Abstract
Innate immunity is an evolutionarily ancient system that provides organisms with immediately available defense mechanisms through recognition of pathogen-associated molecular patterns. We show that in the CNS, specific activation of innate immunity through a Toll-like receptor 4 (TLR4)-dependent pathway leads to neurodegeneration. We identify microglia as the major lipopolysaccharide (LPS)-responsive cell in the CNS. TLR4 activation leads to extensive neuronal death in vitro that depends on the presence of microglia. LPS leads to dramatic neuronal loss in cultures prepared from wild-type mice but does not induce neuronal injury in CNS cultures derived from tlr4 mutant mice. In an in vivo model of neurodegeneration, stimulating the innate immune response with LPS converts a subthreshold hypoxic-ischemic insult from no discernable neuronal injury to severe axonal and neuronal loss. In contrast, animals bearing a loss-of-function mutation in the tlr4 gene are resistant to neuronal injury in the same model. The present study demonstrates a mechanistic link among innate immunity, TLRs, and neurodegeneration.
Keywords: microglia, neuronal injury, lipopolysaccharide, infection
Systemic infection is associated with sustained worsening in many diseases of the CNS, yet the molecular and cellular relationship between infection outside the CNS and potential neuronal loss within the CNS is elusive. Activation of microglia, bone marrow-derived macrophage-like cells that function as the resident immune defense system of the brain (1), is a characteristic feature of most neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, multiple sclerosis, AIDS dementia complex, and amyotrophic lateral sclerosis as well as ischemia and posttraumatic brain injury (2–4). Neurotoxicity induced by β-amyloid or HIV proteins in mixed CNS cultures depends on the presence and activation of microglia (5, 6). Liberatore et al. (7) demonstrated in vivo that microglial inducible nitric oxide synthase plays a crucial role in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in the MPTP mouse model of Parkinson's disease.
The evolutionarily ancient innate immune system provides the first line of host defense against a large variety of pathogens and also controls many aspects of the adaptive immune response (8). Cells of the innate immune system recognize invariant molecular structures of pathogens termed pathogen-associated molecular patterns through a series of genetically conserved and stable cell-surface receptors related to the Drosophila gene toll that thus are referred to as Toll-like receptors (TLRs) (9).
TLR4 functions as the signal-transducing receptor for the endotoxin lipopolysaccharide (LPS) (10), which is a major component of the outer membrane of Gram-negative bacteria. LPS binds to the serum protein LPS-binding protein and the soluble or glycosylphosphatidylinositol-anchored CD14. This complex in turn binds to TLR4 (11) and initiates an intracellular signaling pathway that regulates gene expression through derepression of the transcriptional activator nuclear factor κB (12). To respond efficiently to LPS, TLR4 requires an accessory protein, MD-2, that binds to the extracellular domain of TLR4 and enhances its surface expression (13).
In the CNS, constitutive expression of TLR4 and CD14 transcripts was described in distinct anatomical areas of the brain (14, 15). We recently showed that microglia but not astrocytes or oligodendrocytes express TLR4 and that TLR4 is required for LPS-induced oligodendrocyte death in vitro (16).
The present study elucidates the mechanistic link among activation of innate immunity, TLRs, and neuronal injury in the neonatal CNS. We show that microglia are the major cells in the CNS that express TLR4 and that cortical neurons do not express this receptor. Fluorescently tagged LPS binds to microglia but not to neurons. LPS-induced neuronal cytotoxicity is not cellautonomous but rather requires microglia. In mixed CNS cultures prepared from mice lacking functional TLR4, LPS does not induce death of cortical neurons. Finally, we demonstrate that TLR4 is necessary for neuronal injury triggered by LPS in combination with a subthreshold hypoxic-ischemic insult in vivo.
Materials and Methods
Animals. C.C3H-Tlr4lps-d (lpsd/tlr4 mutant) and BALB/cJ (wildtype) mice were provided by The Jackson Laboratory.
RT-PCR. Relative levels of TLR4 and CD14 mRNA in neurons and microglia were determined by RT-PCR as described (16).
Cell Culture. Primary cultures of cortical neurons were generated from forebrains of embryonic day-17 (E17) BALB/cJ mice, Lpsd mice, or Sprague–Dawley rats (17).
Cortices were triturated and dissociated with papain in Earle's balanced salt solution for 5 min at 37°C, resuspended in 0.25% trypsin inhibitor/0.25% BSA in Earle's balanced salt solution (GIBCO), and incubated at 37°C for 5 min. Cells were harvested by centrifugation at 1,000 × g for 5 min. Cells (1 × 106) in MEM (GIBCO) plus 10% FBS were plated onto poly-d-lysine-coated glass slides (BD Biosciences). Cultures were grown in 5% CO2 at 37°C. Immediately after plating, staining revealed 90–95% purity for neurons. To obtain mixed glia, cells were maintained for 10 days until a mixed glial layer was achieved.
Spinal cord from E17 mice and E13 chicken or dorsal root ganglion cells from E13 chicken were triturated and dissociated with trypsin for 20 min at 37°C, passed through 70-μm mesh filters, and plated on poly-d-lysine-coated glass coverslips in DMEM plus 10% FBS.
Purified oligodendrocytes, microglia, and astrocytes were generated from forebrains of 2-day-old Sprague–Dawley rats as described (16, 18).
Immunfluorescence Microscopy. Cells were fixed and stained with antibodies against neuronal nuclei (NeuN), neurofilament, microtubule-associated protein 2, glial fibrillary acidic protein (Chemicon), O4 (American Type Culture Collection), and F4/80 (Serotec) as described (16). Secondary antibodies were from Jackson Immuno Research. Microglia were stained with isolectin IB4 and Alexa 488-conjugated LPS (Molecular Probes).
LPS in Combination with Hypoxia-Ischemia (HI) in Vivo. LPS treatment and HI were carried out as described (19) with some alterations. BALB/cJ and lpsd mouse pups received a single i.p. dose of LPS (Escherichia coli 0111:B4, List Biological Laboratories, Campbell, CA; 0.3 mg/kg) or vehicle (PBS). This dose was used to simulate a subclinical infection, and it induced no other apparent impairment in the animals. To induce HI in combination with LPS treatment, LPS or vehicle was administered to 7-day-old mice. One hour later pups were subjected to unilateral HI. Ligation of the right common carotid artery was carried out under ether anesthesia. After isolation of the artery, it was cauterized and bisected. The incision was sealed with nylon sutures. Mice were allowed to recover for 1.5 h and then were exposed to 7.7% oxygen in nitrogen at 33°C for 30 min before being returned to room air. The rectal temperature was measured before the injections, before and after surgery, and after HI was induced.
On postnatal day 11 (P11), mice were anaesthetized and perfused intracardially with PBS followed by 4% paraformaldehyde in PBS. Forebrains were postfixed in 4% paraformaldehyde in PBS for 4 h and then cryoprotected in 30% sucrose. Coronal sections (15 μm) were cut through the brain with a cryostat. Immunohistochemical analysis was performed as described (16) by using antibodies against neurofilament or NeuN.
Results
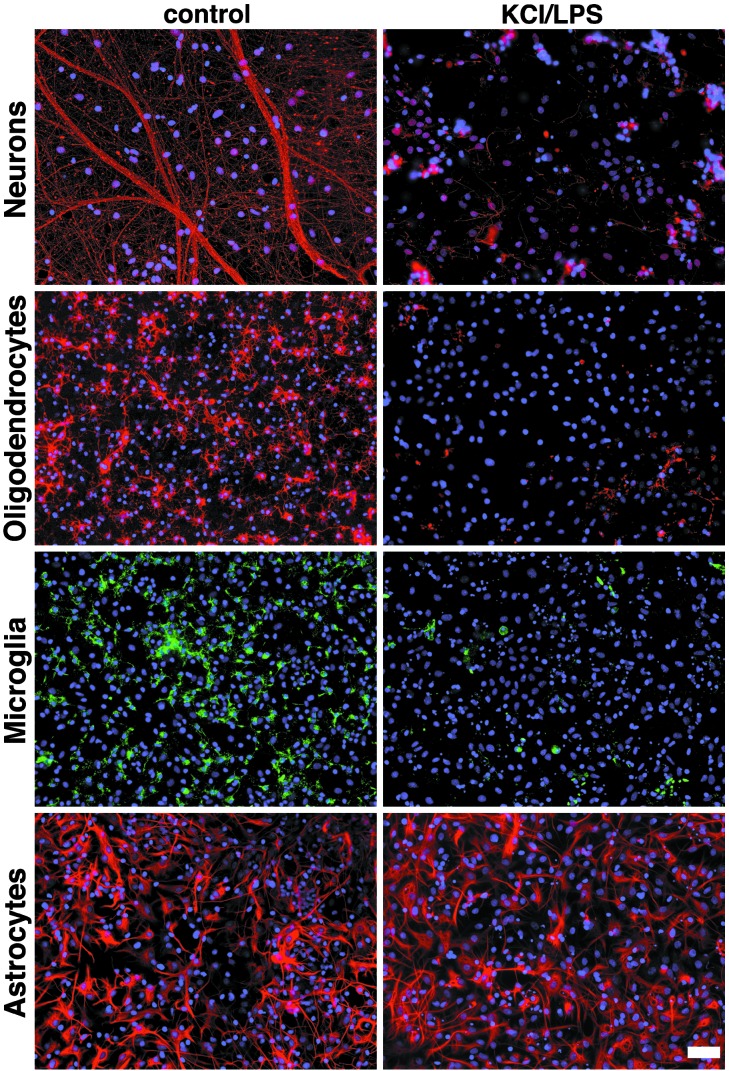
LPS Induces Loss of Axons, Oligodendrocytes, and Microglia in Mixed CNS Cultures. To investigate the effect of LPS on different CNS cell types, we generated mixed CNS cultures from rat forebrains and treated them with 10 μg/ml LPS in combination with 20 mM KCl for 5 days. Cells then were stained with antibodies against neurofilament, O4, glial fibrillary acidic protein, or isolectin B4 to analyze the effects of LPS on axons, oligodendrocytes, astrocytes, and microglia, respectively (Fig. 1). Because extracellular potassium enhances LPS-induced cytotoxicity (20), LPS treatment of cell cultures in further experiments was combined with the addition of 20 mM KCl. Control cultures were treated with KCl alone. Data were analyzed with respect to the total number of cells in culture as assessed by parallel 4′,6-diamidino-2-phenylindole (DAPI) staining. LPS plus 20 mM KCl induced a dramatic reduction in numbers of axons, oligodendrocytes, and microglia. Astrocyte numbers were not altered by LPS toxicity. LPS-treated astrocytes showed a hypertrophic shape compared with astrocytes under control conditions. Neuronal, oligodendrocyte, and microglial cultures treated with KCl alone showed no cell damage or cell loss when compared with untreated cultures.
Fig. 1.
LPS induces axonal, oligodendrocyte, and microglial death. Mixed CNS cultures from rat forebrains were incubated with 10 μg/ml LPS in combination with 20 mM KCl or with PBS for 5 days. Staining with antibodies against neurofilament, O4, and glial fibrillary acidic protein marked axons, oligodendrocytes, and astrocytes, respectively. Microglia were stained with isolectin B4. DAPI staining revealed the total number of cells by staining all nuclei. (Scale bar, 50 μm.)
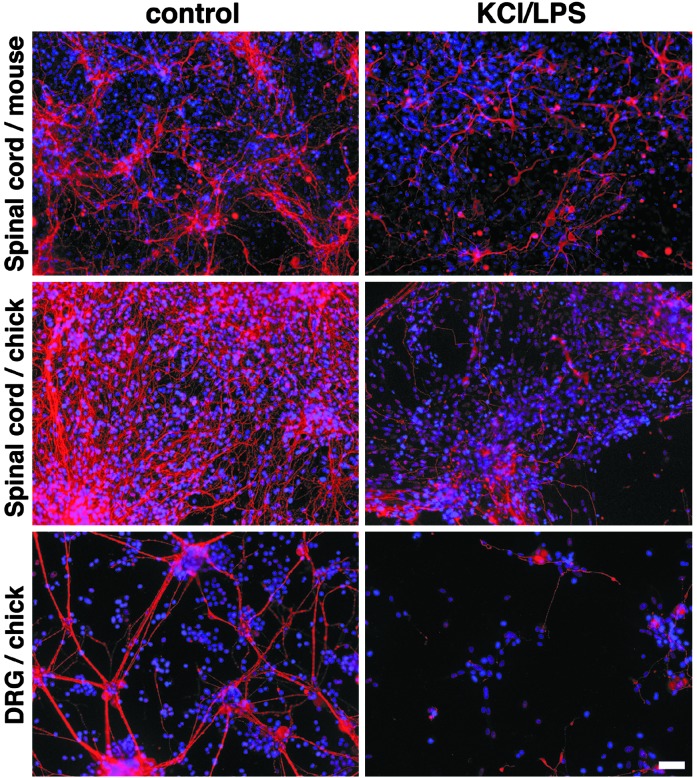
The Cytotoxic Effect of LPS on Neurons Does Not Depend on Animal Species or Neuronal Type. We next addressed the question of whether the effects of LPS on neurons depends on the neuronal cell type or species. We prepared neuronal cultures from mouse and chicken spinal cord and chicken dorsal root ganglion cells. Because dorsal root ganglion cell cultures do not contain glia, we added mixed glial cells from rat to these cultures before treatment with LPS to obtain comparable conditions. Cultures were treated with 10 μg/ml LPS and 20 mM KCl for 5 days and stained with antibodies against microtubule-associated protein 2, neurofilament, and DAPI to analyze cytotoxic effects on neurons (Fig. 2). All LPS-treated cultures showed striking damage and loss of axons and dendrites. These experiments show that the toxic effect of LPS on neurons is a general phenomenon, independent of neuronal subtype or species.
Fig. 2.
LPS-induced neurotoxicity does not depend on cell type or species. Mixed CNS cultures were prepared from mouse and chicken spinal cords. Dorsal root ganglion cells (DRG) were generated from chicken, and mixed glia from rat were added. Cultures were treated with 10 μg/ml LPS in combination with 20 mM KCl or PBS for 5 days. Dendrites and axons were stained with microtubule-associated protein 2 and antibody against neurofilament. Nuclei were stained with DAPI. (Scale bar, 50 μm.)
The Effect of LPS on Neurons Is Not Cell-Autonomous but Requires Microglia. Treatment of neuron/glia cocultures with LPS leads to loss of neurons (21). However, it is not clear whether this phenomenon is cell-autonomous.
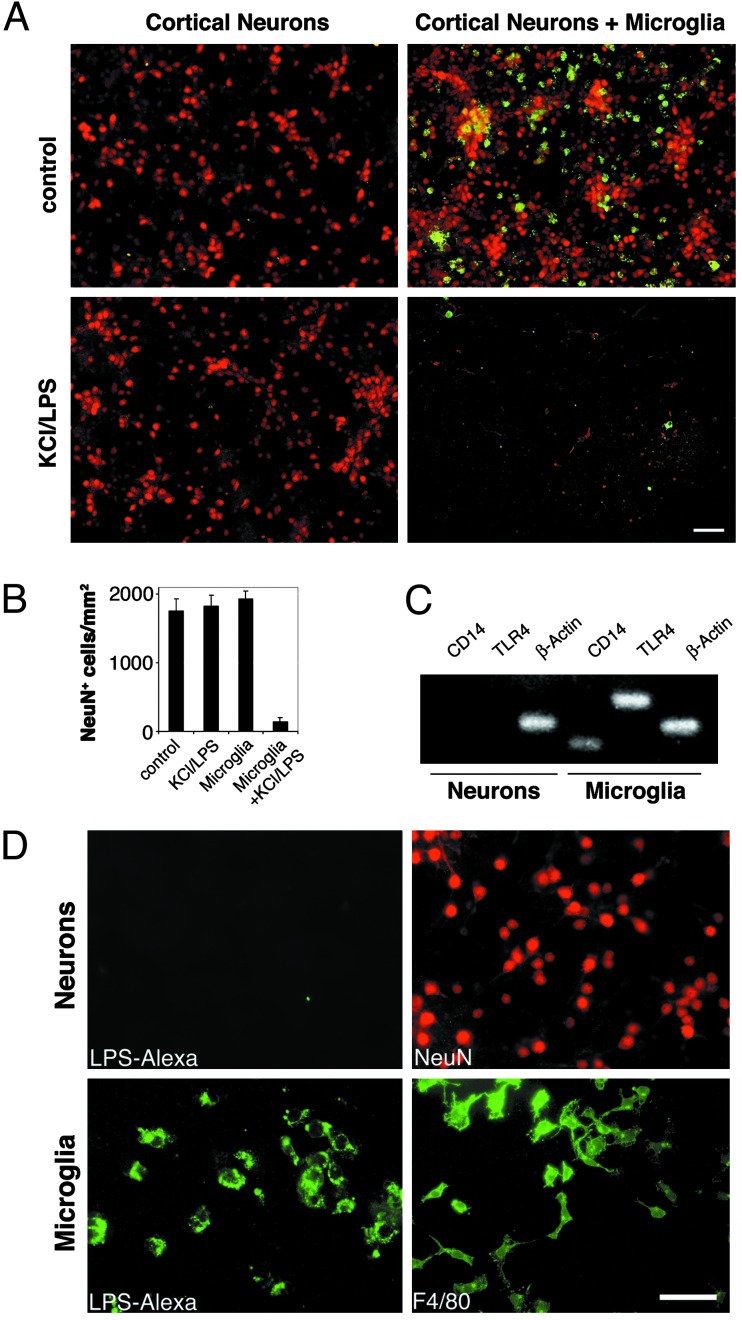
We generated purified cultures of cortical neurons. These cultures were used for studies only if the staining for oligodendrocytes, astrocytes, and microglia demonstrated <5% nonneuronal cells. Purified cultures of cortical neurons were treated with 10 μg/ml LPS and 20 mM KCl for 2 days and then stained with antibodies against NeuN and microglia (Fig. 3A). LPS had no effect on neuronal survival in the absence of microglia. In contrast, when purified microglia were added to highly enriched neurons, we observed an almost complete loss of neurons. The statistical analysis of the ratio of NeuN+ cells per mm2 confirmed this result (Fig. 3B). Microglial cells themselves did not survive treatment with LPS. These data indicate that the toxic effect of LPS on neurons is cell-extrinsic and requires microglia.
Fig. 3.
LPS-induced neurotoxicity is not cell-autonomous but requires microglia. Cortical neurons and microglia were prepared from rat forebrains. (A) Purified neurons and neurons in combination with microglia were incubated with 10 μg/ml LPS/KCl or PBS. After 2 days, cultures were fixed and stained with NeuN and F4/80 to mark neurons and microglia, respectively. (Scale bar, 50 μm.) (B) Quantitation of NeuN+ neurons in purified and microglia-enriched cultures in the presence or absence of LPS/KCl. Results are presented as mean ± SE. P < 0.001. (C) PCR. TLR4 is expressed in microglia but not in neurons. RNA was isolated from purified neuronal and microglial cultures and amplified by RT-PCR with specific primers for TLR4, CD14, or β-actin. (D) Fluorescently labeled LPS binds to microglia but not to neurons. Enriched cultures of microglia and cortical neurons were incubated with Alexa-tagged LPS and analyzed by immunofluorescence. Parallel cultures were stained to identify microglia (F4/80) and neurons (NeuN). (Scale bar, 100 μm.)
Neurons Do Not Express TLR4 or CD14 and Do Not Bind LPS in Vitro. Microglia express TLR4 and CD14 (16, 22). Both receptors are required for the effects of LPS on circulating monocytes (9). Our PCR studies determined that astrocytes and oligodendrocyte precursors express CD14 but not TLR4 (16). To investigate whether neurons express TLR4, CD14, or both, we prepared purified cultures of cortical neurons and microglia from newborn rats. To avoid contaminating signals from microglia, oligodendrocytes, or astrocytes, only neuronal cultures that contained >98% neurons as determined by immunohistochemistry were used. RNA was isolated and reverse-transcribed. PCR was carried out by using primers specific for rat TLR4 and CD14. No transcripts for TLR4 or CD14 were detected in the cortical neurons, whereas microglia expressed both (Fig. 3C).
Microglia bind Alexa-tagged LPS in vitro, whereas oligodendrocytes and astrocytes do not (16). Thus, we next asked whether neurons bind LPS. Primary cultures of purified rat cortical neurons and microglia were incubated with 10 μg/ml LPS-Alexa for 1 h at 37°C. Cells were visualized by fluorescence microscopy after washing with PBS (Fig. 3D). Cortical neurons did not show labeling with LPS-Alexa. The presence of neurons was confirmed by staining with NeuN. In contrast, microglia were stained intensely with LPS-Alexa.
TLR4 Is Necessary for LPS-Induced Neuronal Injury in Vitro. To determine whether TLR4 is necessary for LPS-mediated neuronal injury and death we made use of C.C3H-TLR4lps-d (lpsd) mice. The lpsd mutation originated from the C3H/HeJ mouse and was introduced into the BALB/cJ background by backcrossing C3H/HeJ mice to BALB/cJ mice. The resulting C.C3-TLR4lps-d mice were used in our experiments. The tlr4 mutant mouse C3H/HeJ is characterized by hyporesponsiveness to LPS as a consequence of a functionally defective TLR4 membrane protein due to a mutation of the C-terminal part of TLR4 that interferes with LPS-induced signaling. Macrophages from this strain fail to induce inflammatory cytokines such as tumor necrosis factor α, IL-1, and IL-6 (10, 23, 24).
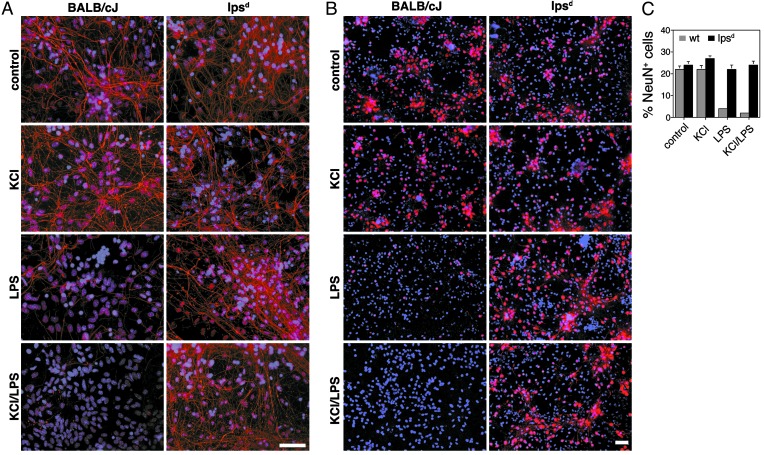
Cortical neurons from E17 forebrains were generated from lpsd and BALB/cJ mice. Staining after 10 days in culture revealed the presence of neurons and astrocytes, oligodendrocytes, and microglia. Cultures were treated with 10 μg/ml LPS or PBS for 5 days. Axons and NeuN were visualized by antineurofilament (Fig. 4A) and NeuN staining (Fig. 4B), respectively. No difference in axonal or neuronal number was observed between control-treated cultures from lpsd and those from BALB/cJ mice. LPS treatment led to a major loss of axons and neurons in mixed CNS cultures derived from BALB/cJ forebrains. LPS treatment had no effect on the number of axons and neurons of lpsd mice. DAPI staining and statistical analysis of the ratio of neuron to DAPI+ cells confirmed these results (Fig. 4C). At baseline, no differences in microglial numbers were detectable between lpsd and BALB/cJ mice (data not shown).
Fig. 4.
TLR4 is necessary for LPS-induced neuronal injury in vitro. Mixed CNS cultures prepared from BALB/cJ and lpsd mouse forebrains were incubated with 10 μg/ml LPS alone or in combination with 20 mM KCl for 5 days. (A) Cultures were stained with antibody against neurofilament to mark axons and with DAPI. (Scale bar, 50 μm.) (B) Cultures were stained with antibody NeuN (neurons) and DAPI. (Scale bar, 50 μm.) (C) Quantitation of NeuN+ neurons from BALB/cJ and lpsd mice in the presence or absence of LPS. Experiments were repeated three times. Results are presented as mean ± SE. P < 0.001.
TLR4 Is Necessary for LPS-Sensitized Hypoxic-Ischemic Neuronal Injury in Vivo. We addressed the question of whether the effect of LPS on neurons observed in vitro is relevant to models of neurodegeneration in vivo. We injected LPS or vehicle i.p. into 7-day-old lpsd and BALB/cJ mice and combined this treatment with exposure to HI. Three lpsd mice were treated with HI alone, and six lpsd mice received LPS i.p. before HI. In parallel, five BALB/cJ mice were treated with HI, and five BALB/cJ mice received an injection of LPS before HI. All 19 animals were studied after 4 days. There was no mortality between experimental treatment on P7 and fixation on P11. A tendency toward lower rectal temperatures after carotid ligation in animals from both strains was observed. The level of starting temperature was always achieved when the animals were allowed to recover after exposure to 7.7% oxygen.
In vehicle-treated animals combined with 30 min HI, no axonal or neuronal damage was observed in lpsd mice or BALB/cJ mice (Fig. 5). Of the total of five animals from the BALB/cJ strain treated with HI in combination with LPS, four showed axonal (Fig. 5A) and neuronal (Fig. 5B) loss in the corpus callosum and underlying structures ipsilateral to the carotid ligation. The contralateral hemisphere revealed neurofilament and NeuN staining comparable to animals that did not receive LPS. In contrast, of the total of six lpsd mice that received LPS in addition to HI, none showed axonal or neuronal damage. No difference in neurofilament and NeuN staining was observed between the ipsilateral and contralateral hemispheres. Quantitative analysis of the pericallosal area of the four affected BALB/cJ mice and six lpsd mice treated with HI and LPS confirmed these results. The total number of NeuN+ cells per field in the ipsilateral hemisphere of BALB/cJ mice was reduced 2.5-fold compared with the contralateral hemisphere, whereas numbers of NeuN+ cells of the ipsilateral hemisphere in lpsd mice showed no significant change compared with the contralateral side. The total number of NeuN+ cells of the contralateral side of BALB/cJ mice did not differ from the numbers of both hemispheres of lpsd mice. The results discussed above indicate an obligatory role for TLR4 in LPS-mediated neuronal injury in vivo.
Fig. 5.
TLR4 is necessary for LPS-induced neuronal injury in vivo. Coronal brain sections of P11 neonatal BALB/cJ and lpsd mice that received a single dose of either LPS or vehicle i.p. before being exposed to HI treatment on P7 were stained with antibodies against neurofilament (A) or NeuN (B). (Scale bar, 50 μm.)
Discussion
In most organ systems, inflammation causes bystander injury that is typically reversible due to the inherent regenerative capacity of the cellular elements of that tissue. However, in the CNS, the stakes are higher. The common consequence of bystander injury in the CNS is irreversible neuronal loss and atrophy due to regenerative failure. Through evolution there is no clear system that has developed to protect the CNS from the effects of inflammation as compensation for its poor regenerative capacity. In this report we elucidated a pathway that links a specific molecular component of Gram-negative infections, LPS, to the resident macrophage-like cell within the CNS, the microglial cell, through TLR4. We showed that activation of microglia by LPS results in neuronal and axonal loss both in vitro and in vivo. In the absence of functional TLR4, neurons and axons were resistant to LPS-mediated injury.
The relationship between neurodegeneration and microglial activation is complicated and unresolved. Traditionally, microglial activation is the normal response to CNS injury. However, in some cases activation of microglia is believed to contribute to, if not cause, neurodegeneration by releasing proinflammatory and cytotoxic factors, including nitric oxide and IL-1 (25, 26). Inhibition of microglial activation by minocycline protects MPTP-induced neuronal death in the MPTP mouse model of Parkinson's disease (27). Activated microglia are critical for N-methyl-d-aspartate-induced neurodegeneration in slice cultures (28). β-Amyloid in combination with IFN-γ triggers the production of reactive nitrogen intermediates and tumor necrosis factor α from microglia with consequent neuronal injury (29). Elevated levels of cytokines such as IL-1β and tumor necrosis factor α mainly produced by microglia are frequently detected in the serum and cerebrospinal fluid of patients with Alzheimer's disease, Parkinson's disease, multiple sclerosis, and amyotrophic lateral sclerosis (30). Activation of microglia is interpreted as an inflammatory response to infection and microglial cytokines are known to induce neuronal death in models of neurodegeneration. Therefore, the question of whether infections may play a role in the aforementioned diseases arises.
TLRs play a central role in the initiation of cellular innate immune responses and serve as pathogen-associated molecular pattern receptors that bind microbial molecular motifs with high specificity. TLR4 is required for transducing LPS signals on monocytes and macrophages (24). We showed that microglia play a role in innate immune actions in the CNS and that microglia are the only nonneuronal cell type that expresses TLR4 (16). In the present study we showed that microglia are not only the sole glial cells that express TLR4 mRNA but that this property also distinguishes microglia from neurons. Consistent with these results and in contrast to microglia, cortical neurons did not bind fluorescently tagged LPS. LPS induced axonal and neuronal loss in different neuronal populations derived from wild-type animals. Axons and neurons, however, were unaffected by LPS in cultures derived from tlr4 mutant mice. Because LPS functions through TLR4 and microglia but not neurons express TLR4, it is consistent that LPS-induced neurotoxicity requires the presence of microglia. It is possible that under conditions of stress as occur in pathologic states, TLR4 expression may be induced in cells other than microglia. Because we did not investigate expression of TLR4 in other neuronal cell types, the possibility that LPS-induced neurotoxicity in other types of neurons is based on a different mechanism than we have observed cannot be excluded.
We observed a rapid loss of microglia in purified cultures during LPS treatment as well as in CNS cultures, although to a lesser extent. It is possible that in vitro microglia “sacrifice” themselves during their normal response to injury or infection. Microglia can be divided into several subtypes based on their morphology and localization (31). Parenchymal microglia differentiate from monocytes that migrate into the CNS during embryonic development (32) and are maintained as a pool with a low turnover rate and limited, if any, replication during adulthood, although the number of microglia increases at sites of CNS reaction after injury. The origin of reactive microglia could either be migrating parenchymal microglia or monocytes induced to differentiate into parenchymal microglia. Other sources of microglia include perivascular microglia or leptomeningeal macrophages derived from circulating monocytes (33). It is also possible that function and survival of microglia require distinct yet unidentified molecules produced by the environment as it exists in vivo but not in vitro.
In some cases, activation of innate immunity may promote CNS regeneration. Zymosan, a yeast-wall extract and a potent pathogen-associated molecular pattern, promotes regeneration of crushed optic nerves (34). Overexpression of transforming growth factor β1 decreases neurotoxic β-amyloid plaque load in a mouse model of Alzheimer's disease through activation of microglia (35). Microglia introduced into the injured spinal cord support growth of injured axons (36). The differences between innate immunity-induced injury and protection may reside in a delicate balance of extracellular signals acting on microglia. With respect to injury, the context within the CNS in which microglia are activated may be critical. In amyotrophic lateral sclerosis, Alzheimer's disease, and stroke, where neurons experience a primary insult that predisposes them to cell death probably through oxidative stress (37, 38), the impact of a secondary insult mediated by microglia may tip the balance to irreversible injury. Consistent with this hypothesis, we show in this report that a subthreshold hypoxic-ischemic insult in mice can be converted to neurodegeneration by activating innate immunity. The CNS is particularly vulnerable to HI (39). Several studies suggest that defects in mitochondrial energy metabolism and excitotoxic neuronal death play a critical role in neurodegenerative diseases. To study the etiology of these diseases, animal models based on HI have been developed. The MPTP model of Parkinson's disease involves a specific defect in mitochondrial energy metabolism induced by MPP+, whereas models of HD are based on defects in oxidative phosphorylation (40, 41). To model the role of innate immunity in neurodegeneration we made use of a paradigm in which brief HI leads to a subthreshold injury in the CNS. When coupled with a specific activator of innate immunity such as LPS, this subthreshold injury is converted to prominent neuronal injury (19). We adapted this model of HI originally designed for the use in rats to 7-day-old mice. We used a low dose of i.p.-injected LPS to stimulate innate immunity, but not cause systemic hypotension, and a limited duration of HI. Although we detected extensive neuronal and axonal loss in the cortex and underlying structures in LPS-treated control animals, no such effect was seen in tlr4 mutant mice. Because we administered LPS systemically we cannot exclude the possibility that in BALB/cJ mice peripheral macrophages became activated and entered the brain through the damaged blood–brain barrier. It is possible that instead of brain-resident microglia, activated peripheral macrophages contributed to neuronal injury by producing neurotoxic molecules in response to activation of the TLR4 pathway.
LPS is only one potential ligand for TLR4. Other exogenous or endogenous ligands may contribute to neurotoxicity and neurodegeneration in disorders not caused by microorganisms. Therefore, LPS should be viewed as an exemplary ligand for TLR4 rather than the sole mechanism to activate microglia and lead to neurodegeneration. Our data demonstrate a key role for TLR4 in LPS-induced neuronal injury in vivo and support the concept that neurons experiencing a single reversible insult (HI) undergo irreversible injury after a second insult (microglial activation).
In summary, this study establishes a general mechanistic link between innate immunity and neuronal injury. Microglia are the only cell type in the resting CNS that expresses TLR4. TLR4 plays a crucial role in LPS-induced neuronal damage and cell death in vitro and in vivo. Further understanding the mechanisms mediated by TLRs may provide insights into potential therapeutic interventions for inflammation-related neurodegenerative diseases.
Acknowledgments
We thank Drs. Clifford Saper, Amyn Habib, Eckart Schott, and the Vartanian Laboratory for helpful comments. This work was supported by National Institute of Neurological Disorders and Stroke Grants P01NS38475 (to T.V., J.J.V., P.A.R., and F.E.J.), K02N502028 (to T.V.), and P30HD18655 and a grant from The Dalis Foundation.
Abbreviations: MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TLR, Toll-like receptor; LPS, lipopolysaccharide; En, embryonic day n; Pn, postnatal day n; NeuN, neuronal nuclei; HI, hypoxia-ischemia; DAPI, 4′,6-diamidino-2-phenylindole.
References
- 1.Kreutzberg, G. W. (1996) Trends Neurosci. 19 312-318. [DOI] [PubMed] [Google Scholar]
- 2.Dickson, D. W., Lee, S. C., Mattiace, L. A., Yen, S. H. & Brosnan, C. (1993) Glia 7 75-83. [DOI] [PubMed] [Google Scholar]
- 3.McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. (1988) Neurology 38 1285-1291. [DOI] [PubMed] [Google Scholar]
- 4.Trapp, B. D., Bo, L., Mork, S. & Chang, A. (1999) J. Neuroimmunol. 98 49-56. [DOI] [PubMed] [Google Scholar]
- 5.Giulian, D., Wendt, E., Vaca, K. & Noonan, C. A. (1993) Proc. Natl. Acad. Sci. USA 90 2769-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald, D. R., Brunden, K. R. & Landreth, G. E. (1997) J. Neurosci. 17 2284-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberatore, G. T., Jackson-Lewis, V., Vukosavic, S., Mandir, A. S., Vila, M., McAuliffe, W. G., Dawson, V. L., Dawson, T. M. & Przedborski, S. (1999) Nat. Med. 5 1403-1409. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov, R. & Janeway, C. A., Jr. (1997) Curr. Opin. Immunol. 9 4-9. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov, R. & Janeway, C., Jr. (2000) Immunol. Rev. 173 89-97. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, K., Takeuchi, O., Kawai, T., Sanjo, H., Ogawa, T., Takeda, Y., Takeda, K. & Akira, S. (1999) J. Immunol. 162 3749-3752. [PubMed] [Google Scholar]
- 11.Wright, S. D. (1999) J. Exp. Med. 189 605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medzhitov, R., Preston-Hurlburt, P., Kopp, E., Stadlen, A., Chen, C., Ghosh, S. & Janeway, C. A., Jr. (1998) Mol. Cell 2 253-258. [DOI] [PubMed] [Google Scholar]
- 13.Shimazu, R., Akashi, S., Ogata, H., Nagai, Y., Fukudome, K., Miyake, K. & Kimoto, M. (1999) J. Exp. Med. 189 1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacroix, S., Feinstein, D. & Rivest, S. (1998) Brain Pathol. 8 625-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laflamme, N. & Rivest, S. (2001) FASEB J. 15 155-163. [DOI] [PubMed] [Google Scholar]
- 16.Lehnardt, S., Lachance, C., Patrizi, S., Lefebvre, S., Follett, P. L., Jensen, F. E., Rosenberg, P. A., Volpe, J. J. & Vartanian, T. (2002) J. Neurosci. 22 2478-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy, T. H., Schnaar, R. L. & Coyle, J. T. (1990) FASEB J. 4 1624-1633. [PubMed] [Google Scholar]
- 18.Vartanian, T., Li, Y., Zhao, M. & Stefansson, K. (1995) Mol. Med. 1 732-743. [PMC free article] [PubMed] [Google Scholar]
- 19.Eklind, S., Mallard, C., Leverin, A. L., Gilland, E., Blomgren, K., Mattsby-Baltzer, I. & Hagberg, H. (2001) Eur. J. Neurosci. 13 1101-1106. [DOI] [PubMed] [Google Scholar]
- 20.Chang, R. C., Hudson, P. M., Wilson, B. C., Liu, B., Abel, H. & Hong, J. S. (2000) Neuroscience 97 757-764. [DOI] [PubMed] [Google Scholar]
- 21.Jeohn, G. H., Kim, W. G. & Hong, J. S. (2000) Brain Res. 880 173-177. [DOI] [PubMed] [Google Scholar]
- 22.Becher, B., Fedorowicz, V. & Antel, J. P. (1996) J. Neurosci. Res. 45 375-381. [DOI] [PubMed] [Google Scholar]
- 23.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Huffel, C. V., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282 2085-2088. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi, S. T., Lariviere, L., Leveque, G., Clermont, S., Moore, K. J., Gros, P. & Malo, D. (1999) J. Exp. Med. 189 615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao, C. C., Hu, S., Molitor, T. W., Shaskan, E. G. & Peterson, P. K. (1992) J. Immunol. 149 2736-2741. [PubMed] [Google Scholar]
- 26.Lee, S. C., Liu, W., Dickson, D. W., Brosnan, C. F. & Berman, J. W. (1993) J. Immunol. 150 2659-2667. [PubMed] [Google Scholar]
- 27.Wu, D. C., Jackson-Lewis, V., Vila, M., Tieu, K., Teismann, P., Vadseth, C., Choi, D. K., Ischiropoulos, H. & Przedborski, S. (2002) J. Neurosci. 22 1763-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ullrich, O., Diestel, A., Eyupoglu, I. Y. & Nitsch, R. (2001) Nat. Cell Biol. 3 1035-1042. [DOI] [PubMed] [Google Scholar]
- 29.Meda, L., Cassatella, M. A., Szendrei, G. I., Otvos, L., Jr., Baron, P., Villalba, M., Ferrari, D. & Rossi, F. (1995) Nature 374 647-650. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Scarano, F. & Baltuch, G. (1999) Annu. Rev. Neurosci. 22 219-240. [DOI] [PubMed] [Google Scholar]
- 31.Rinner, W. A., Bauer, J., Schmidts, M., Lassmann, H. & Hickey, W. F. (1995) Glia 14 257-266. [DOI] [PubMed] [Google Scholar]
- 32.Richardson, A., Hao, C. & Fedoroff, S. (1993) Glia 7 25-33. [DOI] [PubMed] [Google Scholar]
- 33.Hickey, W. F., Vass, K. & Lassmann, H. (1992) J. Neuropathol. Exp. Neurol. 51 246-256. [DOI] [PubMed] [Google Scholar]
- 34.Leon, S., Yin, Y., Nguyen, J., Irwin, N. & Benowitz, L. I. (2000) J. Neurosci. 20 4615-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyss-Coray, T., Lin, C., Yan, F., Yu, G. Q., Rohde, M., McConlogue, L., Masliah, E. & Mucke, L. (2001) Nat. Med. 7 612-618. [DOI] [PubMed] [Google Scholar]
- 36.Prewitt, C. M., Niesman, I. R., Kane, C. J. & Houle, J. D. (1997) Exp. Neurol. 148 433-443. [DOI] [PubMed] [Google Scholar]
- 37.Coyle, J. T. & Puttfarcken, P. (1993) Science 262 689-695. [DOI] [PubMed] [Google Scholar]
- 38.Beal, M. F. (1995) Ann. Neurol. 38 357-366. [DOI] [PubMed] [Google Scholar]
- 39.Lee, J. M., Grabb, M. C., Zipfel, G. J. & Choi, D. W. (2000) J. Clin. Invest. 106 723-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beal, M. F. (1992) Ann. Neurol. 31 119-130. [DOI] [PubMed] [Google Scholar]
- 41.Lee, J. M., Zipfel, G. J. & Choi, D. W. (1999) Nature 399 A7-A14. [DOI] [PubMed] [Google Scholar]