Abstract
Extracellular signal-regulated kinase 5 (ERK5) is a member of the mitogen-activated protein kinase family whose biological function in the CNS has not been defined. In contrast to ERK1 and ERK2, which are activated by neurotrophins (NTs), cAMP, and neuronal activity in cortical neurons, ERK5 is activated only by NTs. Here, we report that ERK5 expression is high in the brain during early embryonic development but declines as the brain matures to almost undetectable levels by postnatal day (P) 49. Interestingly, expression of a dominant-negative ERK5 blocked brain-derived neurotrophic factor protection against trophic withdrawal in primary cortical neurons cultured from embryonic day (E) 17 but not P0. Furthermore, expression of a dominant-negative ERK5 induced apoptosis in E17 but not P0 cortical neurons maintained in the presence of serum. We also present evidence that ERK5 protection of E17 cortical neurons may be mediated through myocyte enhancer factor 2-induced gene expression. These data suggest that ERK5 activation of myocyte enhancer factor 2-induced gene expression may play an important and novel role in the development of the CNS by mediating NT-promoted survival of embryonic neurons.
Neurotrophins (NTs) have profound effects on the development of the CNS and regulate differentiation, survival, and adaptive responses of neurons. For example, NTs protect many types of neurons from apoptosis both during development and in the adult (1–6). Consequently, there is an intense interest in elucidating mechanisms for NT-mediated neuroprotection and its contribution to the development of the CNS. NTs including nerve growth factor, brain-derived neurotrophic factor (BDNF), NT3, NT4/5, and NT6 bind to and activate specific receptor tyrosine kinases of the Trk family (7, 8). Upon binding to and activating Trk, NTs can activate several intracellular signaling transduction systems including the phosphatidylinositol 3-kinase, protein kinase C, and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways (8–11).
Recently, a new member of the mitogen-activated protein (MAP) kinase family, ERK5 (also known as the big MAP kinase 1 or BMK1) was discovered (12, 13). ERK5 is widely expressed in many tissues including brain. Although ERK5 contains a similar TEY dual-phosphorylation motif as ERK1/2 and the N-terminal half of ERK5 shares sequence homology with other members of the MAP kinase family, a large C terminus and a unique loop-12 sequence distinguish it from ERK1/2 and other MAP kinase family members. ERK5 is phosphorylated and activated by MAP kinase kinase (MEK) 5, but not by MEK1 or MEK2 (12, 14). MEK5 is specific for ERK5 and does not phosphorylate ERK1/2, c-Jun N-terminal protein kinase, or p38 MAP kinase (12, 14). A number of substrates have been identified for activated ERK5 including myocyte enhancer factor 2C (MEF2C) (15–17). Like ERK1/2, ERK5 is activated by serum, epidermal growth factor, nerve growth factor, and G protein-coupled receptors (15–19). In contrast to ERK1 and ERK2, which are activated by NTs, cAMP, and neuronal activity in cortical neurons, ERK5 is activated by NTs but not by cAMP or neuronal activity (20).
Although NT activation of phosphatidylinositol 3-kinase and ERK1/2 has been implicated in neuronal differentiation, survival, and neuroplasticity (3–6, 8, 21–27), the biological functions of ERK5 in CNS neurons have not been reported. In this study, we show that ERK5 is expressed developmentally and that it selectively mediates NT-promoted survival of developing CNS neurons but not mature neurons. We also identify MEF2 as a downstream target of ERK5 that mediates BDNF protection of embryonic neurons.
Materials and Methods
Materials. The following plasmids have been described: pON260 (28), the dominant-negative MEK5 and ERK5 (15), the Gal4-MEF2C fusion construct (17), the constitutively active MEF2C-VP16 (29), and the dominant-negative MEF2C-R24L (30). The polyclonal anti-ERK5 antibody was generated as described (20). BDNF and Lipofectamine 2000 were purchased from Invitrogen.
Cell Cultures. Primary cortical neurons were prepared from postnatal day 0 (P0; the day of birth) or embryonic day 17 (E17) Sprague–Dawley rats as described (4, 20, 31, 32). Briefly, dissociated neurons were plated at a density equivalent to 2 × 106 cells per 35-mm dish. Neurons were cultured in basal medium Eagle (Sigma) supplemented with 10% heat-inactivated bovine calf serum, 35 mM glucose, 1 mM l-glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin and maintained in a humidified incubator with 5% CO2 at 37°C. Plates and glass coverslips were coated with poly-d-lysine and laminin. Cytosine β-d-arabinofuranoside (2.5 μM; Sigma) was added to P0 cultures on the second day in vitro (DIV 2) to inhibit the proliferation of nonneuronal cells. Neurons were cultured for 4–5 days (DIV 4–5) before treatment.
Transient Transfection of Primary Cortical Neurons and Cell Death Assays. For cell death assays, neurons were transiently transfected at DIV 3–4 as described by using a calcium-phosphate coprecipitation protocol (4, 33) or Lipofectamine 2000, a lipid-based transfection method (5). Neurons were cotransfected with an expression vector encoding β-galactosidase (pON260) as a marker for transfected cells, and apoptosis was scored by nuclear fragmentation and condensation as described (4, 6, 31, 32). To ensure unbiased counting, slides were coded and apoptosis was scored without knowledge of the treatment. Statistical analysis of the data was performed by using one-way ANOVA.
Trophic Deprivation. At DIV 5–6, or 1–2 days after transfection, trophic deprivation was carried out by withdrawing serum in the presence of 10 μM MK801 as described (6).
Reporter Gene Assays. The Gal4-MEF2C-driven Gal4-luciferase reporter gene expression was assayed as described (20).
Northern Analysis. Total RNA was isolated from whole brains of Sprague–Dawley rats at E15, E18, E20, P0, P7, P14, P21, and P49, and 30 μg of total RNA samples was used for Northern blot analysis.
Western Analysis. Western blot analysis of activated ERK5 was performed as described by using a polyclonal anti-ERK5 antibody (20).
Results
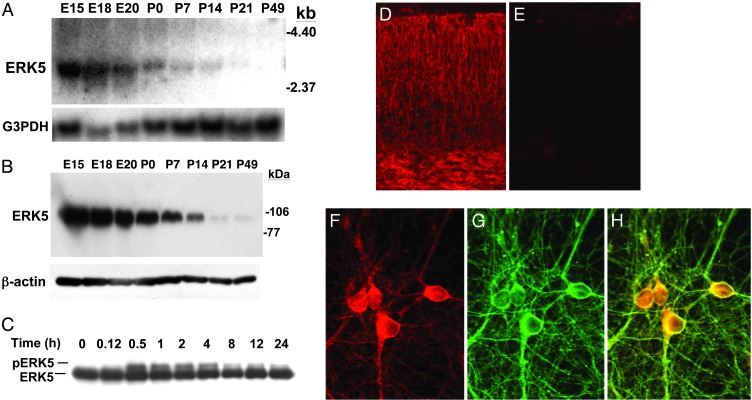
As a starting point for the elucidation of the biological functions of ERK5, we examined ERK5 expression in rat brain during development. Interestingly, ERK5 mRNA and protein levels are very high at E15–18 but gradually decrease to almost undetectable levels by P49 (Fig. 1 A and B). Furthermore, ERK5 proteins were expressed in E18 cortex (Fig. 1 D and E) and in neurons (Fig. 1 F–H). The differential expression of ERK5 from E15 to P49 suggests that ERK5 may play an important role in neurons during CNS development.
Fig. 1.
ERK5 is expressed and activated by BDNF in embryonic cortical neurons. (A) Thirty micrograms of total RNA isolated from whole rat brains at E15–P49 was used for Northern blot analysis for ERK5 mRNA expression. G3PDH was used as an internal loading control. (B) Whole brain extracts were prepared from rats at E15–P49 as indicated. Protein lysates (50 μg) were analyzed for ERK5 protein expression by Western blot analysis by using a polyclonal antibody against ERK5. β-Actin level was used as a loading control. The molecular mass markers are indicated on the right. (C) At DIV5, cortical neurons cultured from E17 rats were treated with BDNF for the indicated times. Cell lysates were prepared, and 20 μg of total protein was submitted to Western blot analysis by using a polyclonal antibody against ERK5. Phosphorylation of ERK5 was observed as a shift in ERK5 mobility, indicative of ERK5 activation. (D and E) ERK5 protein is expressed in cortex. E18 rat brain cortical sections were immunostained with anti-ERK5 antibody (D). When the antibody was preabsorbed with recombinant ERK5 protein (E), there was no staining, demonstrating the specificity of the immunostaining in D. (F–H) ERK5 protein is expressed in neurons. Neurons were stained with anti-ERK5 (F) and anti-β-tubulin III (G), a marker for neurons. The merged image in H demonstrates the costaining of the two proteins. (Magnification: D and E, ×50; F–H, ×500.)
Neurogenesis is regulated by neuronal activity and NTs. We previously reported that ERK5 is activated by NTs (BDNF, NT3, and NT4) but not by neuronal activity in postnatal cortical neurons (20). To determine whether ERK5 is important for NT regulation of neurogenesis, we measured BDNF stimulation of ERK5 in embryonic neurons. Activation of ERK5 was monitored by reduced electrophoretic mobility (phosphorylation shift) (15, 18, 20). Like postnatal neurons, ERK5 was activated by BDNF in E17 cortical neurons (Fig. 1C). Maximum activation of ERK5 occurred at 30–60 min after BDNF treatment, and ERK5 activation persisted for at least 24 h.
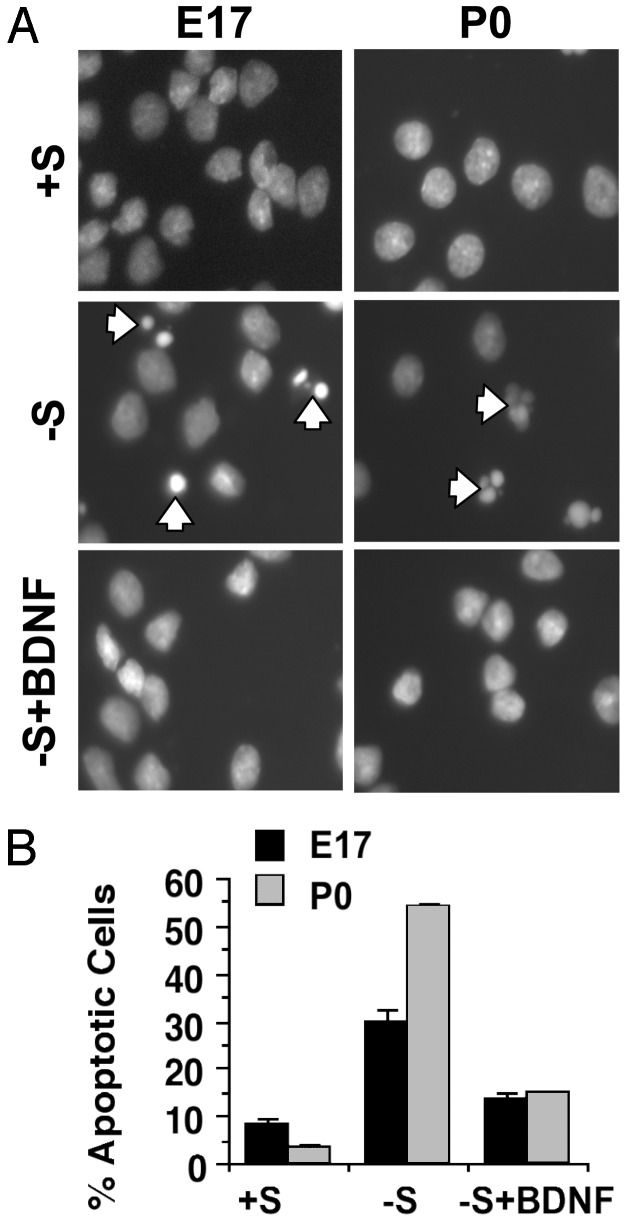
NTs regulate many aspects of neurogenesis including neuronal survival and differentiation. To determine whether ERK5 activation is important for BDNF regulation of neurogenesis, we focused on BDNF-promoted neuronal survival during development. We previously (6) established a trophic deprivation paradigm in which P0 cortical neuron apoptosis is induced by serum withdrawal in the presence of an N-methyl-d-aspartate receptor antagonist, dizocilpine maleate (MK-801). This paradigm for trophic deprivation also induced apoptosis effectively in E17 cortical neurons, which was blocked by BDNF (Fig. 2). Therefore, we chose this paradigm to study mechanisms of BDNF-promoted neuronal survival during development.
Fig. 2.
BDNF protects embryonic and postnatal cortical neurons from trophic deprivation-induced apoptosis. (A) Representative photomicrographs of nuclei morphology of cortical neurons at DIV6 treated for 24 h with control wash (+S) or trophic deprivation in the absence (-S) or presence (-S+BDNF) of BDNF. (Left) Neurons cultured from E17 rat cortex. (Right) Neurons cultured from P0 rat cortex. Nuclei morphology was visualized after Hoechst staining. Arrows identify cells with condensed or fragmented nuclei, characteristic of apoptosis. (Magnification: ×400.) (B) Quantification of apoptosis. Error bars, SEM.
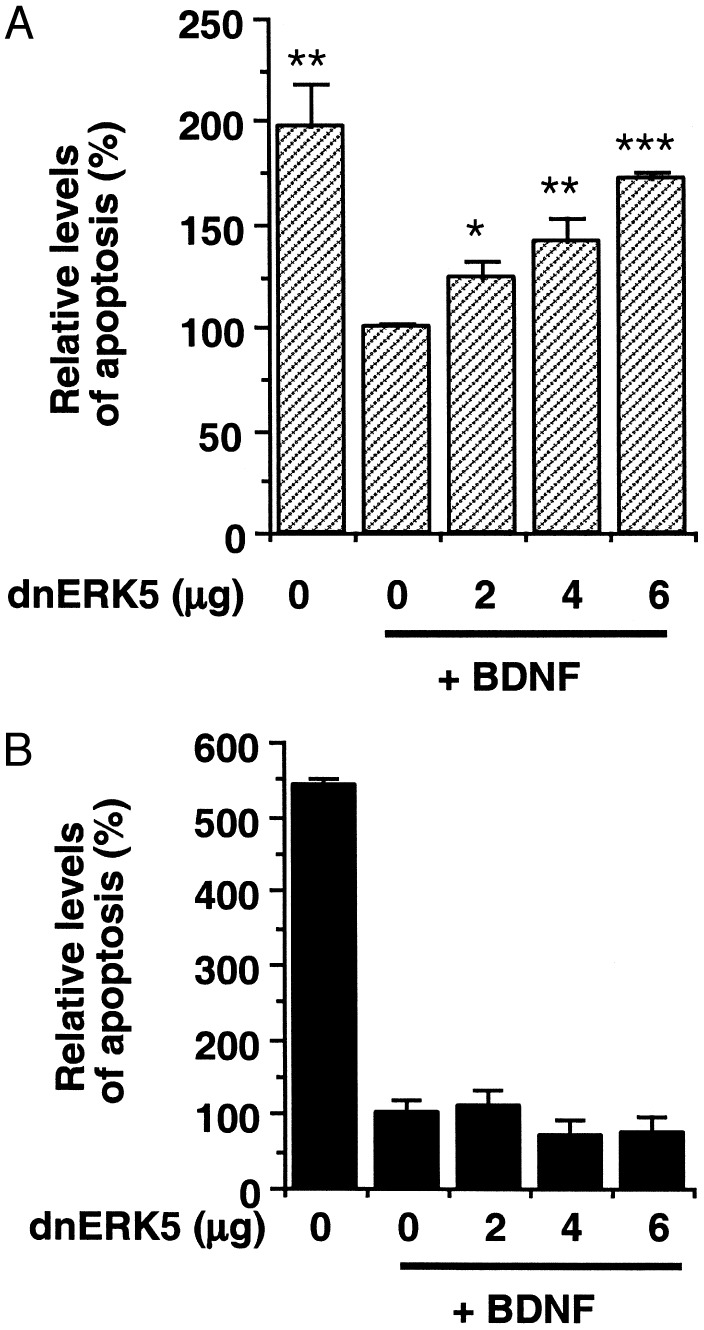
To determine whether ERK5 is important for BDNF neuroprotection, we transiently transfected a dominant-negative ERK5 into E17 and P0 cortical neurons and examined its effect on BDNF neuroprotection against trophic deprivation (Fig. 3). The activating phosphorylation site TEY in this ERK5 mutant was changed to AEP (T218A/Y220P) so that the mutant protein no longer can be phosphorylated or activated by upstream kinase MEK5 (17). Interestingly, expression of this dominant-negative ERK5 significantly reversed BDNF neuroprotection against trophic deprivation in E17 neurons (Fig. 3A). In contrast, expression of dominant-negative ERK5 in P0 neurons did not block BDNF neuroprotection (Fig. 3B). These data suggest that ERK5 activity is required for the neuroprotective activity of BDNF during development of the CNS.
Fig. 3.
Inhibition of the ERK5 pathway blocks BDNF protection against trophic withdrawal in embryonic, but not postnatal, cortical neurons. At DIV3–4, E17 (A) or P0 (B) neurons were transfected with various amounts of DNA encoding a dominant-negative ERK5 (dnERK5). Cells were also cotransfected with a plasmid DNA encoding β-galactosidase (PON260) as a marker for transfection. The pcDNA3 vector DNA was used to supplement so that all plates contained equal amounts of DNA. At 1–2 days after transfection, neurons were subject to trophic deprivation treatment with or without BDNF. Apoptosis in the transfected cell population (β-galactosidase-stained neurons) was scored 24 h after trophic deprivation. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (ANOVA); error bars, SEM.
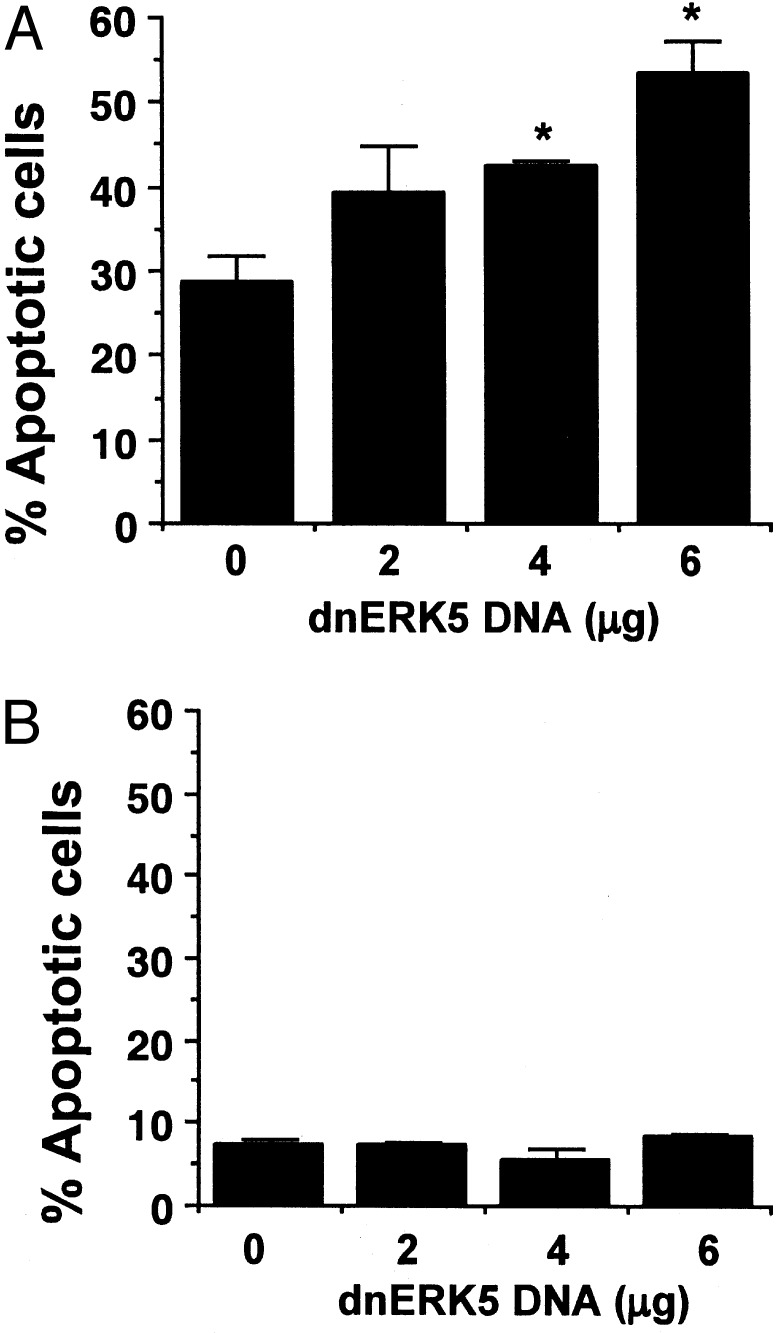
To determine whether ERK5 activity also is critical for serum-promoted neuronal survival, cortical neurons were transiently transfected with the dominant-negative ERK5 and its effect on basal cell survival was examined. Expression of this mutant significantly increased basal cell death even in the presence of serum in embryonic but not postnatal neurons (Fig. 4). These data suggest that the ERK5 pathway plays an important role in serum-promoted neuron survival in early development.
Fig. 4.
Inhibition of ERK5 increases basal cell death in embryonic, but not postnatal, cortical neurons cultured in the presence of serum. E17 (A) or P0 (B) neurons at DIV3–4 were transfected with various amounts of DNA encoding a dominant-negative ERK5 (dnERK5) as described in Fig. 3. Apoptosis in the transfected cell population (β-galactosidase-stained neurons) was scored 24 h (A) or 48 h (B) after transfection. *, P < 0.05 (ANOVA) compared with the sample from 0 μg of dnERK5; error bars, SEM.
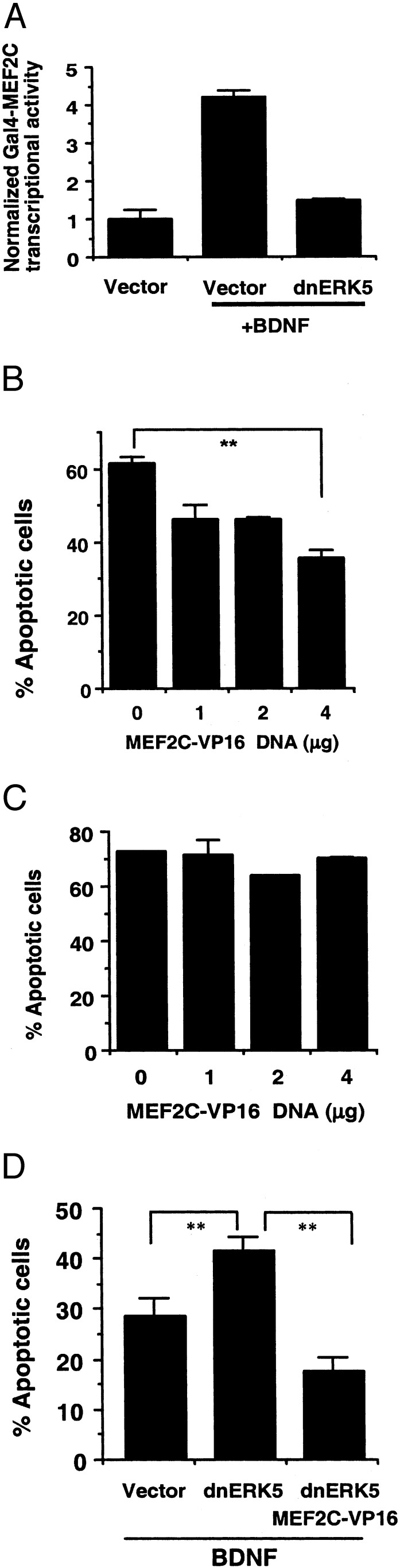
What is the downstream target that mediates the neuroprotective effect of ERK5? MEF2C is a well-established substrate of activated ERK5 in nonneuronal cells (15–17, 34), PC12 cells, and P0 cortical neurons (20). BDNF activated MEF2C transcriptional activity in E17 cortical neurons, and this activation was blocked by expression of a dominant-negative ERK5 (Fig. 5A). These data indicate that BDNF stimulation of MEF2C in embryonic neurons may be mediated by ERK5.
Fig. 5.
Expression of a constitutively active MEF2C is sufficient to protect E17 but not P0 cortical neurons from trophic deprivation. (A) Similar to P0 cortical neurons (20), BDNF activates MEF2C in E17 cortical neurons, which requires ERK5 signaling. To measure MEF2C transcriptional activity, E17 cortical neurons were transiently transfected with a Gal4-luciferase reporter gene and an expression vector for Gal4-MEF2C fusion protein as described (20). To block ERK5 signaling, neurons were cotransfected with a dominant-negative ERK5 (dnERK5; 600 ng DNA per well) or vector control. Two days after transfection, cells were incubated in serum-free medium for 2 h and then treated with BDNF or vehicle control for 6 h. E17 (B) or P0 (C) neurons at DIV3 were transfected with various amounts of DNA encoding MEF2C-VP16, a constitutively active MEF2C. One day after transfection, neurons were subject to trophic deprivation, and apoptosis in transfected cells (β-galactosidase-positive) was scored. (D) At DIV3–4, E17 neurons were transfected with a vector control, a dominant-negative ERK5 (dnERK5, 4 μg of DNA per 35-mm dish), and MEF2C-VP16 (3 μg of DNA per 35-mm dish) as indicated. One day after transfection, neurons were placed in BDNF-containing, serum-free medium. Apoptosis in the transfected cell population (β-galactosidase-stained neurons) was scored 24 h later. **, P < 0.01 (ANOVA); error bars, SEM.
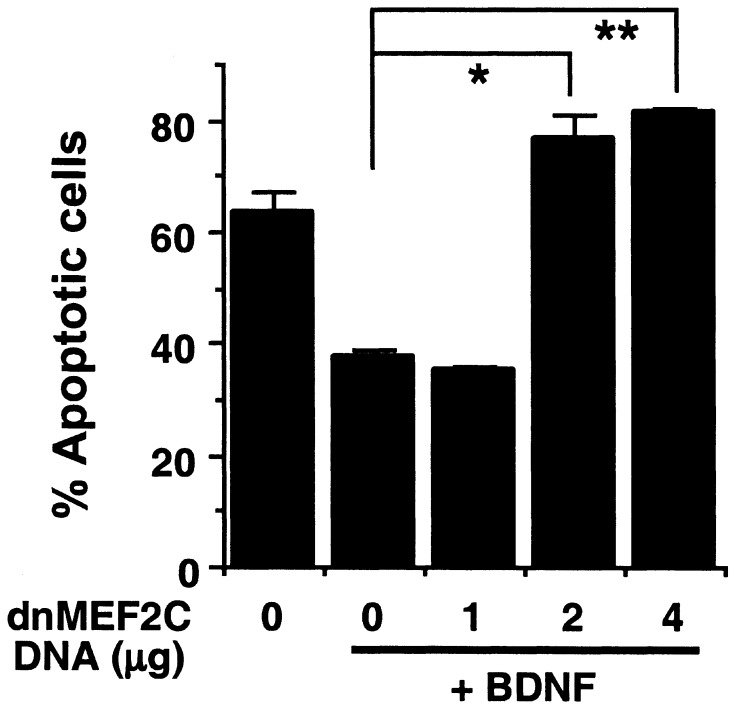
To determine whether MEF2-mediated gene expression contributes to ERK5 neuroprotection, cortical neurons were transfected with an expression vector encoding a constitutively active form of MEF2C (MEF2C-VP16) in which the DNA-binding and dimerization domain of MEF2C was fused to VP16 (29). Because the DNA-binding and dimerization domains of MEF2 isoforms are highly conserved (35), this MEF2C-VP16 mutant is expected to mimic the function of all activated MEF2s and to bind to MEF2 sites within the promoters of target genes. Expression of MEF2C-VP16 significantly protected embryonic (Fig. 5B,P < 0.01), but not postnatal (Fig. 5C), cortical neurons against trophic deprivation. Importantly, MEF2C-VP16 reversed the apoptosis caused by expression of the dominant-negative ERK5 (Fig. 5D). To block MEF2-mediated gene expression, cortical neurons were transfected with MEF2C-R24L, a dominant-negative MEF2C that contains a point mutation in the DNA-binding domain that prevents its binding to the specific MEF2 DNA-recognition site. However, MEF2C-R24L still dimerizes with WT MEF2s and, therefore, inhibits the function of endogenous MEF2s (30). Expression of this dominant-negative MEF2C completely reversed the BDNF neuroprotection against trophic deprivation in embryonic neurons (Fig. 6). Collectively, these data suggest that MEF2-mediated gene expression is both necessary and sufficient for trophic factor-promoted neuronal survival during development.
Fig. 6.
Expression of a dominant-negative MEF2C blocks BDNF protection against trophic deprivation in E17 cortical neurons. E17 cortical neurons at DIV3 were transfected with various amounts of DNA encoding MEF2C-R24L, a dominant-negative MEF2C (dnMEF2C). One day after transfection, neurons were subject to trophic deprivation treatment with or without BDNF. Apoptosis in the transfected cell population (β-galactosidase-positive) was scored 24 h after trophic deprivation. *, P < 0.05; **, P < 0.01 (ANOVA); error bars, SEM.
Discussion
The objective of this study was to determine whether ERK5 signaling contributes to NT protection of embryonic or mature cortical neurons. We report that ERK5 expression in the brain is high during early embryonic development and gradually declines as the brain matures. Similar to postnatal neurons, ERK5 is activated by BDNF and regulates MEF2C-mediated gene expression in embryonic cortical neurons. Although ERK5 is expressed in several adult tissues, including heart and skeletal muscle (12, 13), our data demonstrate a developmentally regulated expression of ERK5 in the CNS. These results suggest the interesting possibility that ERK5 may play a role in neuronal survival early in development when ERK5 levels are high, but not postnatally when ERK5 protein levels begin to decline. Indeed, blocking ERK5 activation induced cell death under normal culture conditions in E17 but not P0 cortical neurons, even in the presence of serum. Furthermore, blockade of ERK5 signaling inhibited the neuroprotective activity of BDNF against trophic deprivation in E17 neurons but not P0 neurons. Moreover, constitutive activation of MEF2 was sufficient to protect E17 cortical neurons against trophic deprivation but not P0 neurons. It also blocked cortical neuron apoptosis induced by dominant-negative ERK5. Inhibition of MEF2 activity completely abrogated BDNF neuroprotection against trophic deprivation in embryonic neurons. These data suggest that ERK5 and ERK5-regulated MEF2 gene expression selectively mediate NT-promoted cortical neuron survival during development.
The physiological function of ERK5 signaling is just beginning to be elucidated. Growth factor activation of ERK5 in nonneuronal cells has been implicated in fibroblast cell proliferation, muscle cell differentiation, and cancer cell transformation (18, 36–38). ERK5 null mice are embryonic-lethal at E9.5–E10.5 before the development of the CNS as a result of defective blood vessel and cardiac development (39, 40). Suzaki et al. (41) reported a potential role for ERK5 activation in PC12 cell survival after hydrogen peroxide treatment. Furthermore, ERK5 has been implicated in nerve growth factor retrograde signaling and survival of the peripheral dorsal root ganglia neurons (42). The data in this study demonstrate a unique function of ERK5 signaling in the survival of cortical neurons and a biological function of ERK5 in CNS neurons.
The MEF2 proteins constitute a family of transcription factors: MEF2A, MEF2B, MEF2C, and MEF2D. MEF2A and MEF2C are known substrates of ERK5, and their transactivating activity can be stimulated by ERK5 via direct phosphorylation (15, 17). In addition to muscle, MEF2A and MEF2C are expressed in developing and adult brain including cortex and cerebellum (43–46). Our data demonstrate that BDNF regulation of MEF2C-mediated gene expression in E17 cortical neurons is ERK5-dependent. Furthermore, expression of a constitutively active MEF2C mutant protected E17 cortical neurons from trophic deprivation and apoptosis induced by dominant-negative ERK5, whereas expression of a dominant-negative MEF2C blocked BDNF protection against trophic deprivation. These data illustrate that ERK5 regulation of MEF2-induced gene expression may play an important role in BDNF neuroprotection of developing cortical neurons. Because the DNA-binding and dimerization domains of MEF2 isoforms are highly conserved, results from these mutant MEF2C proteins alone cannot identify definitively MEF2C as the MEF2 isoform involved in neuroprotection. However, MEF2B and 2D are not regulated by ERK5 (17), ruling out an involvement of these two isoforms. Because cortex contains a relatively higher level of MEF2C protein, whereas cerebellum contains a relatively higher level of MEF2A protein (ref. 47; Z.M., unpublished data), the MEF2C isoform is likely to contribute to the BDNF/ERK5/MEF2-survival pathway.
In summary, we have discovered a mechanism that contributes to the survival of developing but not mature cortical neurons. We also identify MEF2C as a possible downstream target of ERK5 that mediates neuroprotection of embryonic, but not mature neurons, by BDNF. These studies have important implications for the protection of neurons from loss of growth factor support and the proper innervation of neuronal targets during fetal development.
Acknowledgments
This work was supported by Grant APP3010 from a Burroughs Wellcome Fund New Investigator Award in the Toxicological Sciences (to Z.X.) and National Institutes of Health Grants AG19193 (to Z.X.) and HD39446 (to Z.M.).
Abbreviations: NT, neurotrophin; MAP, mitogen-activated protein; MEK, MAP kinase kinase; ERK, extracellular signal-regulated kinase; MEF, myocyte enhancer factor; En, embryonic day n;Pn, postnatal day n; BDNF, brain-derived neurotrophic factor; DIV, day in vitro.
References
- 1.Levi-Montalcini, R. & Booker, B. (1960) Proc. Natl. Acad. Sci. USA 46 384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene, L. A. (1978) J. Cell Biol. 78 747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datta, S. R. & Greenberg, M. E. (1998) in Hormones and Signaling, ed. O'Malley, B. (Academic, San Diego), Vol. 1, pp. 257-306. [Google Scholar]
- 4.Hetman, M., Kanning, K., Cavanaugh, J. E. & Xia, Z. (1999) J. Biol. Chem. 274 22569-22580. [DOI] [PubMed] [Google Scholar]
- 5.Hetman, M., Hsuan, S. L., Habas, A., Higgins, M. J. & Xia, Z. (2002) J. Biol. Chem. 277 49577-49584. [DOI] [PubMed] [Google Scholar]
- 6.Hetman, M., Cavanaugh, J. E., Kimelman, D. & Xia, Z. (2000) J. Neurosci. 20 2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snider, W. D. & Wright, D. E. (1996) Neuron 16 229-232. [DOI] [PubMed] [Google Scholar]
- 8.Segal, R. A. & Greenberg, M. E. (1996) Annu. Rev. Neurosci. 19 463-489. [DOI] [PubMed] [Google Scholar]
- 9.Marshall, C. J. (1995) Cell 80 179-185. [DOI] [PubMed] [Google Scholar]
- 10.Castellino, A. M. & Chao, M. V. (1996) Cytokine Growth Factor Rev. 7 297-302. [DOI] [PubMed] [Google Scholar]
- 11.Klesse, L. J. & Parada, L. F. (1999) Microsc. Res. Tech. 45 210-216. [DOI] [PubMed] [Google Scholar]
- 12.Zhou, G., Bao, Z. Q. & Dixon, J. E. (1995) J. Biol. Chem. 270 12665-12669. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J. D., Ulevitch, R. J. & Han, J. (1995) Biochem. Biophys. Res. Commun. 213 715-724. [DOI] [PubMed] [Google Scholar]
- 14.English, J. M., Vanderbilt, C. A., Xu, S., Marcus, S. & Cobb, M. H. (1995) J. Biol. Chem. 270 28897-28902. [DOI] [PubMed] [Google Scholar]
- 15.Kato, Y., Kravchenko, V. V., Tapping, R. I., Han, J. H., Ulevitch, R. J. & Lee, J. D. (1997) EMBO J. 16 7054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.English, J. M., Pearson, G., Baer, R. & Cobb, M. H. (1998) J. Biol. Chem. 273 3854-3860. [DOI] [PubMed] [Google Scholar]
- 17.Marinissen, M. J., Chiariello, M., Pallante, M. & Gutkind, J. S. (1999) Mol. Cell. Biol. 19 4289-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, Y., Tapping, R. I., Huang, S., Watson, M. H., Ulevitch, R. J. & Lee, J. D. (1998) Nature 395 713-716. [DOI] [PubMed] [Google Scholar]
- 19.Kamakura, S., Moriguchi, T. & Nishida, E. (1999) J. Biol. Chem. 274 26563-26571. [DOI] [PubMed] [Google Scholar]
- 20.Cavanaugh, J. E., Ham, J., Hetman, M., Poser, S., Yan, C. & Xia, Z. (2001) J. Neurosci. 21 434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansour, S. J., Matten, W. T., Hermann, A. S., Candia, J. M., Rong, S., Fukasawa, K., Vande Woude, G. F. & Ahn, N. G. (1994) Science 265 966-970. [DOI] [PubMed] [Google Scholar]
- 22.Seger, R. & Krebs, E. G. (1995) FASEB J. 9 726-735. [PubMed] [Google Scholar]
- 23.Riccio, A., Ahn, S., Davenport, C. M., Blendy, J. A. & Ginty, D. D. (1999) Science 286 2358-2361. [DOI] [PubMed] [Google Scholar]
- 24.Cowley, S., Paterson, H., Kemp, P. & Marshall, C. J. (1994) Cell 77 841-852. [DOI] [PubMed] [Google Scholar]
- 25.Lewin, G. R. & Barde, Y. A. (1996) Annu. Rev. Neurosci. 19 289-317. [DOI] [PubMed] [Google Scholar]
- 26.Casaccia-Bonnefil, P., Gu, C. & Chao, M. V. (1999) Adv. Exp. Med. Biol. 468 275-782. [DOI] [PubMed] [Google Scholar]
- 27.Impey, S., Obrietan, K. & Storm, D. R. (1999) Neuron 23 11-14. [DOI] [PubMed] [Google Scholar]
- 28.Cherrington, J. M. & Mocarski, E. S. (1989) J. Virol. 63 1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han, J., Jiang, Y., Li, Z., Kravchenko, V. V. & Ulevitch, R. J. (1997) Nature 386 296-299. [DOI] [PubMed] [Google Scholar]
- 30.Molkentin, J. D., Black, B. L., Martin, J. F. & Olson, E. N. (1996) Mol. Cell. Biol. 16 2627-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namgung, U. & Xia, Z. (2000) J. Neurosci. 20 6442-6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueroa-Masot, X. A., Hetman, M., Higgins, M. J., Kokot, N. & Xia, Z. (2001) J. Neurosci. 21 4657-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia, Z., Dudek, H., Miranti, C. K. & Greenberg, M. E. (1996) J. Neurosci. 16 5425-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, C. C., Ornatsky, O. I., McDermott, J. C., Cruz, T. F. & Prody, C. A. (1998) Nucleic Acids Res. 26 4771-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Black, B. L. & Olson, E. N. (1998) Annu. Rev. Cell Dev. Biol. 14 167-196. [DOI] [PubMed] [Google Scholar]
- 36.Dinev, D., Jordan, B. W., Neufeld, B., Lee, J. D., Lindemann, D., Rapp, U. R. & Ludwig, S. (2001) EMBO Rep. 2 829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong, F., Gutkind, J. S. & Larner, A. C. (2001) J. Biol. Chem. 276 10811-10816. [DOI] [PubMed] [Google Scholar]
- 38.Pearson, G., English, J. M., White, M. A. & Cobb, M. H. (2001) J. Biol. Chem. 276 7927-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regan, C. P., Li, W., Boucher, D. M., Spatz, S., Su, M. S. & Kuida, K. (2002) Proc. Natl. Acad. Sci. USA 99 9248-9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sohn, S. J., Sarvis, B. K., Cado, D. & Winoto, A. (2002) J. Biol. Chem. 277 43344-43351. [DOI] [PubMed] [Google Scholar]
- 41.Suzaki, Y., Yoshizumi, M., Kagami, S., Koyama, A. H., Taketani, Y., Houchi, H., Tsuchiya, K., Takeda, E. & Tamaki, T. (2002) J. Biol. Chem. 277 9614-9621. [DOI] [PubMed] [Google Scholar]
- 42.Watson, F. L., Heerssen, H. M., Bhattacharyya, A., Klesse, L., Lin, M. Z. & Segal, R. A. (2001) Nat. Neurosci. 4 981-988. [DOI] [PubMed] [Google Scholar]
- 43.McDermott, J. C., Cardoso, M. C., Yu, Y. T., Andres, V., Leifer, D., Krainc, D., Lipton, S. A. & Nadal-Ginard, B. (1993) Mol. Cell. Biol. 13 2564-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leifer, D., Krainc, D., Yu, Y. T., McDermott, J., Breitbart, R. E., Heng, J., Neve, R. L., Kosofsky, B., Nadal-Ginard, B. & Lipton, S. A. (1993) Proc. Natl. Acad. Sci. USA 90 1546-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyons, G. E., Micales, B. K., Schwarz, J., Martin, J. F. & Olson, E. N. (1995) J. Neurosci. 15 5727-5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao, Z., Bonni, A., Xia, F., Nadal-Vicens, M. & Greenberg, M. E. (1999) Science 286 785-790. [DOI] [PubMed] [Google Scholar]
- 47.Lin, X., Shah, S. & Bulleit, R. F. (1996) Mol. Brain Res. 42 307-316. [DOI] [PubMed] [Google Scholar]