tumors in a variety of tissues, including the mammary gland (34, 35). In the present study, we demonstrate the ability of enforced TGF-β signaling to oppose the oncogenic ability of Neu receptors that use distinct signaling pathways for transformation. Moreover, the ability of a dominant negative TβRII to accelerate Neu-induced tumorigenesis demonstrates that the endogenous TGF-β pathway can suppress tumor outgrowth.
Recently, a fusion protein consisting of the extracellular domain of TβRII linked to the Fc component of IgG (Fc:TβRII) was able to suppress lung metastases in both polyoma virus Middle T-induced and Neu-induced mammary tumors but, unlike our studies, no effect was observed on the growth of the primary tumor (36, 37). The failure of the Fc:TβRII antagonist to potentiate polyoma virus Middle T-induced tumorigenesis may reflect the fact that these are aggressively growing tumors that have already lost their sensitivity to TGF-β-mediated growth arrest (36). Therefore, disruption of endogenous TGF-β signaling would not enhance mammary tumor formation in this mouse model. Fc:TβRII locally expressed in the mammary epithelium as a transgene did not affect the growth of Neu-induced mammary tumors (37).
In our study, in vivo tumor cell proliferation induced by Neu(YD) and Neu(YB) was suppressed in the presence of TβRI(AAD) whereas the latency of tumor formation in Neu(YB) mice was decreased by TβRII(ΔCyt). In addition, primary tumor cultures expressing Neu(YD), which signals through Shc, were sensitive to growth inhibition by TGF-β. Interestingly, Neu(YB), which signals through Grb2, conferred partial resistance to growth inhibition by TGF-β in vitro. Despite these suppressive effects on the primary tumor, TβRI(AAD) can no longer reduce the proliferative potential of Neu-induced tumor cells that formed extravasated lung metastases. It should be noted that although TβRI(AAD) is capable of signaling in the absence of TGF-β, the activity of this receptor can be further enhanced by TGF-β stimulation (data not shown). Thus, it is possible that TβRI(AAD) further sensitizes the tumor cells to the action of TGF-β present in the microenvironment by increasing the levels of TβRI on their cell surface. Once the tumor cells reach the lung, the reduced local concentrations of TGF-β may be insufficient to effectively inhibit the outgrowth of the metastatic tumor cells. Alternatively, tumor cells that colonize the lung may have acquired additional mutations that render them insensitive to the growth inhibitory effects of TβRI(AAD), including c-myc overexpression or the loss of cdk inhibitors such as p15Ink4b or p21Cip1.
Our results indicate that exogenous TGF-β signaling enhances the formation of lung metastases whereas inhibition of the endogenous TGF-β pathway can suppress lung metastasis of Neu-induced tumors. Although the percentage of mice developing lung lesions was not significantly altered in monogenic versus bigenic animals, the percentage of lesions representing extravasated metastases was higher in the presence of TβRI(AAD), at least when coexpressed with Neu(YD). Conversely, expression of TβRII(ΔCyt) resulted in a greater proportion of Neu(YB)-induced tumor cell lesions remaining trapped within pulmonary vessels. These results argue that the importance of TGF-β signaling within the tumor is to specifically enhance the ability of the tumor cells to extravasate into the lung or promote their growth in the lung parenchyma (Fig. 5). The similar mitotic potential of cells within extravascular lesions, in the absence of significant levels of apoptosis (data not shown), in monogenic and bigenic animals suggests that TGF-β signaling plays an important role in promoting tumor cell extravasation. TGF-β signaling may facilitate tumor cell extravasation in a cell autonomous fashion by inducing the expression of proinvasive and prometastatic gene products, such as integrins and matrix metalloproteinases (38, 39).
The pleiotropic effects of TGF-β on diverse cell types contributes to multiple steps during the metastatic cascade for those tumor cells that have escaped the growth inhibitory effects of this cytokine (40). With the present observation, we define a prometastatic function for TGF-β during the formation of pulmonary metastases by specifically enhancing the ability of mammary tumor cells to extravasate into the lung parenchyma.
Supplementary Material
Acknowledgments
We are indebted to Ye-Guang Chen for plasmids used in this study. We thank Willie Mark and the Memorial Sloan-Kettering Cancer Center transgenic core facility for performing DNA microinjections, Katia Manova and members of the Memorial Sloan-Kettering Cancer Center cytology core facility for histological and immunohistochemical assistance, Robert Munn and Carlos Cordon-Cardo for photographic assistance, and members of the Massagué laboratory for their helpful insights and discussions. This work was supported by National Institutes of Health Grant P01-CA94060 (to J.M.) and Grant U42-RR14905 from the National Institutes of Health, National Regional Resource Centers program (to R.D.C.). P.M.S. was supported by a fellowship from the Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation (DRG-1532), and J.M. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: TGF-β, transforming growth factor β; TβRI, TGF-β type I receptor; TβRII, TGF-β type II receptor; MMTV, mouse mammary tumor virus.
References
- 1.Massague, J., Blain, S. W. & Lo, R. S. (2000) Cell 103 295-309. [DOI] [PubMed] [Google Scholar]
- 2.Gold, L. I. (1999) Crit. Rev. Oncog. 10 303-360. [PubMed] [Google Scholar]
- 3.Oft, M., Peli, J., Rudaz, C., Schwarz, H., Beug, H. & Reichmann, E. (1996) Genes Dev. 10 2462-2477. [DOI] [PubMed] [Google Scholar]
- 4.Portella, G., Cumming, S. A., Liddell, J., Cui, W., Ireland, H., Akhurst, R. J. & Balmain, A. (1998) Cell Growth Differ. 9 393-404. [PubMed] [Google Scholar]
- 5.Gorsch, S. M., Memoli, V. A., Stukel, T. A., Gold, L. I. & Arrick, B. A. (1992) Cancer Res. 52 6949-6952. [PubMed] [Google Scholar]
- 6.Walker, R. A. & Dearing, S. J. (1992) Eur. J. Cancer 28 641-644. [DOI] [PubMed] [Google Scholar]
- 7.Derynck, R., Akhurst, R. J. & Balmain, A. (2001) Nat. Genet. 29 117-129. [DOI] [PubMed] [Google Scholar]
- 8.Letterio, J. J. & Roberts, A. B. (1998) Annu. Rev. Immunol. 16 137-161. [DOI] [PubMed] [Google Scholar]
- 9.Yin, J. J., Selander, K., Chirgwin, J. M., Dallas, M., Grubbs, B. G., Wieser, R., Massague, J., Mundy, G. R. & Guise, T. A. (1999) J. Clin. Invest. 103 197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers, A. F., Groom, A. C. & MacDonald, I. C. (2002) Nat. Rev. Cancer 2 563-572. [DOI] [PubMed] [Google Scholar]
- 11.Guy, C. T., Webster, M. A., Schaller, M., Parsons, T. J., Cardiff, R. D. & Muller, W. J. (1992) Proc. Natl. Acad. Sci. USA 89 10578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel, P. M., Ryan, E. D., Cardiff, R. D. & Muller, W. J. (1999) EMBO J. 18 2149-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller, W. J., Sinn, E., Pattengale, P. K., Wallace, R. & Leder, P. (1988) Cell 54 105-115. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard, L., Lamarre, L., Tremblay, P. J. & Jolicoeur, P. (1989) Cell 57 931-936. [DOI] [PubMed] [Google Scholar]
- 15.Hynes, N. E. & Stern, D. F. (1994) Biochim. Biophys. Acta 1198 165-184. [DOI] [PubMed] [Google Scholar]
- 16.Dankort, D., Maslikowski, B., Warner, N., Kanno, N., Kim, H., Wang, Z., Moran, M. F., Oshima, R. G., Cardiff, R. D. & Muller, W. J. (2001) Mol. Cell. Biol. 21 1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guy, C. T., Cardiff, R. D. & Muller, W. J. (1992) Mol. Cell. Biol. 12 954-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pullan, S. & Streuli, C. H. (1996) in Epithelial Cell Culture, ed. Harris, A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 97-121.
- 19.Carcamo, J., Zentella, A. & Massague, J. (1995) Mol. Cell. Biol. 15 1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster, M. A., Hutchinson, J. N., Rauh, M. J., Muthuswamy, S. K., Anton, M., Tortorice, C. G., Cardiff, R. D., Graham, F. L., Hassell, J. A. & Muller, W. J. (1998) Mol. Cell. Biol. 18 2344-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonderhaar, B. K. & Greco, A. E. (1979) Endocrinology 104 409-418. [DOI] [PubMed] [Google Scholar]
- 22.Manova, K., Tomihara-Newberger, C., Wang, S., Godelman, A., Kalantry, S., Witty-Blease, K., De Leon, V., Chen, W. S., Lacy, E. & Bachvarova, R. F. (1998) Dev. Dyn. 213 293-308. [DOI] [PubMed] [Google Scholar]
- 23.Gavrieli, Y., Sherman, Y. & Ben-Sasson, S. A. (1992) J. Cell Biol. 119 493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieser, R., Wrana, J. L. & Massague, J. (1995) EMBO J. 14 2199-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huse, M., Chen, Y. G., Massague, J. & Kuriyan, J. (1999) Cell 96 425-436. [DOI] [PubMed] [Google Scholar]
- 26.Chen, Y. G., Liu, F. & Massague, J. (1997) EMBO J. 16 3866-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charng, M. J., Kinnunen, P., Hawker, J., Brand, T. & Schneider, M. D. (1996) J. Biol. Chem. 271 22941-22944. [DOI] [PubMed] [Google Scholar]
- 28.Wieser, R., Attisano, L., Wrana, J. L. & Massagué, J. (1993) Mol. Cell. Biol. 13 7239-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dankort, D. L., Wang, Z., Blackmore, V., Moran, M. F. & Muller, W. J. (1997) Mol. Cell. Biol. 17 5410-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Absi, N. R. & Srienc, F. (2002) J. Biotechnol. 95 63-84. [DOI] [PubMed] [Google Scholar]
- 31.Wakefield, L. M., Yang, Y. A. & Dukhanina, O. (2000) Breast Cancer Res. 2 100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce, D. F., Jr., Johnson, M. D., Matsui, Y., Robinson, S. D., Gold, L. I., Purchio, A. F., Daniel, C. W., Hogan, B. L. & Moses, H. L. (1993) Genes Dev. 7 2308-2317. [DOI] [PubMed] [Google Scholar]
- 33.Jhappan, C., Geiser, A. G., Kordon, E. C., Bagheri, D., Hennighausen, L., Roberts, A. B., Smith, G. H. & Merlino, G. (1993) EMBO J. 12 1835-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bottinger, E. P., Jakubczak, J. L., Haines, D. C., Bagnall, K. & Wakefield, L. M. (1997) Cancer Res. 57 5564-5570. [PubMed] [Google Scholar]
- 35.Gorska, A. E., Joseph, H., Derynck, R., Moses, H. L. & Serra, R. (1998) Cell Growth Differ. 9 229-238. [PubMed] [Google Scholar]
- 36.Muraoka, R. S., Dumont, N., Ritter, C. A., Dugger, T. C., Brantley, D. M., Chen, J., Easterly, E., Roebuck, L. R., Ryan, S., Gotwals, P. J., et al. (2002) J. Clin. Invest. 109 1551-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, Y. A., Dukhanina, O., Tang, B., Mamura, M., Letterio, J. J., MacGregor, J., Patel, S. C., Khozin, S., Liu, Z. Y., Green, J., et al. (2002) J. Clin. Invest. 109 1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duivenvoorden, W. C., Hirte, H. W. & Singh, G. (1999) Clin. Exp. Metastasis 17 27-34. [DOI] [PubMed] [Google Scholar]
- 39.Janji, B., Melchior, C., Gouon, V., Vallar, L. & Kieffer, N. (1999) Int. J. Cancer 83 255-262. [DOI] [PubMed] [Google Scholar]
- 40.Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C., Guise, T. A. & Massagué, J. (2003) Cancer Cell, in press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.