Abstract
Although HIV type 1 (HIV-1) cannot efficiently replicate in simian cells, the mechanism(s) involved in the restriction of virus tropism remain unclear. To investigate this, we have focused on the identification of human cellular factors that can influence the infectivity of HIV-1 derived from African green monkey producer cells. Whereas the infectivity of HIV-1 derived from such cells was only 10–15% of that of human cell-derived virus, expression of human topoisomerase I in the African green monkey cells resulted in a 5-fold increase of the infectivity of progeny HIV-1 virions. Replacement of glutamate-236 and asparagine-237 of human topoisomerase I with the corresponding residues (aspartate and serine, respectively) of the African green monkey enzyme abolished this enhancement of HIV-1 infectivity. This positive effect of human topoisomerase I expression in the African green monkey producer cells seemed to result from the promotion of HIV-1 cDNA synthesis. Thus, human topoisomerase I plays an important role in HIV-1 replication and infectivity, and differences in the species specificity of HIV-1 infection can at least in part be attributed to differences in topoisomerase I activities.
Keywords: African green monkey, simian
In a strictly limited number of animal species, including chimpanzee (1), gibbon (2), and the pigtailed monkey (3), HIV type 1 (HIV-1) is able to infect and replicate. It is now clear that HIV-1 replication would appear in part to depend on a number of host cellular factors. These include the cellular protein Crm1, which mediates the interaction between HIV-1 Rev protein and the nuclear pore complex during export of Rev and incompletely spliced viral transcripts from the nucleus (4). Interactions between the cellular Tsg101 (tumor susceptibility gene 101) protein and the PTAP motif present in the p6 domain of HIV-1 Gag are required for viral budding from the cell membrane (5). Furthermore, the host RNase L inhibitor, HP68, seems to play a role in the conversion of viral assembly intermediates into complete immature capsids (6). Transcription from an integrated HIV-1 long-terminal repeat in murine cells, in which HIV-1 fails to replicate and assemble, is markedly increased by expression of human cyclin T1 (7–9). However, it is unknown if human Crm1, Tsg101, or HP68 might promote HIV-1 replication when expressed in rodent cells as occurs with cyclin T1. Like rodent cells, simian cells do not allow efficient HIV-1 replication (10). Whereas pseudotype viruses of HIV-1 with the vesicular stomatitis virus glycoprotein (VSV-G) are able to enter simian cells, they do not produce infectious HIV-1 (11). However, viral transcription, processing, and assembly are not completely suppressed in simian cells, as HIV-1 virions have been prepared in COS cells (12).
The replication of HIV-1 at postentry steps would seem to be regulated either by a cellular factor(s) present in virions from the producer cell or by a factor(s) present in the target cells, and it is possible that these could play a role in restricting viral tropism. HIV-1 and other retroviral particles contain a number of cellular components, including topoisomerase I (Topo I), which are incorporated from the host cell during replication (13). We have previously shown that Topo I is associated with the HIV-1 Gag protein and enhances HIV-1 cDNA synthesis in vitro (14). In addition, expression of a Topo I mutant that lacks ligation activity in HIV-1 producer cells reduced the infectivity of the resulting virions in target cells and also suppressed viral cDNA synthesis in an endogenous reverse transcription assay (15).
We now show that the infectivity of HIV-1 virions derived from African green monkey cells is markedly reduced compared with that of human cell-derived virus and that expression of human Topo I in HIV-1-producing African green monkey cells greatly increased the infectivity titres of progeny virions. Furthermore, we show that Glu-236 and Asn-237 residues of human Topo I are key determinants of the enhancement of HIV-1 infectivity. Expression of human Topo I in African green monkey cells did not affect the stability of HIV-1 genomic RNA, viral processing, virion production, or viral entry but rather increased the efficiency of HIV-1 cDNA synthesis in target cells. These results suggest that Topo I activity is, at least in part, involved in the determination of the species specificity of HIV-1 infection.
Materials and Methods
Cell Culture. The human cell lines 293T (16), HeLa and SW480 (17), MAGIC5 cells (18), and the monkey cell lines COS-1 (19), CV-1 (20), and Vero were maintained under an atmosphere of 5% CO2 at 37°C in DMEM supplemented with 10% FBS.
Transfection and Luciferase Reporter Virus Assay. For preparation of virus stocks, human or African green monkey cells were transfected with an infectious HIV-1 DNA clone based on pNL4–3 (21), designated pNL-Luc-HXB, which contains HXB3 env and in which the nef gene has been replaced with the firefly luciferase gene (22). The amount of virus in culture supernatants of the human or African green monkey cells was quantified 48 h after transfection by measurement of p24 antigen with a p24 Gag antigen capture ELISA (ZeptoMetrix, Buffalo, NY). Equal amounts of each virus were then used to infect MAGIC5 cells. VSV-G-pseudotyped HIV-1 was produced by cotransfection of cells with the pNL-Luc-E-R+ proviral clone (23) and the VSV-G expression vector pHIT/G (24). For luciferase reporter virus assays, MAGIC5 cells (3 × 104) were infected overnight with 10 ng of p24 antigen of the luciferase reporter virus. After culture for an additional 48 h, the cells were lysed, and luciferase activity was determined (25) with a Lumat LB 96V luminometer (Perkin–Elmer).
Cloning of African Green Monkey Topo I cDNA. Polyadenylated RNA was isolated from Vero cells and subjected to reverse transcription with SuperScript RNaseH- reverse transcriptase (Invitrogen) and the oligonucleotide topo2668R (5′-TGGAGATATTATAAGGGGAGAGCTGAGCC-3′) as a primer. The coding region of African green monkey Topo I was then amplified from the resulting cDNA by the PCR with the primers topoF (5′-ATGAGTGGGGACCACCTCCACAACGA-3′) and topoR (5′-CTAAAACTCATAGTCTTCATCAGCCA-3′). The resulting 2.3-kb PCR product was subcloned into the pSTblue-1 cloning vector (Novagen) and sequenced with a model 377A DNA sequencer (Perkin–Elmer). The BamHI–NotI fragment of the PCR product was cloned into pcDNA4/HisMax-C (Invitrogen), a mammalian expression vector containing the Xpress epitope tag sequence, and the resulting construct was designated pcSimWT.
Mutagenesis of Human Topo I cDNA and Plasmid Construction. The full-length human Topo I cDNA was amplified by PCR with phtop1 (26) as a template and the primers topoEcoRI and topoNotI (5′-AGTGAATTCATGAGTGGGGACCACCTCCAC-3′ and 5′-ACGTGCGGCCGCCTA A A ACTCATAGTCTTC-3′, respectively; the restriction sites for EcoRI and NotI are underlined). The PCR product was digested with EcoRI and NotI and then subcloned into pcDNA4/HisMax-C, yielding pcHuWT. The point mutations V145A, T157I, and E236D/ N237S were introduced into pcHuWT with the use of a QuikChange site-directed mutagenesis kit (Stratagene) and the primer pairs (sense and antisense, respectively) 5′-GATGAGGATGATGTTGATTATAAACCT-3′ and 5′-AGGTTTATAATCAACATCATCCTCATC-3′, 5′-AAAACAGAAGATATCAAGAAGGAGAAG-3′ and 5′-CTTCTCCTTCTTGATATCTTCTGTTTT-3′, and 5′-TATGAGCCTCTTCCAGACAGTGTCAAGTTT-3′ and 5′-AAACTTGACACTGTCTGGAAGAGGCTCATA-3′, respectively. The mutated expression vectors were designated pcV145A, pcT157I, and pcE236D/N237S, respectively. An Asp–Lys insertion between residues 40 and 41 of human Topo I was introduced into pcHuWT by the overlap extension method (27). In brief, the primers pSTB 1F (5′-AAAAAAAAAGAATTCATGAGTGGGGACCAC-3′), SImuPCR R (5′-GGCTTTATATCCTCTTTAGGAGGAACAAAA-3′), SImuPCR F (5′-TTTTGTTCCTCCTAAAGAGGATATAAAGCC-3′), and TOPHin R (5′-AAAAAAAAAAAGCTTGTCGATGAAGTACAG-3′) were used to amplify two insertion fragments, which were then combined in a subsequent PCR. The EcoRI–HindIII fragment of the final PCR product was cloned into pcDNA4/HisMax-C to yield pcIns40/ 41. The vector pcSimC was generated by replacing the EcoRI-HindIII fragment of pcSimWT with that of pcHuWT. Similarly, the sequence encoding the NH2-terminal region of human Topo I in pcHuWT was replaced by the corresponding region of pcSimWT to yield pcSimN.
Antibodies. For detection of Pr55gag, Gag intermediates, p24 capsid protein, and p17 matrix protein, we used a mixture of mouse monoclonal antibodies to p24 (1E4) (28) and to p17 (4D9) (28). Sera of patients infected with HIV-1 and horseradish peroxidase-conjugated sheep antibodies to human IgG (Amersham Pharmacia) were used to detect HIV-1 gp160 and gp120.
Quantitation of Synthesized cDNA. Viral stocks were treated with 75 units of DNase I before infection to digest any remaining plasmid DNA. MAGIC5 cells (1.5 × 105) were infected overnight with 50 ng of p24 antigen of NL-Luc-HXB, and, after culture for 48 h, low molecular weight DNA was extracted from the infected cells (29) and resuspended in Tris-EDTA buffer containing RNase A (10 μg/ml). The DNA was then subjected to real-time PCR for 50 cycles with the primers NL-R 1F (5′-CTCTCTGGTTAGACCAGATCTGAGCCTGGG-3′; corresponding to nucleotides 458–487 of pNL4-3) and J2 (5′-GCCGTGCGCGCTTCAGCAAGC-3′; nucleotides 701–721) (30), a QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA), using a LightCycler instrument (Roche Diagnostics).
Analysis of RNA Stability. COS-1 cells (1.5 × 105) were cotransfected with pNL-Luc-HXB and various Topo I expression vectors. After 36 h, the cells were incubated with actinomycin D (10 μg/ml) to arrest transcription, and total RNA was extracted at various times. Extracted RNA was then subjected to reverse transcriptase real-time PCR with the primers NL-R 1F and J2.
Results
Enhancement of HIV-1 Infectivity by Human Topo I. To investigate potential mechanisms for the preferential replication of HIV-1 in human cells, we focused our investigations on cellular factors present in HIV-1 producer cells. We first examined the infectivity of HIV-1 derived from both human and African green monkey cells. Given that cell type-specific differences in HIV-1 infectivity have been described (31, 32), we used three different human (293T, HeLa, and SW480) and African green monkey (COS-1, CV-1, and Vero) cell lines as viral producer cells. Cells were transfected with an HIV-1 proviral DNA construct (pNL-Luc-HXB) in which the nef gene was replaced with the firefly luciferase gene, and the infectivity of the resulting viruses was determined in human MAGIC5 cells. The luciferase activity of MAGIC5 cells infected with viruses derived from human cells was seven to nine times that of cells infected with HIV-1 derived from African green monkey cells (Fig. 1), suggesting that a human cellular factor (or factors) in the viral producer cells is responsible for the enhancement of HIV-1 infectivity in the indicator cells.
Fig. 1.
Infectivity of human or African green monkey cell-derived HIV-1 in MAGIC5 cells. Human (293T, HeLa, and SW480) and African green monkey (COS-1, CV-1, and Vero) cells were transfected with a HIV-1 proviral DNA construct harboring the firefly luciferase gene in place of nef (pNL-Luc-HXB). Equal amounts of the resulting viruses were incubated with MAGIC5 cells, and the luciferase activity of cell lysates was subsequently determined. Data are expressed as relative light units (RLU) and are means ± SD of values from three independent experiments.
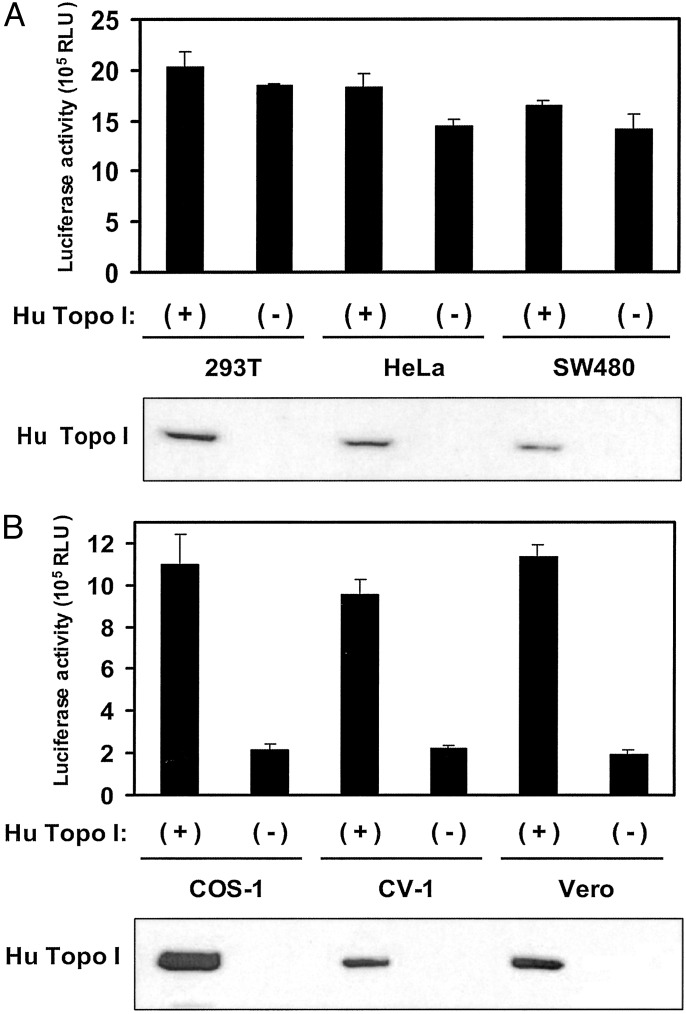
We investigated whether human Topo I could be this factor, as this protein is known to be incorporated into viral particles (13) and has been shown to enhance reverse transcription activity of HIV-1 in vitro (14). In addition, expression of a Topo I mutant that lacks ligation activity in HIV-1-producing cells resulted in a 75% decrease in infectivity compared with that for virus derived from cells expressing wild-type Topo I (15). Expression of recombinant human Topo I in human producer cells only minimally enhanced the infectivity of the resulting HIV-1 in MAGIC5 cells (Fig. 2A). In contrast, the expression of human Topo I in all three African green monkey cell types resulted in a 4- to 5-fold enhancement of viral infectivity (Fig. 2B).
Fig. 2.
Effect of human Topo I on the infectivity of HIV-1 derived from producer cells. Human (A) and African green monkey (B) cells were cotransfected with pNL-Luc-HXB and either a human (Hu) Topo I expression vector (+) or the corresponding empty vector (-). Equal amounts of the resulting viruses were incubated with MAGIC5 cells, and the luciferase activity of cell lysates was subsequently determined. Data are expressed are means ± SD of values from three independent experiments. The expression of ectopic human Topo I in the producer cells was also detected by immunoblot analysis with antibodies to the Xpress epitope tag (shown below each histogram).
To determine whether similar results could be obtained in nontransformed cells, we transfected pNL-Luc-HXB into primary fibroblasts derived from lung or kidney of macaque monkeys. However, neither of these cells was found to produce detectable levels of progeny virus, and as such this question could not be satisfactorily resolved (data not shown).
Role of Glu-236 and Asn-237 of Human Topo I in HIV-1 Replication. To identify the amino acid residues in human Topo I responsible for the restoration of infectivity of HIV-1 derived from African green monkey cells, we isolated a cDNA for African green monkey Topo I (sTopo I) and compared its predicted amino acid sequence with that of human (Fig. 3). sTopo I was found to share 98.4% amino acid sequence identity with the human protein, differing in only eight amino acids (and in 47 nucleotides).
Fig. 3.
Comparison of the predicted amino acid sequences of human and African green monkey Topo I. The eight amino acids that differ between the two proteins are boxed. Numbers above the sequences refer to the residue positions of human Topo I. The cDNA sequence of African green monkey Topo I has been deposited in GenBank under accession no. AB089321.
Expression of recombinant wild-type sTopo I in African green monkey (COS-1) producer cells did not affect the infectivity of the resulting HIV-1, whereas expression of human WT Topo I in these cells resulted in a 5.3-fold increase in infectivity compared with that of viruses derived from cells transfected with vector alone (Fig. 4 A and B). Expression of a chimeric protein (SimC) comprising the NH2-terminal half of human Topo I and the COOH-terminal half of sTopo I enhanced HIV-1 infectivity to an extent similar to that observed with human WT Topo I. In contrast, expression of the reciprocal construct (SimN) had no effect on viral infectivity, indicating that amino acid differences in the NH2-terminal regions of the human and African green monkey proteins are responsible for the difference in the ability to enhance infectivity. Immunoblot analysis revealed no marked differences in the abundance of the ectopic WT and mutant Topo I proteins (Fig. 4C). Insertion of Asp-Lys between Lys-40 and Glu-41 (HuIns40/41), replacement of Val-145 with Ala (HuV145A), or replacement of Thr-157 with Ile (HuT157I) did not affect the ability of human Topo I to enhance HIV-1 infectivity. In contrast, replacement of Glu-236 and Asn-237 of human Topo I with the corresponding residues (Asp and Ser, respectively) present in sTopo I (mutant HuE236D/N237S) negated the enhancement of HIV-1 infectivity. Thus, the ability of human Topo I to enhance HIV-1 infectivity is determined by the amino acids Glu-236 and Asn-237.
Fig. 4.
Identification of the human Topo I residues responsible for enhancement of HIV-1 infectivity. (A) Schematic representation of the structures of Topo I mutants. Asterisks denote the six sites of African green monkey Topo I that differ from the human protein. White and shaded boxes indicate human and African green monkey Topo I, respectively. (B) Comparison of the infectivity of viruses derived from COS-1 cells expressing human WT, African green monkey WT, or mutant Topo I proteins. Data are means ± SD of three independent experiments. (C) Immunoblot analysis of COS-1 cells expressing WT or mutant Topo I with antibodies to the Xpress epitope tag. Lanes 1–8 correspond to the Topo I proteins indicated in A and B; lanes 9 and 10 correspond to cells transfected with a control vector (pcDNA) and to nontransfected cells, respectively.
Lack of Effect of Human Topo I on HIV-1 RNA Stability, Viral Processing, Production, or Viral Entry. We next investigated which steps of viral replication could be influenced by human Topo I. Topo I has been shown to be tightly bound to genomic RNA of Rous sarcoma virus (33) and has also been shown to be incorporated into HIV-1 virions (13), apparently as a result of its interaction with genomic RNA (15, 34). We therefore initially examined whether human Topo I could stabilize HIV-1 RNA. COS-1 cells were cotransfected with pNL-Luc-HXB and an expression vector for human WT, African green monkey WT, or the HuE236D/N237S mutant Topo I protein. Exposure of the cells expressing any of the three recombinant Topo I proteins to actinomycin D resulted in a reduction in the amount of viral RNA similar to that apparent in cells transfected with the corresponding control vector (Fig. 5A). Thus, expression of human Topo I in African green monkey cells does not affect the stability of viral RNA. We next investigated the processing of the HIV-1 Pr55gag protein in the absence or presence of human Topo I. The mature products p17 and p24, as well as unprocessed Pr55gag and the intermediate p41gag, were detected in similar amounts in both human (293T) and African green monkey (COS-1) cells. Furthermore, the abundance of these various proteins was not affected by the expression of recombinant human Topo I in either cell type (Fig. 5B). Similarly, the HIV-1 envelope protein (Env) gp160 and its processed product gp120 were expressed at similar levels in both the human and African green monkey cells, and the amounts of these proteins were not affected by ectopic human Topo I. Thus, human Topo I does not influence the processing of HIV-1 Gag or Env proteins.
Fig. 5.
Lack of effect of human Topo I on the stability of HIV-1 genomic RNA (A) or on viral processing (B), assembly (C), or entry (D). (A) COS-1 cells were cotransfected with pNL-Luc-HXB and either an expression vector for human WT, HuE236D/N237S mutant, or African green monkey WT Topo I proteins or the corresponding empty vector (pcDNA). The amount of HIV-1 RNA remaining at the indicated times after exposure of the transfected cells to actinomycin D is expressed as a percentage of that present at time 0. Data are means ± SD of three independent experiments. (B) Human (293T) or African green monkey (COS-1) cells were cotransfected with pNL-Luc-HXB and either the human Topo I expression vector or empty vector. Cell lysates were subsequently subjected to immunoblot analysis either with sera of patients infected with HIV-1 for detection of gp160 and gp120 (Top and Middle) or with a mixture of monoclonal antibodies to p24 and to p17 for detection of Pr55gag, p41, p24, and p17 (Bottom). Nontransfected cells were similarly analyzed as a control (-). (C) 293T and COS-1 cells were cotransfected with pNL-Luc-HXB and either the human Topo I expression vector (+) or empty vector (-). The amount of p24 in the culture supernatants was subsequently determined and expressed relative to the value for 293T cells expressing recombinant human Topo I as a measure of virion release efficiency. Data are means of triplicates from a representative experiment. (D) 293T or COS-1 cells were cotransfected with pNL-Luc-E–R+, a VSV-G expression vector, and either the human Topo I expression vector (+) or empty vector (-), after which the infectivity of resulting viruses was determined with MAGIC5 cells. Data are means ± SD of triplicates from a representative experiment. Recombinant human Topo I in the producer cells was also detected by immunoblot analysis with antibodies to the Xpress epitope (shown below the histogram).
Given that HIV-1 virion production has been shown to be blocked in murine cells (35), we next examined the efficiency of virion production, as reflected by the amount of p24 antigen in culture supernatants, in human and African green monkey cells transfected with the human Topo I expression vector. The extent of virion production did not differ substantially between human and African green monkey cells and was not affected by ectopic human Topo I (Fig. 5C).
We also examined the possible effect of human Topo I expression in the producer cells on viral entry into MAGIC5 cells with the use of HIV-1 pseudotyped with VSV-G. Consistent with the results shown in Fig. 2, expression of human Topo I in African green monkey cells resulted in a 4- to 5-fold increase in viral infectivity, but the recombinant human protein had no effect on the infectivity of virus produced by human cells (Fig. 5D). In conclusion, our studies show that the enhancement of HIV-1 infectivity by human Topo I is not mediated at the level of Gag or Env processing, virion production, or viral entry, but rather represents a postentry effect.
Human Topo I Promotes HIV-1 cDNA Synthesis. To confirm that human Topo I expression enhances HIV-1 infectivity at a postentry step, we cotransfected COS-1 cells with pNL-Luc-HXB and an expression vector for human WT Topo I, the HuE236D/N237S mutant, or African green monkey WT Topo I, infected MAGIC5 cells with the resulting viruses. Then we determined the number of HIV-1 DNA molecules in the infected cells as a measure of the efficiency of viral cDNA synthesis. The copy number of HIV-1 DNA in MAGIC5 cells infected with virus from the African green monkey cells expressing human WT Topo I was some four times that in MAGIC5 cells infected with virus from the African green monkey cells expressing African green monkey WT Topo I or the human mutant Topo I (Fig. 6A). These results demonstrate that the enhancement of HIV-1 infectivity by the expression of human Topo I in the producer cells results from enhancement of viral cDNA synthesis in the target cells.
Fig. 6.
Effects of human WT, human mutant, or African green monkey WT Topo I expression in producer cells on HIV-1 cDNA synthesis in target cells. COS-1 cells (A) or 293T cells (B) were cotransfected with pNL-Luc-HXB and either an expression vector for human WT, HuE236/N237S mutant, or African green monkey WT Topo I or the corresponding empty vector. The resulting viruses were used to infect MAGIC5 cells, after which the number of synthesized HIV-1 cDNA copies was determined. Data are presented as the relative ratios of the mean copy number of HIV-1 DNA per 10 ng of p24 ± SD with regard to the ratio at human WT as 100% from three independent experiments.
We performed similar experiments with 293T cells as producer cells. The synthesis of HIV-1 cDNA in the infected MAGIC5 cells was reduced by ≈75% as a result of the expression of HuE236D/N237S mutant or African green monkey WT Topo I in 293T cells (Fig. 6B). These findings demonstrate that the human mutant Topo I and African green monkey WT Topo I act in a dominant negative manner in human producer cells.
Discussion
In the present report, we clearly demonstrate an important role for human Topo I in HIV-1 replication and specifically for the effective production of progeny virus. Viruses produced from three African green monkey cell lines all exhibited markedly lower infectivity than those derived from three human cell lines. Consistent with the observations that human Topo I is incorporated into HIV-1 virions (13) and increases both HIV-1 reverse transcriptase activity in vitro (14) and cDNA synthesis in endogenous reverse transcription assays (15), we have now shown that the expression of human Topo I in African green monkey cells greatly enhanced the infectivity titres of progeny HIV-1 virions produced in these cells. Furthermore, we have demonstrated that Glu-236 and Asn-237 are the key amino acid residues of human Topo I necessary for the enhancement of viral infectivity. Expression of human Topo I in the African green monkey producer cells had no effect on viral RNA stability, viral processing, virion release, or viral entry, but instead promoted viral cDNA synthesis in target cells.
Despite the marked effect of human Topo I expression in African green monkey cells, the infectivity titres of virus derived from such cells were still only approximately two-thirds of that of human cell-derived HIV-1. One possible explanation for this difference is that human cells might also express additional factor (or factors) required for the optimal production of infectious HIV-1 that is absent or nonfunctional in African green monkey cells. A second possibility is that the level of human Topo I expression in African green monkey cells was insufficient to overcome fully the activity of simian Topo I. Indeed, cDNA synthesis by 293T cell-derived HIV-1 was reduced by ≈75% as a result of the expression either of simian Topo I or of the human Topo I mutant HuE236D/N237S in the 293T cells, indicative of a dominant negative activity by the ectopic proteins. The complete and selective elimination of endogenous Topo I activity in African green monkey cells, possibly with the use of small interfering RNAs (36), might thus be expected to lead to the total recovery of infectivity of African green monkey cell-derived HIV-1 by expression of human Topo I.
The present studies involved HIV-1 derived from the plasmid pNL-Luc-HXB, which does not encode Nef, a protein that is known to play an important role in viral infectivity. To investigate whether expression of Nef might influence our findings, we infected MAGIC5 cells with HIV-1 derived from COS-1 cells, which were transfected with the pIndie-C1 (18) carrying nef gene and either human Topo I expression vector or an empty vector, and determined the infectivity by staining of β-galactosidase. Expression of human Topo I in COS-1 cells increased the infectivity of progeny virus in a similar fashion to that seen with pNL-Luc-HXB. Therefore, we conclude that Nef does not affect the infectivity enhancement by Topo I.
Our studies with VSV-G–pseudotyped HIV-1 suggest that the expression of human Topo I in African green monkey producer cells promotes viral infectivity at a postentry step. It remains to be determined whether this effect requires the incorporation of human Topo I into virions or whether the human enzyme functions before virion release. However, given that Topo I binds with high affinity to Rous sarcoma virus RNA (33) and HIV-1 RNA (15, 34) as well as enhancing HIV-1 reverse transcriptase activity in vitro (14), it is likely that virion-associated human Topo I promotes HIV-1 cDNA synthesis through a direct interaction with genomic RNA during reverse transcription (15, 34). Furthermore, as studies with chimeric viruses derived from HIV-1 and simian immunodeficiency virus (SIV) have revealed that SIV Gag is essential for viral replication in African green monkey cells (10), the interactions of Topo I and Gag proteins might be also critical for optimal infectivity.
It has been demonstrated that rodent cells show a significant defect in virus release (37). To examine the effect of human Topo I on virion release in rodent cells, we transfected pNL-Luc-HXB, a human cycT1 expression vector (ref. 9; kindly provided by P. D. Bieniasz), and either human Topo I expression vector or a control vector into NIH 3T3 cells and quantified the release of virus. Expression of human Topo I in NIH 3T3 cells could not affect the release of virus from these cells (data not shown).
Hofmann et al. (11) recently investigated species-specific postentry barriers in primate immunodeficiency virus infection and showed that HIV-1 infection is blocked in cells derived from Old World primates (such as the African green monkey, from which the COS-1, CV-1, and Vero cell lines are derived) but not in cells from New World primates. The virus stocks used in these experiments were produced from human 293T cells and should therefore contain human Topo I in the viral particles. This implies the view that Old World primates lack a cellular factor (or factors) required for effective virus replication in target cells.
We have also investigated whether expression of human Topo I in Vero cells as the target cells could improve the infectivity of VSV-G-pseudotyped HIV-1 produced from COS-1 cells. The infectivity in the human Topo I-expressing target Vero cells was similar to that in Vero cells without human Topo I (data not shown), suggesting that human Topo I expressed in target simian cells may not be sufficient to augment reverse transcription.
In conclusion, our studies have demonstrated that the expression of human Topo I in producer cells is required for optimal infectivity of HIV-1, thus explaining at least in part the species specificity of HIV-1 infection and the limitations of viral tropism to a restricted number of cell types. Further characterization of the mechanisms by which human Topo I promotes viral infectivity should assist the further development of targeted therapies against HIV-1 infection.
Acknowledgments
We thank Dr. M. Tatsumi (National Institute of Infectious Diseases) for the gift of MAGIC5 cells. This work was supported in part by grants from the Ministry of Education, Science, Technology, Sports, and Culture of Japan; the Ministry of Health, Labor, and Welfare of Japan; and the Japanese Foundation for AIDS Prevention.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HIV-1, HIV type 1; Topo I, topoisomerase I; VSV-G, vesicular stomatitis virus glycoprotein.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB089321).
References
- 1.Alter, H. J., Eichberg, J. W., Masur, H., Saxinger, W. C., Gallo, R., Macher, A. M., Lane, H. C. & Fauci, A. S. (1984) Science 226 549-552. [DOI] [PubMed] [Google Scholar]
- 2.Lusso, P., Markham, P. D., Ranki, A., Earl, P., Moss, B., Dorner, F., Gallo, R. C. & Krohn, K. J. (1988) J. Immunol. 141 2467-2473. [PubMed] [Google Scholar]
- 3.Agy, M. B., Frumkin, L. R., Corey, L., Coombs, R. W., Wolinsky, S. M., Koehler, J., Morton, W. R. & Katze, M. G. (1992) Science 257 103-106. [DOI] [PubMed] [Google Scholar]
- 4.Neville, M., Stutz, F., Lee, L., Davis, L. I. & Rosbash, M. (1997) Curr. Biol. 7 767-775. [DOI] [PubMed] [Google Scholar]
- 5.Garrus, J. E., von Schwedler, U. K., Pornillos, O. W., Morham, S. G., Zavitz, K. H., Wang, H. E., Wettstein, D. A., Stray, K. M., Cote, M., Rich, R. L., et al. (2001) Cell 107 55-65. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman, C., Klein, K. C., Kiser, P. K., Singh, A. R., Firestein, B. L., Riba, S. C. & Lingappa, J. R. (2002) Nature 415 88-92. [DOI] [PubMed] [Google Scholar]
- 7.Wei, P., Garber, M. E., Fang, S. M., Fischer, W. H. & Jones, K. A. (1998) Cell 92 451-462. [DOI] [PubMed] [Google Scholar]
- 8.Fujinaga, K., Cujec, T. P., Peng, J., Garriga, J., Price, D. H., Grana, X. & Peterlin, B. M. (1998) J. Virol. 72 7154-7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieniasz, P. D., Grdina, T. A., Bogerd, H. P. & Cullen, B. R. (1998) EMBO J. 17 7056-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata, R., Kawamura, M., Sakai, H., Hayami, M., Ishimoto, A. & Adachi, A. (1991) J. Virol. 65 3514-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann, W., Schubert, D., LaBonte, J., Munson, L., Gibson, S., Scammell, J., Ferrigno, P. & Sodroski, J. (1999) J. Virol. 73 10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher, A. G., Ensoli, B., Ivanoff, L., Chamberlain, M., Petteway, S., Ratner, L., Gallo, R. C. & Wong-Staal, F. (1987) Science 237 888-893. [DOI] [PubMed] [Google Scholar]
- 13.Jardine, D., Tachedjian, G., Locarnini, S. & Birch, C. (1993) AIDS Res. Hum. Retroviruses 9 1245-1250. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi, H., Matsuda, M., Kojima, A., Sata, T., Andoh, T., Kurata, T., Nagashima, K. & Hall, W. W. (1995) Proc. Natl. Acad. Sci. USA 92 5694-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi, H., Sawa, H., Hasegawa, H., Shoya, Y., Sata, T., Hall, W. W., Nagashima, K. & Kurata, T. (2002) Biochem. Biophys. Res. Commun. 294 509-517. [DOI] [PubMed] [Google Scholar]
- 16.Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. (1993) Proc. Natl. Acad. Sci. USA 90 8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leibovitz, A., Stinson, J. C., McCombs, W. B., III, McCoy, C. E., Mazur, K. C. & Mabry, N. D. (1976) Cancer Res. 36 4562-4569. [PubMed] [Google Scholar]
- 18.Mochizuki, N., Otsuka, N., Matsuo, K., Shiino, T., Kojima, A., Kurata, T., Sakai, K., Yamamoto, N., Isomura, S., Dhole, T. N., et al. (1999) AIDS Res. Hum. Retroviruses 15 1321-1324. [DOI] [PubMed] [Google Scholar]
- 19.Gluzman, Y. (1981) Cell 23 175-182. [DOI] [PubMed] [Google Scholar]
- 20.Daemer, R. J., Feinstone, S. M., Gust, I. D. & Purcell, R. H. (1981) Infect. Immun. 32 388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi, A., Gendelman, H. E., Koenig, S., Folks, T., Willey, R., Rabson, A. & Martin, M. A. (1986) J. Virol. 59 284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokunaga, K., Greenberg, M. L., Morse, M. A., Cumming, R. I., Lyerly, H. K. & Cullen, B. R. (2001) J. Virol. 75 6776-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor, R. I., Chen, B. K., Choe, S. & Landau, N. R. (1995) Virology 206 935-944. [DOI] [PubMed] [Google Scholar]
- 24.Fouchier, R. A., Meyer, B. E., Simon, J. H., Fischer, U. & Malim, M. H. (1997) EMBO J. 16 4531-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross, T. M. & Cullen, B. R. (1998) Proc. Natl. Acad. Sci. USA 95 7682-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura, H., Kohchi, C., Yamada, R., Ikeda, T., Koiwai, O., Patterson, E., Keene, J. D., Okada, K., Kjeldsen, E., Nishikawa, K., et al. (1991) Nucleic Acids Res. 19 69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho, S. N., Hunt, H. D., Horton, R. M., Pullen, J. K. & Pease, L. R. (1989) Gene 77 51-59. [DOI] [PubMed] [Google Scholar]
- 28.Hoshikawa, N., Kojima, A., Yasuda, A., Takayashiki, E., Masuko, S., Chiba, J., Sata, T. & Kurata, T. (1991) J. Gen. Virol. 72 2509-2517. [DOI] [PubMed] [Google Scholar]
- 29.Hirt, B. (1967) J. Mol. Biol. 26 365-369. [DOI] [PubMed] [Google Scholar]
- 30.Braaten, D., Franke, E. K. & Luban, J. (1996) J. Virol. 70 3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig, E., Silberstein, F. C., van Empel, J., Erfle, V., Neumann, M. & Brack-Werner, R. (1999) J. Virol. 73 8279-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy, K. M., Sweet, M. J., Ross, I. L. & Hume, D. A. (1993) J. Virol. 67 6956-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weis, J. H. & Faras, A. J. (1981) Virology 114 563-566. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi, H., Sawa, H., Hasegawa, H., Sata, T., Hall, W. & Kurata, T. (2002) Biochem. Biophys. Res. Commun. 297 593-599. [DOI] [PubMed] [Google Scholar]
- 35.Mariani, R., Rutter, G., Harris, M. E., Hope, T. J., Krausslich, H. G. & Landau, N. R. (2000) J. Virol. 74 3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411 494-498. [DOI] [PubMed] [Google Scholar]
- 37.Bieniasz, P. D. & Cullen, B. R. (2000) J. Virol. 74 9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]