Abstract
In vivo-induced antigen technology is a method to identify proteins expressed by pathogenic bacteria during human infection. Sera from 10 patients convalescing from cholera infection in Bangladesh were pooled, adsorbed against in vitro-grown El Tor Vibrio cholerae O1, and used to probe a genomic expression library in Escherichia coli constructed from El Tor V. cholerae O1 strain N16961. We identified 38 positive clones in the screen, encoding pili (PilA and TcpA), cell membrane proteins (PilQ, MshO, MshP, and CapK), methyl-accepting chemotaxis proteins, chemotaxis and motility proteins (CheA and CheR), a quorum-sensing protein (LuxP), and four hypothetical proteins. Analysis of immune responses to purified PilA and TcpA in individual patients demonstrated that the majority seroconverted to these proteins, confirming results with pooled sera. These results suggest that PilA and its outer membrane secretin, PilQ, are expressed during human infection and may be involved in colonization of the gastrointestinal tract. These results also demonstrate substantial immune responses to TcpA in patients infected with El Tor V. cholerae O1. In vivo-induced antigen technology provides a simple method for identifying microbial proteins expressed during human infection, but not during in vitro growth.
Vibrio cholerae is a Gram-negative bacillus that causes a severe, dehydrating diarrhea in humans (1). V. cholerae can be differentiated by the lipopolysaccharide in the outer membrane; strains of V. cholerae that produce cholera belong to serogroup O1 or O139. V. cholerae O1 is divided into two biotypes, classical and El Tor; the current global pandemic of V. cholerae O1 infection is caused by El Tor strains.
A major virulence factor for pathogenic strains of V. cholerae is cholera toxin, a protein exotoxin that consists of a single A subunit noncovalently associated with five B subunits (2). A second major virulence factor of V. cholerae is the toxin coregulated pilus (TCP; ref. 3). TCP is essential for colonization and virulence in both mouse models of cholera (3) and human volunteer studies (4). TcpA, the 20.5-kDa major structural subunit of TCP, has homology to the type IV pili of several other bacterial pathogens (5). TcpA from El Tor and classical strains of V. cholerae show ≈80% protein homology; monoclonal antibodies demonstrate epitope differences between these proteins in the two biotypes (6, 7).
In addition to TcpA, the V. cholerae genome encodes two other type IV pili, the mannose-sensitive hemagglutinin (MSHA) and PilA (8). MSHA is a thin, flexible pilus composed of a 17-kDa subunit (9). A strain of V. cholerae deleted in mshA showed no defect in colonization of human volunteers (10). Recently, Fullner et al. (11) described a four-gene cluster, pilABCD, encoding a third type IV pilus in V. cholerae. A deletion of pilA had no effect on colonization in infant mice. The role of PilA in human infection has not been previously examined.
Infection with V. cholerae can induce long-lasting protective immunity against subsequent disease (12, 13), but the full repertoire of immune responses mediating protection is not known. The best characterized of the immune responses induced by V. cholerae is the vibriocidal antibody; elevated vibriocidal titers correlate with protection from subsequent clinical disease in seroepidemiologic studies (14–16). Because V. cholerae is a noninvasive organism, and because there is no disruption of the intestinal epithelium during cholera, a serum complement-fixing antibody response such as the vibriocidal antibody may have minimal activity in the intestinal lumen. The vibriocidal antibody response, therefore, may be a surrogate marker for an intestinal response that is the primary mediator of protective immunity.
Immune responses after cholera have also been examined for a number of other antigens, but none of these responses has been shown to correlate with protection. Approximately 90% of individuals in Bangladesh developed an anti-MSHA response in serum and/or stool after cholera (17). The majority of the anti-cholera toxin (CT) immune response is directed against the nontoxic B subunit (CtxB). Serum anti-CT and anti-CtxB increase substantially after cholera, but these responses have not been shown to protect from subsequent disease (16). Intestinal colonization by V. cholerae is a prerequisite for the development of immune responses during cholera, and TCP has been shown to be required for intestinal colonization of human by V. cholerae (4). However, in volunteer studies in North America, anti-TCP responses were not found in convalescent sera of individuals challenged with V. cholerae. Low-level anti-TCP immune responses were present in convalescent sera from three of six individuals with cholera in Indonesia (18). It is not clear why antibody responses to TCP are not more prominent in normal volunteers, but perhaps immune responses to this pilus require repeated intestinal exposure. Previous studies of immune responses to TCP have also been limited by the lack of purified El Tor TcpA for use in assays; as described above, classical and El Tor TcpA differ immunologically, and assay of responses after El Tor cholera by using classical TcpA as antigen may not detect immune responses appropriately.
All of the antigens described above against which immune responses have been characterized were identified by using in vitro-grown V. cholerae. To identify additional microbial antigens uniquely expressed during infection, a number of newer techniques have been applied to identify gene expression specifically in vivo, in animal models.
The first of these techniques, recombinase in vivo expression technology (RIVET) (19, 20), identified a number of genes in V. cholerae specifically induced during infection of infant mice, including a methyl-accepting chemotaxis protein and vieSAB, encoding a sensor kinase and two distinct response regulatory proteins (20, 21). RIVET has subsequently been used to study the spatiotemporal expression of selected V. cholerae genes (tcpA and ctxAB) during infection in infant mice (22), as well as in a mutagenesis screen to identify V. cholerae genes required for expression of ctxAB and toxT in mice (23). This latter screen identified several chemotaxis genes, a methyl-accepting chemotaxis protein, capK (involved in biofilm-associated exopolysaccharide synthesis), rtxB (encoding the transport gene for the RTX toxin of El Tor V. cholerae; ref. 24), vieS, mfrha (the mannose/fucose-resistant hemagglutinin), and other genes, as important regulators of colonization in mice. Signature-tagged mutagenesis (25) has also been applied to V. cholerae, identifying a number of genes necessary for colonization and growth in infant mice, including genes in the TCP cluster, as well as pta, ptlA, and genes involved in purine biosynthesis (26).
Both RIVET and signature-tagged mutagenesis require an animal model that mimics human infection. Because humans are the only known natural hosts for V. cholerae infection, determination of genes expressed during infection in animal models may not identify genes uniquely required for human infection.
In vivo-induced antigen technology (IVIAT) is a method that circumvents limitations of animal models, allowing direct identification of microbial proteins expressed at sufficient levels during human infection to be immunogenic (27); these same proteins may be important for protective immunity after both natural infection and vaccination. The aim of this study was to use IVIAT to identify V. cholerae genes specifically expressed during human infection.
Materials and Methods
Strains, Plasmids, and Media. Strains and plasmids used in this study are listed in Table 1. Strain N16961 was used to construct the genomic library described below; the genome sequence of this strain has been published (8). Bacterial strains were grown in vitro in LB or AKI media (28), and maintained at -70°C in LB broth containing 15% glycerol.
Table 1. Bacterial strains and plasmids used in this study.
| Genotype and/or phenotype | Reference of source | |
|---|---|---|
| Strains | ||
| N16961 | WT V. cholerae O1 El Tor strain; genomic sequence available: Smr | Lab strain |
| Plasmids | ||
| pET30(abc) | Expression vectors allowing cloning of fragments in each of three reading frames; Kanr | Novagen |
| pLHEAB1 | pET30(a) with intact pilQ gene amplified by PCR from N16961 (1734 bp), and cloned in NdeI and XhoI sites in vector; Kanr | This work |
| pLHEAB2 | pET30(a) with intact pilA gene amplified by PCR from N16961 (459 bp), and cloned in NdeI and XhoI sites in vector; Kanr | This work |
| pLHEAB3 | pET30(a) with intact tcpA gene amplified by PCR from N16961 (672 bp), and cloned in NdeI and XhoI sites in vector; Kanr | This work |
| pLHMA1 | pET30(a) with intact tcpF gene amplified by PCR from N16961 (1014 bp), and cloned in NdeI and XhoI sites in vector; Kanr | This work |
| pLH1 | pET30(a) with intact mshA gene amplified by PCR from N16961 (534 bp), and cloned in EcoRV and XhoI sites in vector; Kanr | This work |
Smr, streptomycin-resistant; Kanr, kanamycin-resistant; Apr, ampicillin-resistant
Genetic Methods and Strain Construction. Oligonucleotides used for PCR and DNA sequencing were obtained from Operon Technologies (Alameda, CA). PCR was performed with TaKaRa Taq polymerase (New England Biolabs) or Pfx DNA polymerase (Invitrogen), and an MJ Research (Cambridge, MA) Thermocycler (model PTC 100). PCR templates were prepared by boiling a single colony of V. cholerae strain N16961 in distilled H2O, followed by centrifugation and recovery of supernatant. DNA sequencing was performed at the DNA Sequencing Core Facility, Department of Molecular Biology, Massachusetts General Hospital. DNA sequences were assembled and ORFs assigned with the GENE WORKS ANALYSIS software package (Institute for Genomic Research, Rockville, MD). Plasmids pLHEAB1, pLHEAB2, pLHEAB3, pLHMA1, and pLH1 for expressing intact PilQ, PilA, TcpA, TcpF, and MshA from V. cholerae strain N16961, respectively, were constructed as in Table 1.
Patient and Control Sera. Serum was collected from patients at the International Centre for Diarrhoeal Disease Research in Dhaka, Bangladesh (ICDDR,B); each patient had culture-confirmed acute diarrheal illness due to El Tor V. cholerae O1, and each demonstrated a 4-fold or greater rise in vibriocidal antibody titer between acute and convalescent sera. Patients seen at the ICDDR,B during the same time period but who were not infected with V. cholerae (by culture and paired serum vibriocidal assay) provided control sera. For both groups, sera were obtained on days 0, 9, and 23 after presentation, and stored at -80°C until use. Patients were entered after informed consent, and the study was approved by the Institutional Review Boards at both the ICDDR,B and Massachusetts General Hospital.
Adsorption of Sera. We pooled equal volumes of convalescent sera (both day 9 and day 23) from 10 patients infected with V. cholerae and extensively adsorbed this pool against an El Tor V. cholerae strain isolated from one of the patients in the study. Pooled sera were serially adsorbed against in vitro-grown V. cholerae whole cells, then French-press extracts, and finally heat-denatured extracts, as described (27). Resultant adsorbed serum was aliquotted and stored at -80°C.
The pool of sera was assayed at various steps of the adsorption process by using an ELISA reaction against either whole V. cholerae extracts, or the in vivo-expressed protein CtxB. To assay antibodies in the serum reactive with whole V. cholerae extracts, a French-pressed lysate was diluted 1:2 in carbonate buffer (pH 9.6), and 100 μl of this lysate was added per well to an ELISA plate. After blocking and washing, serum samples were added at dilutions of 1:200 to 1:25,600, and binding was quantitated by addition of peroxidase-conjugated goat anti-human affinitypurified Ig (ICN), which is reactive with all classes of human immunoglobulins. Reactivity was measured by using the substrate 2,2′azino-bisethylbenzthiazolinesulfonic acid (Sigma), and optical density readings at 405 nm were recorded by using a Vmax microplate reader (Molecular Devices). To assay antibodies specific for CtxB, wells in an ELISA plate were sequentially coated with ganglioside (1 μg in 110 μl of carbonate buffer, pH 9.6) and 100 ng of purified CtxB (List Biological Laboratories, Campbell, CA). After blocking and washing, pooled sera were serially diluted from 1:50 to 1:25,600 and added to each well. Reactivity was measured as above.
Construction of a Genomic Expression Library of V. cholerae O1 Strain N16961. An expression library was constructed by using pETabc expression vectors (Novagen); these vectors allow cloning of inserts in each of three reading frames under transcriptional control of a T7 promoter. Vector DNA was digested with BamHI, gel-purified by using the QIAEX II Gel Extraction Kit (Qiagen, Valencia, CA), and treated with shrimp alkaline phosphatase. Genomic DNA from V. cholerae strain N16961 was partially digested with Sau3A, and two pools of genomic DNA fragments ranging in size from 0.5–1.5 and 1.0–3.0 kbp were gel purified. Vector and genomic DNA fragments from each pool were ligated to create two genomic libraries, and each was electroporated into competent Escherichia coli DH5α and spread on LB plates containing kanamycin. After overnight incubation at 37°C, growth on plates was collected by scraping, and plasmid DNA was recovered from an aliquot of the library and electroporated into E. coli BL21 (DE3).
Purification of Proteins. A derivative of TcpA from El Tor V. cholerae O1, with a histidine tag fused at the amino terminus of residue 29 of mature TcpA, and a glutathione S-transferase derivative of TcpF from classical V. cholerae O1, were overexpressed and purified as described (29, 30). Purified MshA from El Tor V. cholerae O1 was purified as described (17). PilA from N16961 was overexpressed with a carboxyl-terminal hexahistidine tail and purified by using a combination of nickel affinity chromatography and nondenaturing preparative gel electrophoresis (unpublished results).
Screening for Proteins Uniquely Expressed in Vivo by V. cholerae. To screen the genomic library with adsorbed pooled sera, an aliquot of the library in expression host BL21 (DE3) was diluted and spread on LB plates containing kanamycin to produce ≈300 colonies per plate. After overnight growth at 37°C, colonies were lifted by using nitrocellulose membranes, replica plated onto LB plates containing kanamycin and isopropyl-β-d-thiogalactoside (1 mM), and incubated overnight at 37°C to induce expression of genes in cloned inserts. After overnight incubation, plates were exposed to chloroform for 15 min to partially lyse bacteria and release induced proteins, then overlaid with a nitrocellulose membrane for 15 min at room temperature. Each membrane was removed, blocked with 10% nonfat skim milk, and reacted with a 1:100 dilution of adsorbed pooled sera at room temperature for 1h with mildagitation.Clones reacting with antibody in adsorbed sera were detected by using peroxidase-conjugated goat, anti-human Ig at a 1:5,000 dilution, and developed by using an ECL chemiluminescence kit (Amersham Pharmacia Biotech). Reactive clones were identified by their position on the master plate; each positive clone was purified at least two additional times and confirmed as reactive with adsorbed sera. Plasmids from individual reactive clones were purified, and the V. cholerae DNA inserted in the vector was sequenced in both directions by using pET30-specific primers (Novagen). PCR with these same primers was used to estimate the size of inserted DNA. Altogether, ≈30,000 clones from the two differently sized DNA expression libraries were screened, and 38 positive clones were identified, confirmed on multiple occasions, and sequenced. Proteins encoded in the cloned insert fragments were identified by using the genomic sequence of V. cholerae strain N16961.
To further confirm specific recognition of PilA, PilQ, TcpA, TcpF, and MshA by pooled, adsorbed convalescent sera from cholera patients, we used expression plasmids for each intact protein in a dot-blot hybridization assay identical to the IVIAT screen of the library above, to confirm immunoreactivity. As a control, results were compared with those with empty plasmid vector.
Analysis of RNA Transcription of Selected Genes in Vitro by RT-PCR. After overnight culture of strain N16961 in LB broth or AKI media, RNA was isolated by using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA was purified with an RNeasy Mini Kit (Qiagen) and treated with an RNase-Free DNase kit (Qiagen) according to the manufacturer's instructions. RNA was eluted in diethyl pyrocarbonate-treated water and treated with DNase I (Ambion, Austin, TX). Control PCRs were performed on the purified RNA preparations to ensure absence of amplification from contaminating DNA. Reverse transcription to cDNA was done by using a Reverse Transcription System (Promega). Primers specific for tcpA, pilA, pilQ, and rpoB (as a control) were used in PCRs on cDNA; primers were obtained from the Tufts University Core Facility (Boston). PCR products were detected on a 1.5% agarose gel with ethidium bromide.
Results and Discussion
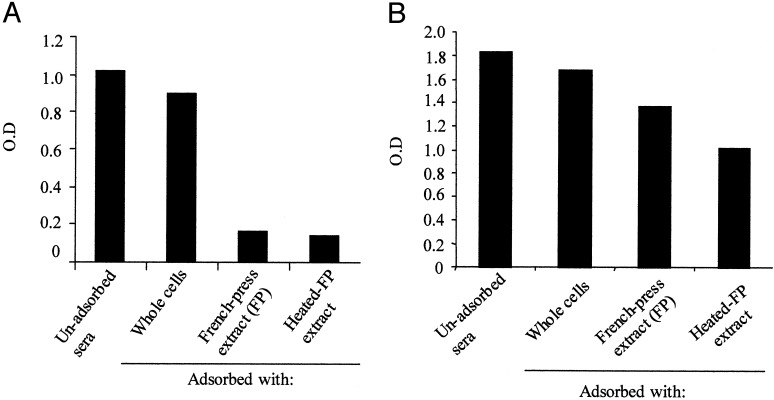
Adsorption of a Pool of Sera from Patients Convalescing from Cholera Removes Antibodies Against Most V. cholerae Proteins Expressed in Vitro but Not Those Expressed in Vivo. Pooled sera from patients convalescing from cholera in Bangladesh were successively adsorbed against in vitro grown V. cholerae. As shown in Fig. 1A, there was a progressive decrease in reactivity of the pooled sera with in vitro-grown V. cholerae, particularly after the French press lysate adsorption step. In contrast, there was substantially less removal of antibodies recognizing CtxB (Fig. 1B). Cholera toxin is not significantly expressed by El Tor V. cholerae during growth in LB broth, but is expressed during human infection.
Fig. 1.
ELISA results after sequential steps in adsorption of pooled, patient sera; OD values are corrected for background and for dilution during adsorption steps. (A) Shown are ELISA plates coated with whole V. cholerae extracts. (B) Shown are ELISA plates coated with GM1 ganglioside and CtxB. FP, French press.
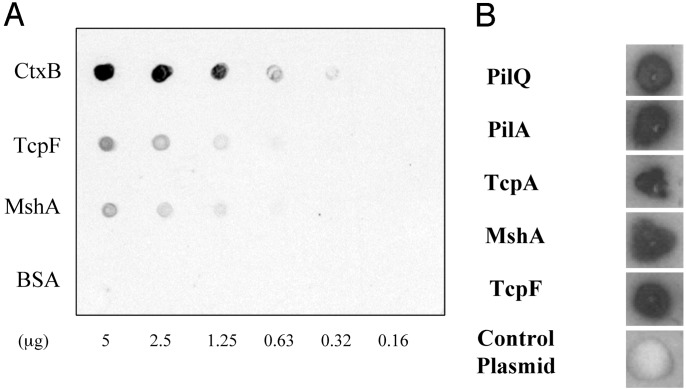
Retention of immunoreactivity to in vivo-expressed V. cholerae proteins in pooled convalescent sera after adsorption was further assessed by using individual purified proteins. In addition to CtxB, we included purified MshA and TcpF, a protein in the TCP gene cluster that is secreted by V. cholerae (30). Genes in the TCP cluster in El Tor V. cholerae are not expressed during growth in LB broth but are expressed during human infection. As shown in Fig. 2A, substantial reactivity to in vivo-expressed V. cholerae proteins was retained after adsorption of convalescent sera.
Fig. 2.
Results using pooled, convalescent sera after complete adsorption. (A) Individual, purified proteins were spotted on a nitrocellulose membrane and the membrane developed by using pooled convalescent sera. (B) Individual E. coli BL21 strains containing plasmids expressing specific proteins (or control plasmid, pET30a) were transferred to a nitrocellulose membrane and developed by using pooled patient sera after adsorption.
Use of Adsorbed Convalescent Sera to Probe a Genomic DNA Expression Library of V. cholerae Strain N16961. Screening of the two expression libraries with the adsorbed convalescent sera yielded 38 clones that were persistently reactive after at least three rounds of purification. The identity of proteins encoded by clones reactive with convalescent sera is shown in Table 2; these reactive clones include proteins encoding two of the type IV pili in V. cholerae (PilA and TcpA), a minor component of the MSHA pilus (MshO) and a related outer membrane protein (MshP), a cell membrane protein (PilQ) that acts as an outer membrane secretin for PilA in other organisms, a cell membrane protein (CapK) that is involved in biofilm-associated exopolysaccharide synthesis, three methyl-accepting chemotaxis proteins, two proteins involved in chemotaxis and motility (CheA and CheR), a quorum-sensing protein (LuxP), and four hypothetical ORFs. Most of the reactive clones contained only a portion of the coding sequence of the relevant protein product. A number of reactive clones expressed V. cholerae proteins previously detected by screens in infant mice by using RIVET and signature-tagged mutagenesis, including TcpA (which was identified eight times in the screen), three different methyl-accepting chemotaxis proteins, and CapK.
Table 2. V. cholerae proteins encoded in clones identified by IVIAT.
| Functional classes of encoded protein products | Name of encoded protein product | Gene designation in V. cholerae genome sequence | Encoded on chromosome I or II | No. of clones identified in IVIAT screen |
|---|---|---|---|---|
| Pili | PilA | VC2423 | I | 17 |
| TcpA | VC0828 | I | 8 | |
| Cell membrane proteins | PilQ | VC2630 | I | 1 |
| MshO* | VC0412 | I | 1 | |
| MshP* | VC0413 | I | ||
| CapK | VC0924 | I | 1 | |
| Methyl-accepting chemotaxis proteins | MCP | VCA0176 | II | 1 |
| MCP | VCA1056 | II | 1 | |
| MCP | VC0216 | I | 1 | |
| Chemotaxis and motility proteins | CheA | VC1397 | I | 1 |
| CheR | VC1399 | I | 1 | |
| Quorum-sensing protein | LuxP | VCA0737 | II | 1 |
| Hypothetical proteins | VCA0536 | II | 1 | |
| VCA0884 | II | 1 | ||
| VCA0931 | II | 1 | ||
| VC1431 | I | 1 |
These two genes were encoded on the same recovered fragment
IVIAT also identified several clones containing genes not detected in previous screens in infant mice, including mshO, mshP, cheA, cheR, luxP, and four hypothetical ORFs. LuxP is a periplasmic protein that is a component of one of three recently described quorum-sensing systems in V. cholerae (31). Of the four hypothetical ORFs identified, three (VCA0536, VCA0884, and VCA0931) are predicted to encode cytoplasmic membrane proteins (PSORT program), and one (VC1431) has a signal peptide (SIGNALP program; www.cbs.dtu.dk/services/SignalP-2.0/) and a motif homologous to a bacterial chemotaxis sensory transducer (MOTIF program); PSORT and MOTIF analyses were done with tools available at free web-based resources such as the Baylor College of Medicine (http://searchlauncher.bam.tmc.edu). Interestingly, three of the four hypothetical ORFs identified are encoded on the smaller chromosome II of V. cholerae (32). Chromosome II has many fewer genes required for growth of V. cholerae in laboratory media, but contains many genes necessary for adaptation and growth of V. cholerae in unique environments (8). The fact that three of four hypothetical ORFs identified by IVIAT are encoded on chromosome II raises the possibility that these genes may encode functions specifically required for growth in human intestine.
The V. cholerae gene sequence identified most frequently by IVIAT was that encoding PilA, the structural subunit of a third type IV pilus in V. cholerae (identified 17 separate times in the IVIAT screen). In addition, IVIAT identified antibody in convalescent sera to PilQ. Sera from control patients did not recognize clones encoding PilA or PilQ. The fact that PilA and PilQ are expressed and immunogenic during human infection, but that mutation of pilA has no effect on colonization of infant mice by V. cholerae, raises the possibility that PilA and its assembly apparatus may play a specific role in colonization of human intestine.
To confirm the results of the IVIAT screen, we constructed plasmids specifically expressing TcpA, TcpF, MshA, PilA, and PilQ and examined reactivity of adsorbed patient sera with E. coli containing each of these plasmids. As shown in Fig. 2B, each selected clone showed prominent reactivity with adsorbed convalescent sera whereas a strain containing the control plasmid did not.
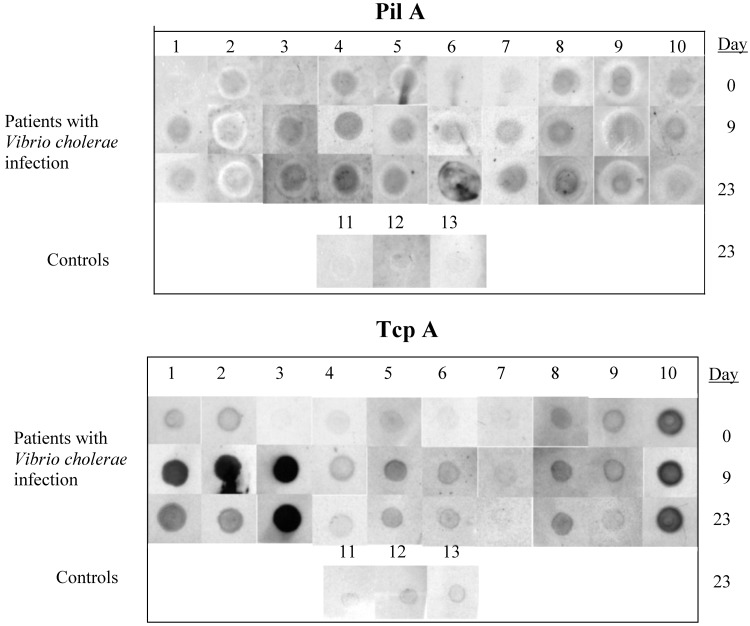
Individual Patients with El Tor V. cholerae Infection Develop Specific Immune Responses to Purified TcpA and PilA After Infection. Because of the importance of type IV pili in colonization of the gastrointestinal tract, and because adsorbed, convalescent sera from patients specifically recognized all three V. cholerae type IV pili, we focused on further analysis of immune responses to these pili. We examined whether TcpA and PilA reactivity was present at high level in a subset of patients from whom sera was pooled, or if most or all of individual patients recovering from cholera had antibody reactive to these proteins. For this purpose, we used immunoscreening with purified TcpA and PilA (see Materials and Methods) and unadsorbed sera from days 0, 9, and 23 after V. cholerae infection, comparing results to control patients who did not have V. cholerae infection; because the quantities of purified TcpA and PilA were limited, we used a dot-blot assay for analysis. Results in Fig. 3 show that, between day 0 and day 23, 6 patients increased immunoreactivity to PilA and 4 patients (numbers 2, 8, 9, and 10) showed immunoreactivity that did not change or decreased, suggesting that infection with V. cholerae may have begun earlier than study entry. For TcpA, 6 of 10 patients showed increasing reactivity, 3 patients (numbers 8, 9, and 10) showed immunoreactivity that did not change (or decreased) and 1 patient (patient 7) did not show immunoreactivity to TcpA, despite having culture-documented El Tor V. cholerae O1 infection and antibody to PilA. Control patients did not demonstrate significant immunoreactivity with either protein.
Fig. 3.
Comparison of reactivity of unadsorbed sera from individual patients collected on days 0, 9, and 23 after V. cholerae infection, and control patients from day 23, against purified El Tor PilA or TcpA.
Type IV pili share homology at the amino terminus (5). We considered the possibility that the serologic responses might be directed to epitopes shared between TcpA and PilA. However, the histidine-tagged form of TcpA used in our assay is missing the first 28 aa of the mature protein, the area of highest homology between type IV pili. Therefore, it is unlikely that the results represent crossreactivity between these two different pilus proteins. In addition, IVIAT identified reactivity with other components of each of the type IV pilus systems of V. cholerae, and these components do not share the same homology as the mature pilus subunits. These findings document strong human immune responses to TcpA and PilA after El Tor V. cholerae O1 infection.
Assessment of Expression of Selected Genes in Vitro by RT-PCR. We assessed expression of selected genes in vitro by using RT-PCR (data not shown). Transcription of tcpA was not detected after growth in LB broth but was present after growth in AKI media, as previously reported (22). Expression of pilA was detected after overnight growth in both LB broth and AKI media, even though antibodies reactive with PilA were not removed during the adsorption process. Expression of pilQ could not be detected after growth in either media. The control gene, rpoB, was expressed in both media as expected.
Because the RT-PCR results suggested no transcription of pilQ in LB broth, one possibility is that pilA is transcribed, but PilA is not assembled on the cell surface because of the absence of PilQ (and perhaps other components of the assembly machinery). This result might lead to premature degradation of PilA when V. cholerae is grown in LB broth.
Xu et al. (33) analyzed genes specifically expressed by El Tor V. cholerae during growth in a rabbit ileal loop, by using a gene microarray. They demonstrated that genes for mshABCD are highly expressed in rabbit ileal loop compared with in vitro, as are genes for motility and chemotaxis. Merrell et al. (34) demonstrated that V. cholerae recovered directly from human stool are hyperinfectious for infant mice compared with organisms grown in LB broth. Transcriptional profiling of V. cholerae in human stool suggested induction of genes involved in nutrient acquisition and motility, perhaps correlating with enhanced infectivity to subsequent hosts. Bina et al. (35) examined gene expression in V. cholerae in stools rapidly frozen at the bedside of cholera patients. Their results showed that several genes involved in pathogenesis were more highly expressed in stool compared with growth in LB broth; these more highly expressed genes included ctxAB, mshK, mshO, mshP, pilE, the pilOPQ operon, and cheA; of these genes, cheA and the pilOPQ operon were particularly strongly expressed during human infection. The genes for tcpA and mshA were less strongly expressed.
IVIAT identified many of the genes that were strongly expressed in both rabbit ileal loops and human stool, particularly genes encoding cell surface proteins (such as PilQ, MshO, MshP, CapK, the methyl-accepting chemotaxis proteins, and the hypothetical ORFs), or cytoplasmic proteins that are particularly strongly expressed in human stool, such as CheA. However, IVIAT did not identify all genes strongly expressed in human stool, particularly those whose protein products are not surface localized. This result may in part be due to the fact that our screen of the V. cholerae genome is not saturated, as demonstrated by the fact that we did not yet identify clones for cholera toxin, despite the fact that its in vivo expression is well established. It is also of interest that all of the genes from chromosome II of V. cholerae identified as expressed during human infection by IVIAT were expressed poorly in human stool by using microarray technology (35). One possibility is that genes on chromosome II turn off expression particularly quickly during the transit from the upper gastrointestinal tract to stool. IVIAT seems to preferentially identify proteins that are surface localized and highly immunogenic during human infection with a mucosal pathogen such as V. cholerae. Because these same proteins are likely involved in generating protective immune responses, IVIAT may provide data that enhance microarray technology to identify relevant antigens expressed in vivo.
The fact that convalescent sera from cholera patients specifically recognizes PilA and PilQ suggests that this pilus may be uniquely expressed during human infection, and may play a role in V. cholerae pathogenesis not suspected by previous animal model experiments. IVIAT may provide a new technology to detect genes specifically expressed during human infection. An important next step will be to use IVIAT in a more complete screen of the V. cholerae genome.
Acknowledgments
We appreciate the thoughtful comments of Drs. John J. Mekalanos, David Sack, and Firdausi Qadri. Dr. Ann-Mari Svennerholm provided MshA for the study, and Drs. A. S. G. Faruque, M. A. Salam, and G. J. Fuchs were involved in a study in which sera used here were collected. This work was supported by Grants UO1 HD39165 (to S.B.C.), RO1 AI44487 (to S.B.C.), RO1 AI25096 (to R.K.T.), RO1 AI40725 (to E.T.R.), and RO1 DE13523 (to M.H.) from the National Institutes of Health and Grant D43 TW05572 (to S.B.C.) from the Fogarty International Center.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TCP, toxin coregulated pilus; MSHA, mannose-sensitive hemagglutinin; RIVET, recombinase in vivo expression technology; IVIAT, in vivo-induced antigen technology.
References
- 1.Kaper, J. B., Morris, J. G. J. & Levine, M. M. (1995) Clin. Microbiol. Rev. 8 48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill, D. M. (1976) Biochemistry 15 1242-1248. [DOI] [PubMed] [Google Scholar]
- 3.Taylor, R. K., Miller, V. L., Furlong, D. B. & Mekalanos, J. J. (1987) Proc. Natl. Acad. Sci. USA 84 2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrington, D. A., Hall, R. H., Losonsky, G., Mekalanos, J. J., Taylor, R. K. & Levine, M. M. (1988) J. Exp. Med. 168 1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel, P., Marris, C. F., Mattick, J. S., Ruehl, W. W., Taylor, R. K. & Koomey, M. (1991) Infect. Immun. 59 4674-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun, D. X., Mekalanos, J. J. & Taylor, R. K. (1990) J. Infect. Dis. 161 1231-1236. [DOI] [PubMed] [Google Scholar]
- 7.Rhine, J. A. & Taylor, R. K. (1994) Mol. Microbiol. 13 1013-1020. [DOI] [PubMed] [Google Scholar]
- 8.Heidelberg, J. F., Eisen, J. A., Nelson, W. C., Clayton, R. A., Gwinn, M. L., Dodson, R. J., Haft, D. H., Hickey, E. K., Peterson, J. D., Umayam, L., et al. (2000) Nature 406 477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonson, G., Holmgren, J. & Svennerholm, A. M. (1991) Microb. Pathog. 11 433-441. [DOI] [PubMed] [Google Scholar]
- 10.Tacket, C. O., Taylor, R. K., Losonsky, G., Lim, Y., Nataro, J. P., Kaper, J. B. & Levine, M. M. (1998) Infect. Immun. 66 692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fullner, K. J. & Mekalanos, J. J. (1999) Infect. Immun. 67 1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cash, R. A., Music, S. I., Libonati, J. P., Snyder, M. J., Wenzel, R. P. & Hornick, R. B. (1974) J. Infect. Dis. 129 45-52. [DOI] [PubMed] [Google Scholar]
- 13.Cash, R. A., Music, S. I., Libonati, J. P., Craig, J. P., Pierce, N. F. & Hornick, R. B. (1974) J. Infect. Dis. 130 325-333. [DOI] [PubMed] [Google Scholar]
- 14.Mosley, W. H., Benenson, A. S. & Barui, R. (1968) Bull. W. H. O. 38 327-334. [PMC free article] [PubMed] [Google Scholar]
- 15.Glass, R. I., Becker, S., Huq, M. I., Stoll, B. J., Khan, M. U., Merson, M. H., Lee, J. V. & Black, R. E. (1982) Am. J. Epidemiol. 116 959-970. [DOI] [PubMed] [Google Scholar]
- 16.Glass, R. I., Svennerholm, A. M., Khan, M. R., Huda, S., Huq, M. I. & Holmgren, J. (1985) J. Infect. Dis. 151 236-242. [DOI] [PubMed] [Google Scholar]
- 17.Qadri, F., Jonson, G., Begum, Y. A., Wenneras, C., Albert, M. J., Salam, M. A. & Svennerholm, A. M. (1997) Clin. Diagn. Lab. Immunol. 4 429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall, R. H., Losonsky, G., Silveira, A. P., Taylor, R. K., Mekalanos, J. J., Witham, N. D. & Levine, M. M.. (1991) Infect. Immun. 59 2508-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilli, A., Beattie, D. T. & Mekalanos, J. J. (1994) Proc. Natl. Acad. Sci. USA 91 2634-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camilli, A. & Mekalanos, J. J. (1995) Mol. Microbiol. 18 671-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, S. J., Angelichio, M. J., Mekalanos, J. J. & Camilli, A. (1998) J. Bacteriol. 180 2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S. H., Hava, D. L., Waldor, M. K. & Camilli, A. (1999) Cell 99 625-634. [DOI] [PubMed] [Google Scholar]
- 23.Lee, S. H., Butler, S. M. & Camilli, A. (2001) Proc. Natl. Acad. Sci. USA 98 6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, W., Fullner, K. J., Clayton, R., Sexton, J. A., Rogers, M. B., Calia, K. E., Calderwood, S. B., Fraser, C. & Mekalanos, J. J. (1999) Proc. Natl. Acad. Sci. USA 96 1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensel, M., Shea, J. E., Gleeson, C., Jones, M. D., Dalton, E. & Holden, D. W. (1995) Science 269 400-403. [DOI] [PubMed] [Google Scholar]
- 26.Chiang, S. L. & Mekalanos, J. J. (1998) Mol. Microbiol. 27 797-805. [DOI] [PubMed] [Google Scholar]
- 27.Handfield, M., Brady, L. J., Progulske-Fox, A. & Hillman, J. D. (2000) Trends Microbiol. 8 336-339. [DOI] [PubMed] [Google Scholar]
- 28.Murley, Y. M., Behari, J., Griffin, R. & Calderwood, S. B. (2000) Infect. Immun. 68 3010-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig, L., Taylor, R. K., Pique, M. E., Adair, B. D., Arvai, A. S., Singh, M., Lloyd, S. J., Shin, D. S., Getzoff, E. D., Yeager, M., et al. (2003) Mol. Cell 11 1139-1150. [DOI] [PubMed] [Google Scholar]
- 30.Kirn, T. J. & Taylor, R. K. (2003) Mol. Microbiol., in press.
- 31.Miller, M. B., Skorupski, K., Lenz, D. H., Taylor, R. K. & Bassler, B. L. (2002) Cell 110 303-314. [DOI] [PubMed] [Google Scholar]
- 32.Trucksis, M., Michalski, J., Deng, Y. K. & Kaper, J. B. (1998) Proc. Natl. Acad. Sci. USA 95 14464-14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu, Q., Dziejman, M. & Mekalanos, J. J. (2003) Proc. Natl. Acad. Sci. USA 100 1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrell, D. S., Butler, S. M., Qadri, F., Dolganov, N. A., Alam, A., Cohen, M. B., Calderwood, S. B., Schoolnik, G. K. & Camilli, A. (2002) Nature 417 642-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bina, J., Zhu, J., Dziejman, M., Faruque, S., Calderwood, S. & Mekalanos, J. (2003) Proc. Natl. Acad. Sci. USA 100 2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]