Abstract
Some individuals claim that they are very sensitive to pain, whereas others say that they tolerate pain well. Yet, it is difficult to determine whether such subjective reports reflect true interindividual experiential differences. Using psychophysical ratings to define pain sensitivity and functional magnetic resonance imaging to assess brain activity, we found that highly sensitive individuals exhibited more frequent and more robust pain-induced activation of the primary somatosensory cortex, anterior cingulate cortex, and prefrontal cortex than did insensitive individuals. By identifying objective neural correlates of subjective differences, these findings validate the utility of introspection and subjective reporting as a means of communicating a first-person experience.
The conscious experience of a sensory event is derived from a complex convolution of afferent information arising from peripheral sensory transducers with cognitive information about the present context, past history, and future implications of the stimulus. Accordingly, the experience of a specific stimulus is unique to a given individual. Thus, a fundamental problem inherent in the exploration of any conscious sensory experience is how a third-person observer can appreciate the first-person experience of another individual (1). In many instances, when a stimulus is available to multiple observers, concurrence among observers serves to reinforce the idea that the experience of each observer is highly similar to that of the others. Yet, in other cases, such external referents are either unavailable or present inaccurate information to outside observers, rendering the first-person perspective on the sensory experience relatively private and inaccessible. Thus, questions about the contents of consciousness have long resided in the domain of philosophers (2). However, an individual's experience of pain, particularly pain of pathological origin, underscores the practical importance of appreciating a first-person experience from a third-person perspective (2). Undetectable physical differences in injuries or disease processes can result in chronic pain for one individual but only minimal deficits for another. Furthermore, an individual's subjective experience of pain can vary substantially from day-to-day despite being evoked by a temporally invariant stimulus (3).
In an effort to facilitate description of the subjective experience, a variety of psychophysical techniques have been developed to provide a rigorous framework for standardizing the communication about a sensory experience between the individual and an observer (4, 5). However, in the absence of any reliable objective anchor, individual differences in psychophysical ratings are frequently viewed as artifacts of scale usage rather than a reflection of true experiential differences (6). Brain imaging studies of multiple subjects have identified significant within-subject relationships between regional brain activity and subjective reports of pain evoked by different intensities of stimuli (7–9). Yet, no study to date has identified the neural correlates of an individual's subjective experience of pain and characterized them in relation to those of other individuals receiving exactly the same stimulus. Thus, 17 normal volunteers were recruited to participate in a combined psychophysical and functional MRI (fMRI) study of interindividual differences in pain sensitivity.
Methods
Subjects. A total of 17 normal, healthy subjects (8 women and 9 men) participated in this study. All participants were white and ranged in age from 21 to 40 yr (mean age 26 yr). All procedures were approved by the Institutional Review Board of Wake Forest University School of Medicine. All volunteers gave written, informed consent acknowledging that (i) they would undergo brain imaging and experience experimental pain stimuli, (ii) all methods and procedures were clearly explained, and (iii) they were free to withdraw from the experiment at any time.
Psychophysical Training and Assessment. Thermal stimuli were delivered by a 16 × 16-mm peltier device (Medoc, Ramat-Yishai, Israel; TSA-II) and were assessed with a 10-unit mechanical visual analog scale (VAS) for pain intensity (5). Subjects first participated in a psychophysical training session in which they rated 32 stimuli (35°C, 43–49°C, 5-s duration) applied to their nondominant ventral forearm (10). These data are not reported further. Subjects then provided a description of the time course of pain intensity by continuously rating a set of stimuli identical to those presented during the functional imaging session by using a computerized VAS. Individual responses were then normalized to a range of 0 to 1 and averaged together to characterize the time course of perceived pain intensity. Poststimulus ratings of pain intensity were also acquired for these stimuli to enable direct comparison with those obtained during the scanning session.
Functional Imaging. Each of the subjects underwent functional imaging during thermal stimulation of the skin of the right lower leg (overlying the inferior aspect of the popliteal fossa and superior portions of the medial and lateral heads of the gastrocnemius muscle). For painful stimulation, five 30-s duration epochs of 49°C stimulation were interleaved with six 30-sduration epochs of 35°C stimulation (with rise and fall rates of 6°C/s). Each functional image series consisted of 110 volumes acquired with a 2D spiral sequence [28 × 5-mm-thick slices per volume, with 3.75 × 3.75-mm in-plane resolution, repetition time (TR) = 3 s, echo time (TE) = 40 ms, a = 88°] at 1.5 T (General Electric Horizon LX). Two such series were acquired for each subject during 49°C stimulation. At the end of each 330-s functional imaging series, subjects provided a psychophysical rating of pain intensity. The average of these two ratings was used to assign subjects to a high-, moderate-, or low-sensitivity subgroup. A high-resolution structural volume (3D spoiled gradient recalled echo sequence) also was acquired for each subject for anatomic localization of functional changes.
All image processing operations and statistical analyses were accomplished with FSL software [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, Oxford]. Functional data were movement corrected, normalized for global signal change, smoothed with a 7.5-mm Gaussian filter, and temporally filtered (sigma = 45.0 s). These data were then registered with structural data by using a 7-parameter transform and transformed into stereotaxic space by using a 12-parameter transform calculated from the structural volume (11, 12).
For statistical analyses of pain intensity-related activation, the hemodynamic response function was modeled by first scaling the averaged time course of pain intensity ratings such that the peak rating of the time course data was equal to that obtained at the end of each scanning series for each individual. This scaled rating was then convolved with a gamma function (3-s SD, 6-s lag) to better approximate hemodynamic changes. For each individual, fixed effects general linear modeling (GLM) analyses were used to identify brain activation associated with the modeled hemodynamic response function, with significant activation being determined by Z > 3.1 and a cluster significance of P < 0.005 (13–15). These individual statistical maps then were binarized and summed to produce separate images of the frequency of activation for high-sensitivity and low-sensitivity subgroups. To further confirm results from frequency analyses, multi-subject GLM analyses were performed to identify pain intensity-related activation within the high- and low-sensitivity subgroups. Within-subgroup analyses were performed by using regressors that were scaled by each individual's psychophysical rating.
To identify brain regions that were activated more frequently in the high-sensitivity individuals, the frequency map of the low-sensitivity group was subtracted from that of the high-sensitivity group. Regions with frequency differences ≥4 were defined as statistically significant (Fisher's exact test, P < 0.05). To determine whether the high-sensitivity subgroup exhibited significantly larger signal changes than the low-sensitivity subgroup, an additional multisubject GLM analysis was performed. Importantly, this analysis used the same nonscaled pain intensity time course regressor for each individual to ensure that assessments of brain activation would not be confounded by psychophysical differences. As with the individual GLM analyses, statistically significant activations were determined with a Z > 3.1 and a cluster significance of P < 0.005. In order for a given brain region to be considered to be differentially activated between high- and low-sensitivity subgroups, it was required to exhibit both a frequency difference of ≥4 and a Z > 3.1 in the between-group GLM comparison. With the sole exception of the thalamus, we have limited our discussion to brain regions meeting these criteria.
Results
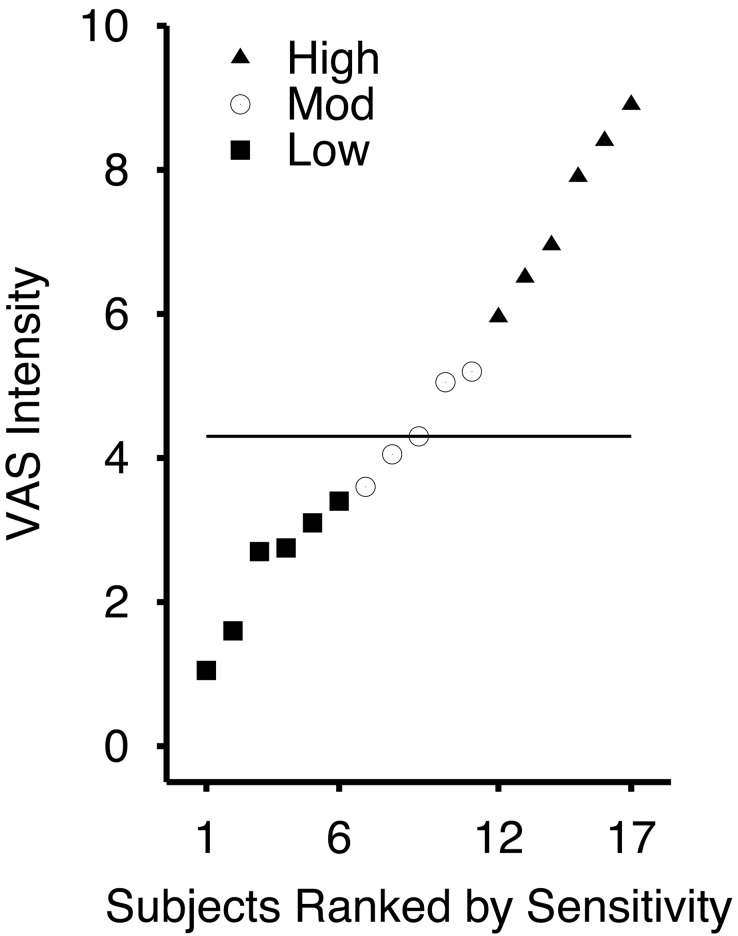
Psychophysical Differences Between Individuals. According to subjective VAS reports, the individual experience of pain intensity evoked by a 49°C noxious stimulus delivered to the posterior aspect of the lower right leg differed substantially across subjects (Fig. 1). The most sensitive subject rated the 49°C stimulus as 8.9/10, whereas the least sensitive individual rated the 49°C stimulus as 1.05/10. To identify the neural correlates underlying these interindividual experiential differences, subjects were first divided into three subgroups representing the most sensitive third (mean VAS rating = 7.43), least sensitive third (mean VAS rating = 2.43), and the middle third (mean VAS rating = 4.44) of the sampled population (Fig. 1). ANOVA followed by Fisher's probable least-squares difference (PLSD) tests confirmed that pain intensity ratings were significantly different between subgroups (main effect of sensitivity, F(2,14) = 42.165, P < 0.0001; high vs. low sensitivity, P < 0.0001; high vs. middle sensitivity, P < 0.0001; middle vs. low sensitivity, P < 0.0036). The gender distribution between subgroups was generally similar in that 2/6 of the subjects of the least sensitive subgroup and 4/6 of the subjects of the most sensitive subgroup were female (Fisher's exact test, not significant). Similarly, the mean age of subjects was 26 yr in the least sensitive subgroup and 28 yr in the highly sensitive subgroup.
Fig. 1.
The distribution of pain intensity ratings obtained during functional MRI scanning shows that the subjective experience of pain intensity evoked by a 49°C stimulus differed markedly across individuals. The horizontal bar indicates the median of all 17 individuals.
Ratings of pain intensity evoked by 49°C stimulation of the leg were highly correlated between training and functional imaging sessions (r = 0.89, P < 0.0001), despite the fact that these sessions may have been separated by up to several days. Consistent with previous data, the average of the absolute value of the session-to-session difference was 0.9 (VAS) for the highly sensitive subgroup and 0.8 (VAS) for the least sensitive subgroup (3).
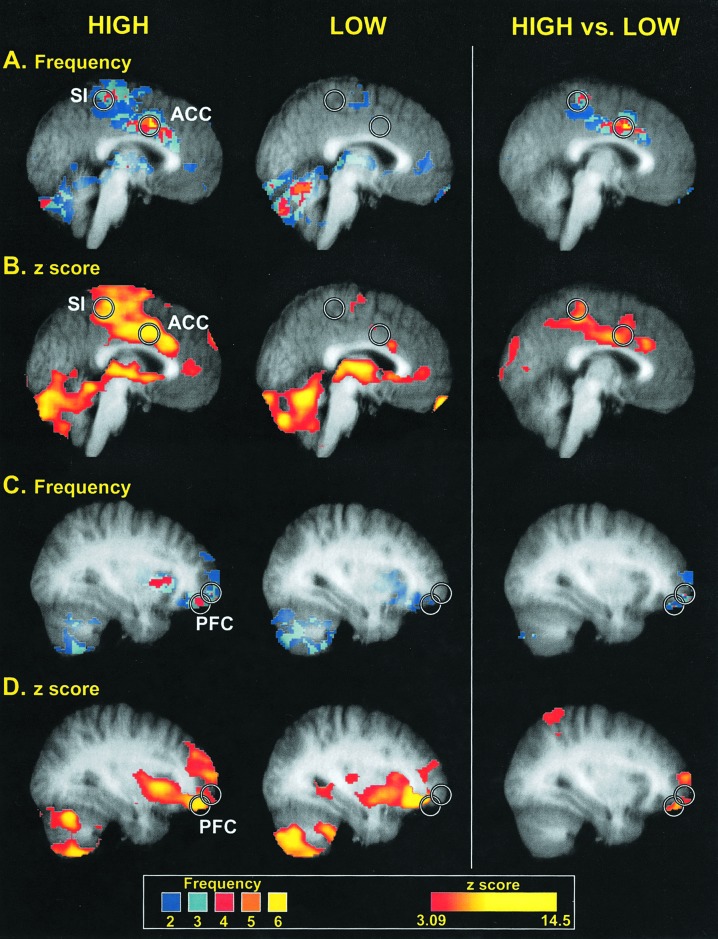
Differences in Cerebral Cortical Activation in Individuals with High and Low Pain Sensitivity. Cerebral cortical regions important in sensation, attention, and affect were activated significantly more frequently in individuals who were highly sensitive to the 49°C stimulus than in those who were insensitive (Table 1, Fig. 2). The most robust distinction in the frequency of activation between the high-sensitivity and low-sensitivity subgroups was located within a portion of the anterior cingulate cortex (ACC), where 6/6 of the highly sensitive subjects, but none of the insensitive subjects, displayed statistically significant activation (Fig. 2). However, significant differences between subgroups extended from a caudal portion of the ACC, which is activated in many studies of pain, to a more rostrally located region associated with pain affect (16, 17). Similar to the ACC, a large portion of the primary somatosensory cortex (SI) exhibited significantly more frequent activation in highly sensitive individuals than in insensitive individuals (Table 1, Fig. 2). This activation was focused on the medial wall of the contralateral hemisphere in a region corresponding to the representation of the lower limb (18, 19). Spatially restricted regions of the ipsilateral (right) prefrontal cortex (PFC) in the vicinity of the frontal pole also were activated more frequently in highly sensitive individuals (Table 1, Fig. 2). Other cortical regions known to be involved in pain intensity processing, such as the secondary somatosensory cortex and insular cortex, exhibited no statistically reliable differences in the frequency of activation.
Table 1. Brain regions displaying differential activation between high- and low-sensitivity subgroups.
| Region | Coordinates (mediolateral, anterior-posterior, and dorsoventral, mm) | High sensitivity | Low sensitivity | Difference between high-sensitivity and low-sensitivity subgroups |
|---|---|---|---|---|
| ACC (caudal) | -2, 8, 36 | Freq = 6, z = 9.38 | Freq = 0, z = NS | Freq = 6*, z = 5.50 |
| ACC (perigenual) | -4, 18, 24 | Freq = 5, z = 6.58 | Freq = 1, z = 4.8 | Freq = 4*, z = 3.2 |
| SI | -4, -34, 58 | Freq = 4, z = 7.25 | Freq = 0, z = NS | Freq = 4*, z = 4.7 |
| PFC | 30, 64, 0 | Freq = 4, z = 3.75 | Freq = 0, z = NS | Freq = 4*, z = 3.76 |
| PFC | 32, 62, -8 | Freq = 4, z = 3.80 | Freq = 0, z = NS | Freq = 4*, z = 3.4 |
| PFC (ventral) | 32, 52, -18 | Freq = 4, z = 8.21 | Freq = 0, z = NS | Freq = 4*, z = 5.98 |
| Thalamus | -18, -20, 14 | Freq = 4, z = 6.85 | Freq = 3, z = 9.63 | Freq = 1, z = NS |
Numbers of subjects (freq) demonstrating significant activation and z-scores (z) of pain intensity-related activation are displayed for high- and low-sensitivity subgroups. Statistically significant differences in the frequency of activation between the high- and low-sensitivity subgroups are defined by Fisher's exact test (P < 0.05, whereas significant activations (or differences) in subgroup analyses were defined by z-scores ≥3.1 and cluster significance of P < 0.005. Nonsignificant functional MRI signal changes are denoted NS
, right column
Fig. 2.
Brain regions displaying different frequencies of activation between high- and low-sensitivity subgroups. Circles are centered on regions where the peak differences between groups were located. Colors in A and C correspond to the number of individuals displaying statistically significant activation at a given voxel (frequency), whereas colors in B and D correspond to the z-score of the subgroup analysis. Slice locations in A and B are -2 mm from the midline, whereas slice locations in B and C are 32 mm from the midline (in standard stereotaxic space). Structural MRI data (gray) are averaged across all individuals involved in corresponding functional analysis.
Group-based analyses of the magnitude of pain-induced activation both confirm and extend the results of the individual analyses of frequency of activation. The ACC, SI, and PFC all exhibited significantly greater magnitudes of activation in the high-sensitivity subgroup than the low-sensitivity subgroup (Table 1, Fig. 2).
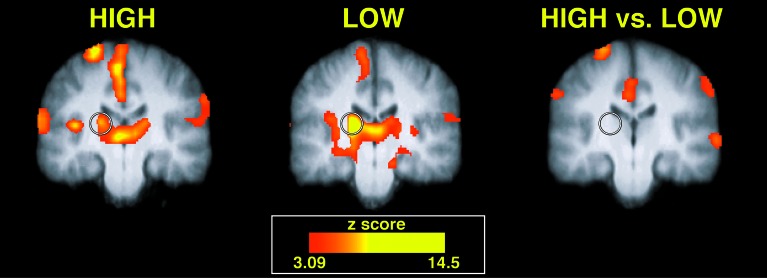
Similar Thalamic Activation in both High- and Low-Sensitivity Subgroups. Activation within the contralateral thalamus differed markedly from that observed within the SI, ACC, and PFC. Neither the frequency nor magnitude of thalamic activation was significantly different between the high- and low-sensitivity subgroups (Table 1, Fig. 3). It is unlikely that this absence of reliable differences could be attributed to inadequate statistical power because (i) the frequency analysis revealed that nearly equal numbers of sensitive and insensitive individuals activated this region and because (ii) within-subgroup analyses revealed a tendency toward a more robust activation of this region in the insensitive rather than the sensitive subgroup (Table 1, Fig. 3).
Fig. 3.
Pain-evoked thalamic activation displayed no significant differences between high- and low-sensitivity subgroups. Both high-sensitivity (left image) and low-sensitivity (center image) subgroups exhibited statistically significant activation of the contralateral (left) thalamus (denoted by a circle). Slices are located -20 mm from the anterior commissure.
Discussion
Although the neural substrates of conscious experience of pain remain elusive, the present concurrence between multiple individuals' patterns of regional brain activation and their subjective reports of pain provides an objective context in which to assess the subjective report of any given individual. Cerebral cortical regions such as the ACC, SI, and PFC exhibited more frequent and more robust activation in individuals who were highly sensitive to pain vs. individuals who were insensitive to pain. In contrast, thalamic regions known to be critically involved in the afferent transmission of nociceptive information exhibited no statistically reliable differences in activation between highly sensitive and insensitive individuals. This dichotomy between cerebral cortical and thalamic patterns of activation provides insight into the CNS mechanisms that may account for interindividual differences in pain sensitivity.
Cerebral Cortical Regions Reflect Interindividual Differences in Pain Sensitivity. A substantial body of evidence indicates that the cerebral cortical regions activated more frequently and more robustly in the highly sensitive individuals play important roles in the pain experience (20). In within-subjects studies, all three of these regions (i.e., SI, ACC, and PFC) have been shown to exhibit increasing activation as noxious stimulus intensities increase (7–9). Here, we provide a demonstration that these regions may be critically important in processes leading to between-individual differences in pain sensitivity.
Although SI, ACC, and PFC all exhibit responses that are related to pain intensity, each region may make a differential contribution to various aspects of the pain experience. Interindividual differences in the activation of SI were centered along the medial wall of the cortical hemisphere contralateral to stimulation, a region consistent with the representation of the lower leg (18, 19). Consistent with this clear somatotopic organization, activation of SI is thought to contribute to early levels of pain localization processing (20). Interindividual differences in activation of the ACC extended across two distinct subregions of this complex structure. The caudal activation focus occurred in an area that is activated in most studies of acute heat pain and may be involved in motivation and goal-oriented cognitive processes (16). The anterior focus was located in a region that has been shown to exhibit activation that is significantly related to the affective component of pain and that is anatomically well situated to contribute to processes providing the negative emotional valence to the experience of pain (16, 17, 20). Interindividual differences in the activation of the PFC were located near the lateral aspect of the frontal pole. During some stimulation paradigms, this region of the PFC exhibits activation that is positively related to perceived pain intensity, whereas it exhibits a more complex pattern of activation in other stimulation paradigms (7, 8). Although its role in pain is far from clear, this region is thought to play important roles in working memory, affect, and attention (8, 21, 22).
Individual Differences in Pain Sensitivity May Result from Cortical Elaboration of Afferent Information. Given that the thalamus is the primary relay for afferent transmission of nociceptive information, the absence of detectable functional MRI differences in the thalamus (in combination with robust differences in the SI, ACC, and PFC) suggests that generally similar afferent input was conveyed to thalamic levels in both high- and low-sensitivity individuals. Accordingly, a large portion of the variability of interindividual differences in both the subjective experience of pain and activation of SI, ACC, and PFC is likely attributable to factors other than differential sensitivity of spinal or peripheral afferent mechanisms. Therefore, supraspinally mediated factors that fall into the cognitive domain may account for significant portions of interindividual differences in the subjective experience of pain. For example, expectations about a stimulus and previous experience have a marked impact on the subjective experience of pain (2, 23, 24). Psychological factors, such as hypnotic manipulation of the subjective experience of pain, have been shown to produce significant changes in the activity of SI and ACC (17, 25), but not the thalamus,¶ underscoring how these cerebral cortical regions may be involved either as effectors and/or targets of cognitive modulation of the subjective experience of pain.
Importance of the Subjective Report. Different pain experiences are characterized by different patterns of supraspinal activation. For example, noxious chemical stimuli infrequently evoke activation of the parietal operculum whereas noxious thermal stimuli produce robust activation of this structure (20). Similarly, some forms of chronic pain evoke a paradoxical asymmetric decrease in thalamic blood flow whereas the majority of acute pain states are characterized by contralateral or bilateral increases in the activation of this region (20, 26, 27). Thus, generalizations between different pain states may be misleading. Pain is defined by the first-person experiential perspective and must be diagnosed and treated with significant consideration of the subjective report. Thus, even if unique patterns of brain activity have been characterized in large numbers of patients for a given chronic pain state, the subjective report will likely remain the single most reliable index of the magnitude of pain.
Importantly, the present findings validate the subjective report and provide insight into the utility of introspection as a means of assessing a conscious experience. First-person introspection is a necessary component of the process of generating a subjective report for communication to a third-person observer. Therefore, the finding that individuals with similar patterns of activation of SI, ACC, and PFC provided similar subjective reports of pain magnitude suggests that they can accurately capture their conscious experience via introspection.
Acknowledgments
We thank Steven Smith and the group at the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain for the fsl software package, Christian Grefkes for the probabilistic maps of SI, and Nancy Stein and Peter Redgrave for their helpful comments on the manuscript. This research supported by the National Institute of Neurological Disorders and Stroke (RO1 NS 39426).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: VAS, visual analog scale; GLM, general linear modeling; ACC, anterior cingulate cortex; SI, primary somatosensory cortex; PFC, prefrontal cortex.
Footnotes
Derbyshire, S., Whalley, M. & Oakley, D. (2003) J. Pain 4, Suppl. 1, 39 (abstr.).
References
- 1.Chalmers, D. J. (1996) The Conscious Mind (Oxford Univ. Press, New York).
- 2.Price, D. D. (1999) Psychological Mechanisms of Pain and Analgesia (Int. Assoc. for the Study of Pain, Seattle).
- 3.Rosier, E. M., Iadarola, M. J. & Coghill, R. C. (2002) Pain 98, 205-216. [DOI] [PubMed] [Google Scholar]
- 4.Stevens, S. S. (1975) Psychophysics (Wiley, New York).
- 5.Price, D. D., Bush, F. M., Long, S. & Harkins, S. W. (1994) Pain 56, 217-226. [DOI] [PubMed] [Google Scholar]
- 6.Algom, D. & Marks, L. E. (1984) J. Exp. Psychol. Gen. 113, 571-593. [DOI] [PubMed] [Google Scholar]
- 7.Derbyshire, S. W. G., Jones, A. K. P., Gyulai, F., Clark, S., Townsend, D. & Firestone, L. L. (1997) Pain 73, 431-445. [DOI] [PubMed] [Google Scholar]
- 8.Coghill, R. C., Sang, C. N., Maisog, J. M. & Iadarola, M. J. (1999) J. Neurophysiol. 82, 1934-1943. [DOI] [PubMed] [Google Scholar]
- 9.Porro, C. A., Cettolo, V., Francescato, M. P. & Baraldi, P. (1998) J. Neurophysiol. 80, 3312-3320. [DOI] [PubMed] [Google Scholar]
- 10.Coghill, R. C., Mayer, D. J. & Price, D. D. (1993) J. Neurophysiol. 69, 703-716. [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson, M. & Smith, S. (2001) Med. Image Anal. 5, 143-156. [DOI] [PubMed] [Google Scholar]
- 12.Talairach, J. & Tournoux, P. (1988) Co-planar Stereotaxic Atlas of the Human Brain (Thieme, New York).
- 13.Worsley, K. J., Evans, A. C., Marrett, S. & Neelin, P. (1992) J. Cereb. Blood Flow Metab. 12, 900-918. [DOI] [PubMed] [Google Scholar]
- 14.Friston, K. J., Worsley, K. J., Frackowiak, R. S. J., Mazziotta, J. C. & Evans, A. C. (1994) Hum. Brain Mapp. 1, 210-220. [DOI] [PubMed] [Google Scholar]
- 15.Woolrich, M. W., Ripley, B. D., Brady, M. & Smith, S. M. (2001) Neuroimage 14, 1370-1386. [DOI] [PubMed] [Google Scholar]
- 16.Vogt, B. A. & Sikes, R. W. (2000) Prog. Brain Res. 122, 223-235. [DOI] [PubMed] [Google Scholar]
- 17.Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. (1997) Science 277, 968-971. [DOI] [PubMed] [Google Scholar]
- 18.Geyer, S., Schleicher, A. & Zilles, K. (1999) Neuroimage 10, 63-83. [DOI] [PubMed] [Google Scholar]
- 19.Penfield, W. & Boldrey, E. (1937) Brain 60, 389-443. [Google Scholar]
- 20.Coghill, R. C. (2002) in Surgical Management of Pain, ed. Burchiel, K. J. (Thieme, New York), pp. 919-932.
- 21.Fuster, J. M. (1997) The Prefrontal Cortex (Lippincott–Raven, Philadelphia).
- 22.Coghill, R. C., Gilron, I. & Iadarola, M. J. (2001) J. Neurophysiol. 85, 2602-2612. [DOI] [PubMed] [Google Scholar]
- 23.Dar, R., Ariely, D. & Frenk, H. (1995) Pain 60, 189-193. [DOI] [PubMed] [Google Scholar]
- 24.Price, D. D., Milling, L. S., Kirsch, I., Duff, A., Montgomery, G. H. & Nicholls, S. S. (1999) Pain 83, 147-156. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer, R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. (2001) J. Neurophysiol. 86, 402-411. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh, J. C., Belfrage, M., Stone-Elander, S., Hansson, P. & Ingvar, M. (1995) Pain 63, 225-236. [DOI] [PubMed] [Google Scholar]
- 27.Iadarola, M. J., Max, M. B., Berman, K. F., Byas-Smith, M. G., Coghill, R. C., Gracely, R. H. & Bennett, G. J. (1995) Pain 63, 55-64. [DOI] [PubMed] [Google Scholar]