Abstract
Sialorphin is an exocrine and endocrine signaling mediator, which has been identified by a genomic approach. It is synthesized predominantly in the submandibular gland and prostate of adult rats in response to androgen steroids and is released locally and systemically in response to stress. We now demonstrate that the cell surface molecule to which sialorphin binds in vivo in the rat kidney is the membrane-anchored neutral endopeptidase (neprilysin; NEP, EC 3.4.24.11). NEP plays an important role in nervous and peripheral tissues, as it turns off several peptide-signaling events at the cell surface. We show that sialorphin prevents spinal and renal NEP from breaking down its two physiologically relevant substrates, substance P and Met-enkephalin in vitro. Sialorphin inhibited the breakdown of substance P with an IC50 of 0.4–1 μM and behaved as a competitive inhibitor. In vivo, i.v. sialorphin elicited potent antinociceptive responses in two behavioral rat models of injury-induced acute and tonic pain, the pin-pain test and formalin test. The analgesia induced by 100–200 μg/kg doses of sialorphin required the activation of μ- and δ-opioid receptors, consistent with the involvement of endogenous opioid receptors in enkephalinergic transmission. We conclude that sialorphin protects endogenous enkephalins released after nociceptive stimuli by inhibiting NEP in vivo. Sialorphin is a natural systemically active regulator of NEP activity. Furthermore, our study provides evidence that it is a physiological modulator of pain perception after injury and might be the progenitor of a new class of therapeutic molecules.
The mammalian submandibular gland produces various signaling polypeptides, including growth factors, homeostatic proteases, regulatory peptides, and protease inhibitors. These mediate many local and peripheral functions participating in the adaptive responses to stress (1–5).
In a genomic approach, an androgen-regulated gene has been identified, which is predominantly expressed in the submandibular gland and prostate of adult rats (6, 7). The gene encodes a precursor protein, submandibular rat1 protein (SMR1), which gives rise to three structurally related peptides: SMR1-undecapeptide, SMR1-hexapeptide, and SMR1-pentapeptide. In common with other peptide hormones, the three peptides are selectively matured from the precursor in vivo by cleavage at multibasic sites by a paired basic amino acid-converting enzyme (probably furin). They are differentially accumulated in the submandibular gland, in an age-dependent and sex-related manner, and are released both locally and systemically in response to neuroendocrine stimuli (8).
Sialorphin, the final mature peptide generated from SMR1, was previously named SMR1-pentapeptide. We now call it sialorphin, because it is an endogenous salivary antinociceptive modulator. It is synthesized predominantly in response to androgen steroids and is constitutively released into the blood-stream under basal conditions, and acutely secreted in response to environmental stress. Circulating sialorphin thus is rapidly and selectively taken up by peripheral targets via specific binding sites. The main sites are on the renal proximal tubular absorptive epithelium, trabecular and alveolar bone, and dental dentine remodeling units (9). The distribution of most sialorphin target sites within the major tissues involved in ion capture, transport, and regulation suggests that sialorphin modulates systemic mineral ion homeostasis. The fact that the androgen-regulated sialorphin is acutely secreted in response to environmental stress led us to postulate that this signaling peptide helps mediate the adaptative homeostatic responses of male rats to stressful situations. It thus may participate in the control of typical intraspecific social behavior, such as aggressive response to territorial challenge and/or sexual intercourse (5, 9).
We have therefore attempted to elucidate the pathway by which sialorphin acts on its targets. This article describes the molecular identity of the major sialorphin-binding sites on renal cell membranes and the implications for its function in vitro and in vivo. The present study provides evidence that sialorphin is a natural inhibitor of the cell surface neutral endopeptidase (neprilysin, NEP; EC 3.4.24.11) in mammals and a physiological modulator of painful responses to threatening environments in rats.
Materials and Methods
Molecular Characterization. Male Wistar rats (6 weeks old, Charles River Breeding Laboratories) were anaesthetized with pento-barbital (45 mg/kg of body weight, i.p., Sanofi-Synthelabo Research, Malvern, PA). The receptor sites for sialorphin in living rat tissues were labeled with Gln-His-Asn-(3H)Pro-Arg (3H-sialorphin) and cross-linked with divalent aldehydes as described (9). Organs were rapidly removed, and tissues were dissected out and then incubated in fresh fixative (5 h at 4°C). The tissues were rinsed in cold Dulbecco's PBS (GIBCO), and free aldehyde groups were saturated by immersion in 0.2 M ethanolamine, pH 7.5 (3 h at 4°C). Tissue samples were homogenized at 4°C in 10 vol of 10 mM ammonium acetate, pH 9 containing a mixture of peptidase inhibitors (9). The homogenate was centrifuged at 1,000 × g (15 min at 4°C), and the pellet was homogenized once more. The combined supernatants were centrifuged at 4°C and 7,700 × g for 10 min, and the resulting supernatant was centrifuged at 100,000 × g for 1 h. The resulting pellet was incubated overnight at 4°C in 10 vol of 0.5% SB3–14 plus 0.1% Triton X-100 (Fluka) and centrifuged at high speed as above. The resulting supernatant was the solubilized membrane extract used for further purification.
Soluble renal membrane extracts first underwent preparative liquid isoelectric focusing (IEF) in a preformed pH gradient 3–10 (30% yield, Pharmacia Biotech ampholytes, and Bio-Rad Rotofor cell). Radioactive IEF fractions were concentrated on a Centri/Por 50-kDa MWCO concentrator (60% yield, Spectrum-Biovalley, Marnela Vallée, France), mixed with 10 mM DTT, and subjected to RP-HPLC on a Pep RPC column (58% yield, Pharmacia Biotech). Elution was with two-linear gradients, one from 1–10% acetonitrile for 15 min, and the other from 10–99% acetonitrile for 25 min at 1 ml/min. The major radioactive peaks were analyzed by denaturizing gel electrophoresis in a 4–10% polyacrylamide gradient gel or 7% SDS/PAGE (1% SDS and 10 mM DTT), followed by silver staining or enhanced chemiluminescence (ECL) Western blotting (Pharmacia Biotech) or β-imager analyses (9). Based on the β-imager data, the IEF-purified renal receptor sites complexed with 3H-sialorphin migrated as three radioactive protein bands with apparent molecular masses of 150, 110, and 80 kDa. The ECL data of RP-HPLC fractions, using goat polyclonal IgG specific for NEP and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology), delineated a NEP-staining band in the vicinity of 120 kDA after SDS/PAGE of radioactive fractions.
The radioactive fractions obtained from successive steps of purification were pooled, concentrated, and submitted to lysine endopeptidase digestion. Peptide fragments were separated by RP-HPLC. The amino acid sequences of these digested fragments were determined by the automated Edman procedure (Plateforme d'Analyze et de Microséquençage des Protéines, Institut Pasteur).
Measurement of Ectopeptidase Activity. Male Wistar rats were anaesthetized as above and killed by cardiac puncture. The organs were rapidly removed and washed in ice-cold oxygenated Krebs–Ringer containing bicarbonate (27 mM) and glucose (6 mM) and saturated under 95% O2–5% CO2 atmosphere. The slices of fresh tissues and membrane preparations were prepared as described (10).
Membrane endopeptidase activity was assayed by measuring the breakdown of two NEP-sensitive peptides, Met-enkephalin (ME) and substance P (SP). We used native ME (Peninsula-Biovalley, Marnela Vallée, France) and modified tritiated SP [(3,4,3H)Pro-2-Sar-9-Met(O2)11]-SP (NEN). Hydrolysis of substrates was measured in the presence and absence of specific inhibitors. These were added to the preincubation medium: bestatin, phosphoramidon, and dipcptidylaminopeptidase inhibitor II (Calbiochem), thiorphan, captopril, and GEMSA (Sigma). The standard reaction mixture consisted of tissue (membranes or slices) in 50 mM Tris·HCl (pH 7.2) or oxygenated Krebs–Ringer containing bicarbonate (27 mM) and glucose (6 mM), both containing 10 μM bestatin and 0.1% BSA. The substrate was added after preincubation for 10 or 15 min, and the hydrolysis was carried out for 10 or 20 min at 25°C in a constantly shaken water bath. The reaction was terminated by cooling to 4°C and adding HCl (0.3 M final concentration). The reaction tubes were then centrifuged (4,700 × g for 15 min at 4°C), and the remaining intact substrate and its metabolites were measured. Time and temperature of incubation and protein concentrations of membrane enzyme were defined according to conditions of initial velocity measurement.
C-18 Sep-Pak cartridges (Waters) were used to analyze the hydrolysis of tritiated SP (3H-SP). Two major N-terminal 3H-metabolites were successively isolated by elution (4 ml) with H20–0.1% trifluoroacetic acid (TFA) and then with 20% methanol–0.1% TFA. The intact 3H-SP was eluted with 75–100% methanol–0.1% TFA.
RP-HPLC coupled to a spectrophotometer (SP8800, Spectra-Physics and L3000, Merck) was used to analyze the hydrolysis of ME (C-18 LUNA column, AIT, Le Menil le Roi, France). Elution with a 30-min linear gradient from 0.1% TFA in water to 0.1% TFA in 100% acetonitrile, at 1 ml/min, separated the two ME metabolites (Tyr-Gly-Gly YGG: 5.8 ± 0.2; Phe-Met FM: 12.8 ± 0.1 min retention time) and the intact ME (YGGFM: 18.8 ± 0.2 min). Their identities and relative quantities (peak height) were checked by monitoring the column outflow at 264 nm. The disappearance of the initial ME substrate was also quantified by RIA. The assay used anti-ME antibody (11) and 125I-ME (80 TBq/mmol, NEN).
The proteins in tissue extracts and membrane suspensions were determined by using the Bio-Rad DC protein assay. All data are expressed as means ± SD of at least two independent duplicate determinations, and data analysis was carried out by using assayzap, Microsoft excel, and kaleidagraph software.
Behavioral Assays. The pin-pain model, derived from that of Hebert et al. (12) was used to test the activity of sialorphin on mechanical pain response. Male Wistar rats (350–400 g body weight, Charles River Breeding Laboratories) were placed in the experimental device, an open area (45 × 45 × 40 cm) divided into nine equal squares (15 × 15 cm), eight of them peripheral and one central. The peripheral squares were overlaid with stainless steel pins (2/cm2; length, 8 mm; diameter, 0.6 mm). The rat was placed in the central square and its behavior was recorded for a 3-min test. The rats were placed in the experimental device without pins for 20 min 2 days before the pain test, so as to reduce the stress linked to the spatial neophobia.
The formalin test was used to test the activity of sialorphin on chemical pain response. The formalin test was done by injecting 50 μl of a 2.5% formalin solution under the surface of the hind paw. The duration of paw licking was recorded for a period of 15 min after formalin administration. The rats were habituated to the test environment during 2 days (30 min per day) before pain test.
Results are expressed as means ± SEM. The significance of differences between groups was evaluated by using ANOVA with a subsequent Student's unpaired t test (pin pain) or Mann–Whitney U test (formalin pain). All statistical analyses were carried out by using the STATVIEW 5 statistical package (SAS Institute, Cary, NC). In all experiments, the care and euthanasia of study animals were in accordance with the European community standards on the care and use of laboratory animals.
Results and Discussion
Sialorphin, a Physiological Ligand of Membrane-Bound NEP. The rat renal outer medulla bears a prominent density of sialorphin receptor sites (9). We used the 3H-labeled ligand, given systemically, to obtain further information on sialorphin receptor molecules (9). We specifically isolated and identified the radio-labeled receptor sites that were securely cross-linked to [3H]-sialorphin.
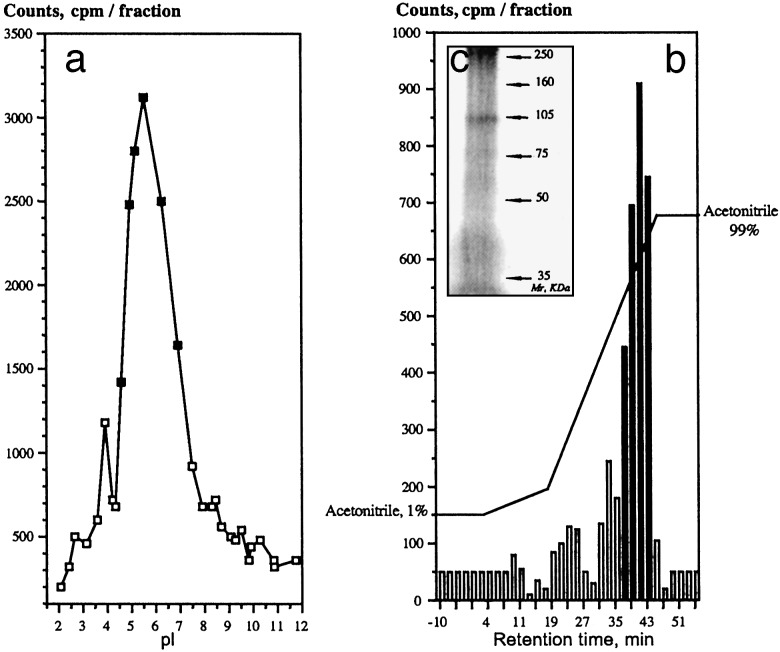
Most of the cross-linked radioactive peptide in the rat kidney extracts was membrane-associated (74–80%) and was released by treating the membrane extract with a mixture of sulfobetaine SB3–14 and Triton X-100 detergents. The solubilized target cell surface sialorphin receptors were purified to apparent homogeneity by a three-step procedure according to their isoelectric point (preparative liquid IEF), molecular mass (ultrafiltration/ concentration), and hydrophobic characteristics (C18–RP-HPLC). The major 3H-labeled renal membrane molecules with a pI of 5.4 ± 0.2 (n = 16) (Fig. 1a) had hydrophobic characteristics (Fig. 1b), and migrated as ≈100-kDa protein band under denaturizing gel electrophoresis (Fig. 1c). Its partial amino acid sequence demonstrated that the cell surface receptor that bound sialorphin in vivo shares sequence identity to a mammalian integral plasma membrane zinc-containing endopeptidase, neutral endopeptidase NEP (EC 3.4.24.11) (neprilysin, also referred to as enkephalinase). Interestingly, one of the sequences identified, KDGDLVDWFTQQSANN, corresponding to the rat NEP599–614 (W607) peptide segment, lies in the interior of the hydrophobic cavity containing the active site and near the critical motifs for zinc coordination, catalysis, and substrate or inhibitor binding (583HEXXH587 and 646EXXA/GD650) (13). NEP is a glycosylated membrane-anchored protein of 749 aa and an apparent molecular mass of 86–110 kDa; it has a highly hydrophobic membrane-spanning sequence and a pI of 5.5 (14). Its molecular characteristics are very similar to those of renal sialorphin-receptor sites. Furthermore, its renal distribution mainly on the membrane of proximal tubules of the outer stripe of the outer medulla and the deep inner cortex coincides with that of sialorphin sequestration sites in vivo (9, 15). And finally, the purified membrane-bound molecule targeted by sialorphin in this tissue in vivo was immuno-stained positively for NEP (Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org).
Fig. 1.
Molecular characterization of the major 3H-sialorphin binding sites on renal cell membranes in vivo. (a) Representative IEF profile of the renal membrane radioactive extract. (b) Representative RP-HPLC profile of the major IEF radioactive peak (▪ in a). (c) Analysis by SDS/PAGE (7% gel) and silver staining of the major RP-HPLC radioactive peak (black bars in b).
NEP-24.11 is located at the surface of cells in nervous and systemic tissues, where it functions as an ectoenzyme catalyzing the postsecretory processing or metabolism of a number of neuropeptides and regulatory peptides. The main physiologically relevant substrates for NEP are the enkephalins, SP, and atrial natriuretic peptide. These mammalian signal peptides are involved in the control of central and peripheral pain perception, inflammatory phenomena, arterial tone, and mineral homeostasis (16). Their physiological importance and the critical role of NEP ectoenzyme in modulating their functional potency made it important to investigate the biological consequences of the interaction between sialorphin and NEP.
Sialorphin, a Physiological NEP Inhibitor. The kidney, which contains the highest membrane-anchored NEP activity, is the major site of sialorphin binding. This raises the question of whether sialorphin is a physiological substrate of NEP to be cleared from the extracellular space or an inhibitor that protects signaling peptide-hormones from NEP-metabolizing activity? Several observations indicated that sialorphin is a natural inhibitor of NEP activity, rather than a NEP substrate. Autoradiographic visualization of the distribution of in vivo radio labeling showed a good spatial and temporal correlation between the distribution of target organs accessible to circulating 3H-sialorphin, and that to [3H]-HACBO-Gly, a potent selective NEP-inhibitor (9, 17). The characteristics of tissue uptake were consistent with a potent interaction between sialorphin and the enzyme in vivo. Sequestration would be unlikely if the circulating sialorphin was a substrate for the membrane-bound NEP and cleared from the extracellular space. Furthermore, the overall characteristics of the tissue-bound ligand revealed that the uptake of sialorphin by renal tissue is kinetically and biochemically stable in vivo (9) and in vitro (Table 1, which is published as supporting information on the PNAS web site), indicating that sialorphin is not hydrolyzed at the surface of kidney cells. Accordingly, the molecular interaction of renal NEP and sialorphin established in an in vitro enzyme assay using purified rabbit kidney NEP and artificial fluorogenic Dansyl-Gly-(pNO2)Phe-βAla as substrate (18) provided direct evidence that sialorphin inhibited renal NEP activity. The inhibitory potencies (IC50) of pGlu1 and Gln-1-sialorphin were 5.9 and 6.3 × 10-7 M, respectively.
Using a more biological assay, we explored the effects of sialorphin on cell surface NEP peptidase activity by using cell membranes as enzyme source. We first explored the effect of sialorphin on rat kidney NEP. This tissue contains SP released from sensory nerve terminals and acting on nearby target cells through neurokinin receptors (19). Thus, SP can be regarded as a biologically relevant NEP substrate at the renal level. The breakdown of this NEP-sensitive peptide was measured by using tritiated synthetic substrate (3H-SP) and chromatographic analysis. However, SP is readily cleaved by NEP, angiotensin-converting enzyme, and dipeptidylaminopeptidase IV membrane-bound peptidases (20). The specificity of the peptidase assay was assessed by measuring the hydrolysis of 3H-SP by the tissue-membrane enzymes in the presence and absence of selective peptidase inhibitors, and by quantifying the formation of 3H products of the reaction, as indicated in Materials and Methods. The NEP is strongly inhibited by the Streptomyces product phosphoramidon and the synthetic NEP inhibitor thiorphan (21, 22). Bestatin was also added to the incubation medium to block membrane aminopeptidase (ANP) activity.
The breakdown of SP by renal membranes gave two N-terminal 3H-metabolites under our experimental conditions of initial velocity measurement, one of which was major (85%). Phosphoramidon (10 μM) and thiorphan (1 μM) inhibited the formation of the major breakdown product by 61 ± 10% and captopril (10 μM), which blocks angiotensin-converting enzyme activity, by 38 ± 12%. In male rat kidney tissue, the specific SP-hydrolyzing activity was 197 ± 25 pM/min per μg of membrane proteins, of which 60 ± 5% (n = 9) was inhibited by sialorphin (4 μM, Fig. 2), as effectively as phosphoramidon. And the inhibition by sialorphin of 3H-SP endoproteolysis by rat kidney was strictly concentration dependent (r = 0.970, n = 20) (Fig. 2 a and b). The effective doses for sialorphin ranged from 400 to 4,000 nM (maximum), being half-maximal (IC50) at 1 μM and similar to those obtained by using purified rabbit renal NEP (0.6 μM). These results indicate that the interaction between sialorphin and renal membrane-anchored NEP shown by tissue uptake in vivo could lead to a physiological action, such as the protection of regulatory peptides from breakdown by NEP. The kidney, which contains the highest NEP activity, seems also to be a major site of atrial natriuretic peptide (ANP) metabolism (23). And, as several peripheral effects of circulating ANP are regulated mainly by NEP ectopeptidase, we postulate that sialorphin may help potentiate the physiological actions of ANP at periphery, especially at the renal site (23, 24).
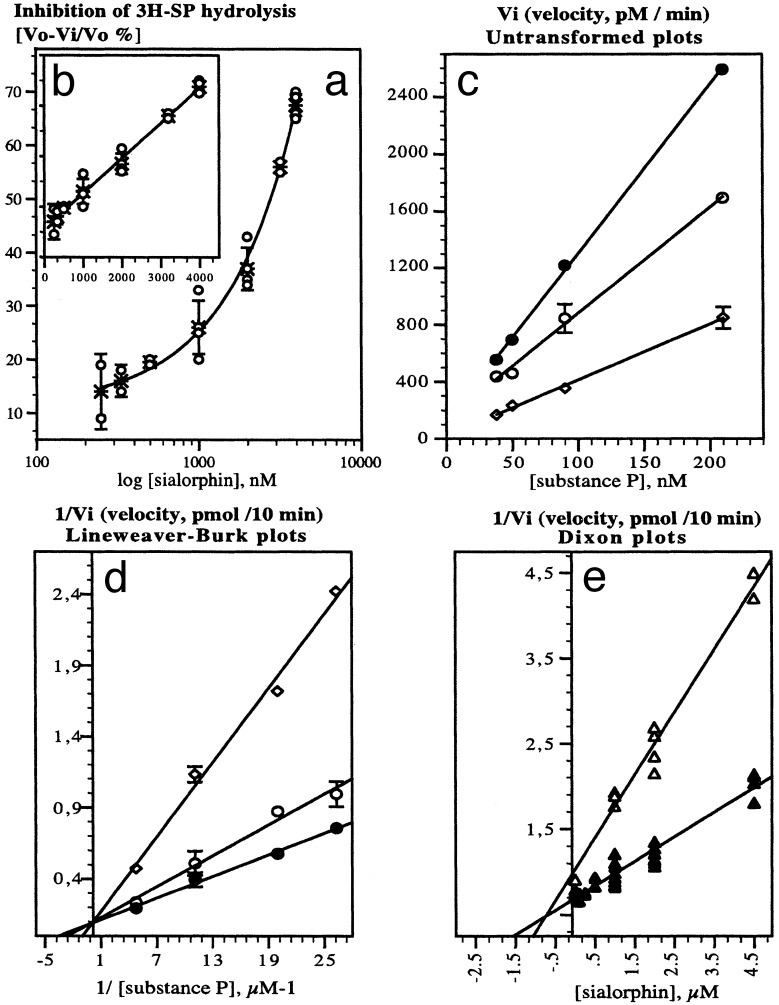
Fig. 2.
Inhibition by sialorphin of 3H-SP breakdown by rat renal membranes ex vivo. (a and b) Concentration-dependent inhibition by sialorphin of 3H-SP endoproteolysis by renal membranes. Each point (○) represents the percentage of 320 nM intact 3H-SP recovered and calculated as: percentage of velocity without inhibitor - velocity in the presence of inhibitor/velocity without inhibitor; * represents the mean ± SD of four determinations. Sialorphin concentration expressed in nM is plotted on a log scale in a. Representative untransformed (c) and double reciprocal (d) and Dixon (e) plots of the inhibition by sialorphin of SP breakdown by rat renal membranes. Inhibition by sialophin was measured by using 3H-SP as substrate at the concentrations indicated for c and d, and at 24 nM (▵) or 105 nM (▴) for e. The concentrations of inhibitor for c and d were 0 (•), 1.5 (○), and 4.5 μM (open rhombs).
We also identified the inhibitory action of sialorphin by plotting the initial velocities of the renal enzyme reaction against the substrate concentration for several inhibitor concentrations or against inhibitor concentration for given substrate concentrations. The pattern of the untransformed plots (Fig. 2c), double reciprocal plots (Fig. 2d), and Dixon plots (Fig. 2e) for the inhibition of 3H-SP hydrolysis by sialorphin all indicated that inhibition is competitive. The kinetic behavior of sialorphin, plus the fact that the interaction with its membrane receptor site in vivo involves multivalent mineral ions (9), suggests that sialorphin interacts optimally with groups, such as a zinc coordinating ligand, in the enzyme active site.
Sialorphin, an Inhibitor of the SP and ME Catabolism by Spinal Tissue. Although the kidney is the best source of NEP in mammals, studies on the metabolism of neuropeptides in the brain and spinal cord first established the physiological roles of NEP. Therefore, we explored the effects of sialorphin on NEP peptidase activity by using tissues from the dorsal zone of the rat spinal cord (10). The physiologically relevant substrates of NEP in the brain and spinal cord are enkephalins and SP (10, 22). The breakdown of these NEP-sensitive peptides was measured by using 3H substrate and chromatographic analysis for SP, as above, or using natural substrate and RP-HPLC or RIA analyses for ME. Peptidase inhibitors were used to confirm that the neuropeptide fragments detected are metabolic products formed through enzymatic action ex vivo (fresh spinal slices) or in vitro (spinal membranes).
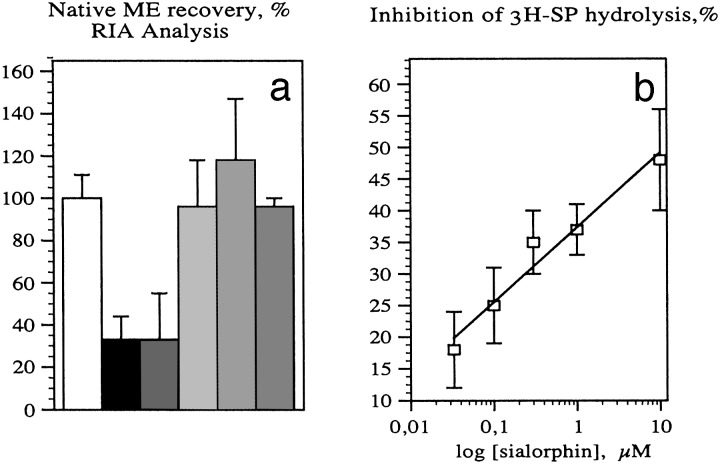
RIA (Fig. 3a) and RP-HPLC analyses showed that 67–74% of the exogenous ME were destroyed by spinal cord slices after incubation for 20 min at 25°C, in the absence of NEP inhibitors or sialorphin. The breakdown of ME was not prevented by bestatin (10 μM), an inhibitor of rat brain APN activity. In contrast, phosphoramidon (10 μM) completely inhibited Metenkephalinase activity, whereas thiorphan (1 μM) blocked 80– 100% of the ME breakdown by fresh spinal cord tissue. This inhibition of enkephalinase activity by thiorphan or phosphoramidon provided maximal protection of the exogenous peptide, indicating that ex vivo the hydrolysis of enkephalin was caused entirely by spinal NEP ectopeptidase. As expected, the carboxypeptidase inhibitor (GEMSA) and dipeptidylaminopeptidase IV inhibitor (10 μM) did not inhibit the breakdown of ME by fresh spinal slices. Adding 10 μM sialorphin to this selective NEP assay prevented 70–96% of the breakdown of ME by the spinal enkephalinase, indicating that sialorphin efficiently protects ME from breakdown by spinal cell surface endopeptidase ex vivo.
Fig. 3.
Effects of metallo-ectopeptidase inhibitors and sialorphin on the breakdown
of exogenous ME and SP by rat spinal cord slices or membranes. (a)
Met-enkephalinase activity in spinal cord slices was determined in the
presence of APN inhibitor alone (10 μM bestatin, ▪) or with peptidase
inhibitors: NEP inhibitors, phosphoramidon (10 μM,  ) or thiorphan (1
μM,
) or thiorphan (1
μM,  ), carboxypeptidase and dipcptidylaminopeptidase (DAPIV)
inhibitors, GEMSA and DAPIV inhibitor II (10 μM, ▪), and sialorphin
(10 μM,
), carboxypeptidase and dipcptidylaminopeptidase (DAPIV)
inhibitors, GEMSA and DAPIV inhibitor II (10 μM, ▪), and sialorphin
(10 μM,  ). Control (○) represents the native ME recovery in the
absence of tissue slice. Values represent the percentage of 10 μM intact ME
determined by RIA and recovered after incubation with fresh tissue slice in
oxygenated Krebs–Ringer containing bicarbonate (27 mM) and glucose (6
mM) medium. (b) Concentration-dependent inhibition by sialorphin of
3H-SP catabolism by rat spinal cord membranes (□). Sialorphin
concentration expressed in μM is plotted on a log scale. Each point
represents the percentage of the mean recovery of 25 nM intact
3H-SP after incubation with spinal membranes in Tris·HCl
buffer.
). Control (○) represents the native ME recovery in the
absence of tissue slice. Values represent the percentage of 10 μM intact ME
determined by RIA and recovered after incubation with fresh tissue slice in
oxygenated Krebs–Ringer containing bicarbonate (27 mM) and glucose (6
mM) medium. (b) Concentration-dependent inhibition by sialorphin of
3H-SP catabolism by rat spinal cord membranes (□). Sialorphin
concentration expressed in μM is plotted on a log scale. Each point
represents the percentage of the mean recovery of 25 nM intact
3H-SP after incubation with spinal membranes in Tris·HCl
buffer.
The breakdown of SP by spinal cord tissue gave two N-terminal 3H-metabolites, one of which was major (96%). Phosphoramidon (10 μM) and thiorphan (1 μM) inhibited the formation of the major breakdown product by 60–65%. In contrast, captopril (10 μM), which blocks angiotensin-converting enzyme activity, had no effect on its formation from spinal tissue. However, dipeptidylaminopeptidase IV (DAPIV) inhibitor (10 μM) prevented 40% of the breakdown of SP by fresh spinal slices, indicating that DAPIV in parallel to NEP was responsible for the primary cleavage of SP by spinal endopeptidases ex vivo or in vitro. This finding agrees with other observations on the metabolism of SP in vivo, suggesting that brain NEP and aminopeptidases (DAPIV or prolyl endopeptidase) were involved in primary SP cleavages, whereas angiotensin-converting enzyme was involved in secondary cleavages (25). Sialorphin (10 μM) inhibited the hydrolysis of SP by spinal tissue (membranes or slices) by 55%; spinal endopeptidase activity gave rise to 242 ± 33 fmol/min per mg of membrane protein without sialorphin, and only 109 ± 15 fmol/min per mg of membrane protein when it was present. The amount of sialorphin that inhibited by 50% (IC50) the breakdown of 3H-SP by spinal cord membranes (Fig. 3b) was 3.9 × 10-7 M. This finding agrees with studies on superfused rat spinal cord slices, in which the degradation of exogenous ME was reduced by 50% by 4.7 10-6 M of the potent synthetic mixed NEP and APN inhibitor, kelatorphan (10). Besides, from other studies, it has been shown that the endogenous peptide, calcitonin gene-related peptide acts in the spinal cord as a natural inhibitor of SP degradation by competing for the same catabolic endopeptidase NEP (26).
Thus our data indicate that sialorphin is a potent natural inhibitor of the rat spinal cell surface NEP ex vivo and in vitro.
Sialorphin Displays Analgesic Activity. NEP plays a pivotal role in controlling activity of the neuropeptide signals that convey sensory information from the peripheral sensory nerve endings to pain-processing centers in the spinal cord and brain. Prominent among these mediators are SP and enkephalins, which are released on noxious chemical, mechanical, or thermal stimuli and are implicated in spinal processing of nociceptive information and analgesic mechanism (27). We have shown here that sialorphin prevents, as effectively as synthetic NEP inhibitors, their extracellular breakdown by NEP in rat nerve tissues in vitro or ex vivo. Besides the synthetic inhibitors of membrane-bound metallopeptidases, NEP and APN, which both rapidly inactivate enkephalins, gave potent analgesic responses in various models of pain (28). To evaluate the effects of the natural NEP inhibitor sialorphin in vivo on pain responses we used two behavioral rat models of peripheral injury-induced acute and tonic pain, the pin-pain test and formalin test.
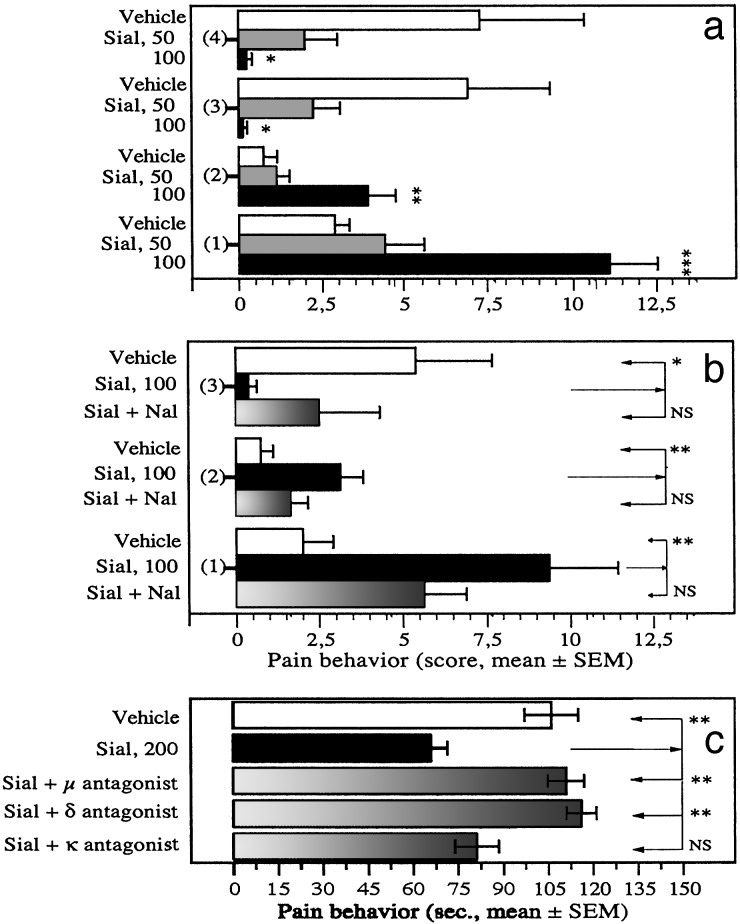
The pin-pain test involves the exposure of rats to a natural stimulus, the acute mechanical stimulation of the paws in a context conducive to the expression of a range of behavioral responses (12). An i.v. injection of sialorphin (50 or 100 μg per kg of body weight) resulted in the rats emitting less vocalization than vehicle controls and they displayed locomotor and exploratory activities in the peripheral pin-areas (P ≤ 0.001 by ANOVA, Fig. 4a). For instance, sialorphin (100 μg/kg) significantly increased the frequency at which peripheral squares were crossed from 2.9 ± 0.4, n = 8 (vehicle) to 11.1 ± 1.4, n = 8 (P ≤ 0.001), and that of rearing on pin area from 0.7 ± 0.4 to 3.9 ± 0.8 (P ≤ 0.005), a behavior that increases pressure on the footpads and would lead to enhanced pain. It also significantly decreased the number of audible vocalizations from 7.2 ± 3.1 (vehicle) to 0.25 ± 0.16 (P ≤ 0.05) and avoidance responses to aversive pin-areas from 6.9 ± 2.5 to 0.1 ± 0.1 (P ≤ 0.05), both behavioral reactions that involve substantial supraspinal integration. Hence, sialorphin-treated rats did not behave as though they experienced contact with the pins as painful, suggesting an antinociceptive action for sialorphin in the perception of sharp painful stimuli. The effect of sialorphin on these responses to noxious stimuli was reduced by 20–63% by the broad-spectrum opioid antagonist naloxone (Fig. 4b). Sialorphin-treated rats also spent significantly less total time (58 ± 21 s) in the central area of the open field, which was not pin-overlaid, than controls (155 ± 14 s, P ≤ 0.002) and this behavior was reversed by 56% by nalaxone (112 ± 17 s) (data not shown). Thus the opiate receptors are required for the antinociceptive effect exerted by sialorphin during acute mechanical stimuli.
Fig. 4.
Pain response of rats given sialorphin (i.v.) to mechanical and chemical noxious stimulus. (a and b) Frequencies of various behavioral responses in the pin-pain test. The 3-min test was performed 5 min after injecting rats with sialorphin (Sial) or vehicle via the tail vein. We measured activity in peripheral squares (overlaid with pins) during the test. 1 = number of times squares were crossed; 2 = number of rearing on squares; 3 = number of escape responses; 4 = number of audible vocalizations. (a) Dose-dependent antinociceptive effects of sialorphin. (b) Effects of sialorphin (100 μg/kg) in the absence or presence of the opioid antagonist naloxone (Nal, 0.3 mg/kg i.p. given 20 min before the test). (c) Duration of paw licking during the early phase of the formalin test (0–15 min). The test was performed 10 min after injecting rats with sialorphin (Sial) or vehicle via the tail vein. Effects of sialorphin (200 μg/kg) in the absence or presence of the μ-opioid antagonist β-funaltrexamine (20 mg/kg i.p. given 20 h before the test), the δ-opioid antagonist naltrindole (10 mg/kg i.p. given 15 min before the test), or the κ-opioid antagonist nor-binaltorphimine (5 mg/kg i.p. given 3 h before the test). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (n = 6–8 animals for each condition).
Formalin injection into the hind paw produces an immediate noxious stimulus by inducing tissue damage, thus providing a model for inescapable acute pain (29). Systemic administration of sialorphin inhibited the early phase of paw licking of the formalin-injected hind paw. For instance, sialorphin (200 μg/kg) significantly reduced the time spent by treated rats in paw licking from 106.3 ± 9.3 s, n = 8 (vehicle) to 66.3 ± 5.5 s, n = 8 (P ≤ 0.004) (Fig. 4c). Pretreatment with either selective μ-or δ-opioid receptor antagonist abolished the antinociceptive effect exerted by sialorphin during the early phase of formalin test: 110.8 ± 5.7 s, n = 6 (β-funaltrexamine, P ≤ 0.003 vs. sialorphin alone) and 115.8 ± 4.8 s, n = 6 (naltrindole, P ≤ 0.002 vs. sialorphin alone) (Fig. 4c). In contrast, administration of the selective κ-receptor antagonist norbinaltorphimine did not significantly affect the antinociceptive response produced by sialorphin. These data indicate that sialorphin inhibits nociception induced by acute chemical stimulus by activating μ- and δ-opioid receptors, consistent with the involvement of endogenous opioidergic pathway in sialorphin-induced hypoalgesia. This finding agrees well with studies demontrating that pain-suppressive effect can be obtained on formalin-induced nociceptive stimuli after systemic administration of enkephalin-catabolizing enzyme inhibitors, such as RB 101, a synthetic mixed NEP-APN inhibitor (2.5–5 mg/kg) (30).
Sialorphin inhibits both mechanical- and chemical-evoked acute and tonic pain behavior, and the μ- and δ-opiate receptors are required for the full hypoalgesia induced by sialorphin. We conclude that sialorphin produces at least part of its analgesic effects by potentiating endogenous μ- and δ-opioid-dependent pathways. Mu-receptor dependent opioidergic pathways are essential for the spinal and supraspinal feedback control of nociceptive inputs and in morphine-induced analgesia (31, 32). We propose that the pharmacological effect of sialorphin leads to potentiated inhibitory control of nociceptive inputs, involving the endogenous opioid peptides, enkephalins, which interact with both μ- and ∂-opioid receptor subtypes. The powerful inhibition of spinal Met-enkephalinase by sialorphin observed ex vivo suggests that the hypoalgesic action of sialorphin is caused by the blockade of enkephalin-inactivating ectopeptidases in vivo, allowing protection of enkephalin released after nociceptive inputs to the spinal cord. Owing to the complementary roles of NEP and APN in enkephalin inactivation, only mixed NEP-APN synthetic inhibitors induce naloxone-reversible antinociceptive responses in various models of pain (16, 30). According to this, our current findings support the possibility that, in vivo, sialorphin elicits μ- and δ-opioid-dependent analgesic responses by entirely protecting enkephalins from inactivation by both enkephalin-degrading enzymes. In contrast, in accord with our ex vivo data and studies monitoring the in vivo metabolism of SP in brain rat (25), it is likely that sialorphin does not protect endogenous SP from cleavage by all spinal SP-inactivating ectopeptidases in vivo. This finding also argues that in both pain tests sialorphin potentiated spinal enkephalin-dependent antinociveptive mechanisms rather than those of SP-mediated nociception.
Sialorphin is an exocrine and endocrine peptide messenger, whose expression is under activational androgenic regulation and secretion is evoked under adrenergic-mediated response to environmental stress, in the male rat. In such stressful contexts, it is acutely released, rapidly distributed, and lasting, taken up by its membrane-associated targets (9). The physiological range of the circulating sialorphin occurring in rats on stress situations is only 20-fold less than the systemic concentration of sialorphin giving analgesic responses to noxious stimuli (100 μg/kg). Its kinetics of bioavailability may help regulate membrane-bound NEP activity in urgent situations and thus potentiate the action of endogenous enkephalins released after specific stimuli. Opioid system modulates responses to threatening environmental stimuli regulating anxiety, aggression, and dominance in addition to nociception (33). From an integrative point of view, the combined physiological and genomic information accrued support the possibility that endogenous sialorphin is dynamically released and recruited to mediate adaptative responses of male rats to major stressors such as pain, injury, or invasion of territory.
The VCSA1 gene encoding the precursor of sialorphin belongs to a multigene family whose members have been identified in the rat, but also in the mouse and human (34). To our knowledge, sialorphin is the only identified physiological systemically active regulator of the membrane-bound NEP activity in rats. This finding raises the question of the existence of such endogenous NEP-ectopeptidase inhibitor in human. And, importantly, sialorphin could spawn a new class of therapeutic compounds, as its target NEP ectopeptidase is highly conserved between species; the rat and human enzymes have high sequence homology (≥93%) (14, 35).
Supplementary Material
Acknowledgments
We thank I. Rosinski-Chupin, C. Barale, and G. Langsley for their critical review of the manuscript and helpful comments. The purified rabbit renal NEP was a gift from B. Roques (Universite Rene Descartes– Unité de Formation et de Recherche des Sciences Pharmaceutiques et Biologiques). We also thank Dr. Roques for help in measuring the inhibitory potency of sialorphin in the enzyme-fluorometric assay. These studies were supported by grants from the Direction de la Valorisation et des Partenariats Industriels, Institut Pasteur.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NEP, neutral endopeptidase (neprilysin); SMR1, submandibular rat1 protein; IEF, isoelectric focusing; SP, substance P; ME, Met-enkephalin; TFA, trifluoroacetic acid; APN, aminopeptidase.
References
- 1.Sabatini, L. M., Warner, T. F., Saitoh, E. & Azen, E. A. (1989) J. Dent. Res. 68 1138-1145. [DOI] [PubMed] [Google Scholar]
- 2.Chao, J., Chai, K. X., Chen, L. M., Xiong, W., Chao, S., Woodley-Miller, C., Wang, L. X., Lu, H. S. & Chao, L. (1990) J. Biol. Chem. 265 16394-16401. [PubMed] [Google Scholar]
- 3.Shugars, D. C., Alexander, A. L., Fu, K. & Freel, S. A. (1999) Arch. Oral Biol. 44 445-453. [DOI] [PubMed] [Google Scholar]
- 4.Nishiura, T. & Abe, K. (1999) Arch. Oral Biol. 44 15-26. [DOI] [PubMed] [Google Scholar]
- 5.Rougeot, C., Rosinski-Chupin, I., Mathison, R. & Rougeon, F. (2000) Peptides 21 443-455. [DOI] [PubMed] [Google Scholar]
- 6.Rosinski-Chupin, I., Tronik, D. & Rougeon, F. (1988) Proc. Natl. Acad. Sci. USA 85 8553-8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosinski-Chupin, I., Rougeot, C., Courty, Y. & Rougeon, F. (1993) J. Histochem. Cytochem. 41 1645-1649. [DOI] [PubMed] [Google Scholar]
- 8.Rougeot, C., Rosinski-Chupin, I., Njamkepo, E. & Rougeon, F. (1994) Eur. J. Biochem. 219 765-773. [DOI] [PubMed] [Google Scholar]
- 9.Rougeot, C., Vienet, R., Cardona, A., Le Doledec, L., Grognet, J. M. & Rougeon, F. (1997) Am. J. Physiol. 273 R1309-R1320. [DOI] [PubMed] [Google Scholar]
- 10.Bourgoin, S., Le Bars, D., Artaud, F., Clot, A. M., Bouboutou, R., Fournie-Zaluski, M. C., Roques, B. P., Hamon, M. & Cesselin, F. (1986) J. Pharmacol. Exp. Ther. 238 360-366. [PubMed] [Google Scholar]
- 11.Gros, C., Pradelles, P., Rougeot, C., Bepoldin, O., Dray, F., Fournie-Zaluski, M. C., Roques, B. P., Pollard, H., Llorens-Cortes, C. & Schwartz, J. C. (1978) J. Neurochem. 31 29-39. [DOI] [PubMed] [Google Scholar]
- 12.Hebert, M. A., Ardid, D., Henrie, J. A., Tamashiro, K., Blanchard, D. C. & Blanchard, R. J. (1999) Physiol. Behav. 67 99-105. [DOI] [PubMed] [Google Scholar]
- 13.Oefner, C., D'Arcy, A., Hennig, M., Winkler, F. K. & Dale, G. E. (2000) J. Mol. Biol. 296 341-349. [DOI] [PubMed] [Google Scholar]
- 14.Malfroy, B., Schofield, P. R., Kuang, W. J., Seeburg, P. H., Mason, A. J. & Henzel, W. J. (1987) Biochem. Biophys. Res. Commun. 144 59-66. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida, K., Kanazawa, M., Casley, D. J., Katopothis, A. & Johnston, C. I. (1998) J. Cardiovasc. Pharmacol. 32 702-708. [DOI] [PubMed] [Google Scholar]
- 16.Roques, B. P., Noble, F., Dauge, V., Fournie-Zaluski, M. C. & Beaumont, A. (1993) Pharmacol. Rev. 45 87-146. [PubMed] [Google Scholar]
- 17.Sales, N., Dutriez, I., Maziere, B., Ottaviani, M. & Roques, B. P. (1991) Regul. Pept. 33 209-222. [DOI] [PubMed] [Google Scholar]
- 18.Goudreau, N., Guis, C., Soleilhac, J. M. & Roques, B. P. (1994) Anal. Biochem. 219 87-95. [DOI] [PubMed] [Google Scholar]
- 19.McCarson, K. E. (1999) Neuroscience 93 361-370. [DOI] [PubMed] [Google Scholar]
- 20.Wang, L., Sadoun, E., Stephens, R. E. & Ward, P. E. (1994) Peptides 15 497-503. [DOI] [PubMed] [Google Scholar]
- 21.Roques, B. P., Fournie-Zaluski, M. C., Soroca, E., Lecomte, J. M., Malfroy, B., Llorens, C. & Schwartz, J. C. (1980) Nature 288 286-288. [DOI] [PubMed] [Google Scholar]
- 22.Matsas, R., Kenny, A. J. & Turner, A. J. (1984) Biochem. J. 223 433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb, R. L., Yasay, G. D., McMartin, C., McNeal, R. B. & Zimmerman, M. B. (1989) J. Cardiovasc. Pharmacol. 14 285-293. [DOI] [PubMed] [Google Scholar]
- 24.Kenny, A. J. & Stephenson, S. L. (1988) FEBS Lett. 232 1-8. [DOI] [PubMed] [Google Scholar]
- 25.Michael-Titus, A. T., Fernandes, K., Setty, H. & Whelpton, R. (2002) Neuroscience 110 277-286. [DOI] [PubMed] [Google Scholar]
- 26.Nyberg, F., Le Greves, P. & Terenius, L. (1988) Biochimie 70 65-68. [DOI] [PubMed] [Google Scholar]
- 27.Dickenson, A. H. (1995) Br. J. Anaesth. 75 193-200. [DOI] [PubMed] [Google Scholar]
- 28.Chen, H., Noble, F., Coric, P., Fournie-Zaluski, M. C. & Roques, B. P. (1998) Proc. Natl. Acad. Sci. USA 95 12028-12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubuisson, D. & Dennis, S. G. (1977) Pain 4 161-174. [DOI] [PubMed] [Google Scholar]
- 30.Nieto, M. M., Wilson, J., Walker, J., Benavides, J., Fournie-Zaluski, M. C., Roques, B. P. & Noble, F. (2001) Neuropharmacology 41 496-506. [DOI] [PubMed] [Google Scholar]
- 31.Matthes, H. W., Maldonado, R., Simonin, F., Valverde, O., Slowe, S., Kitchen, I., Befort, K., Dierich, A., Le Meur, M., Dolle, P., et al. (1996) Nature 383 819-823. [DOI] [PubMed] [Google Scholar]
- 32.Sora, I., Takahashi, N., Funada, M., Ujike, H., Revay, R. S., Donovan, D. M., Miner, L. L. & Uhl, G. R. (1997) Proc. Natl. Acad. Sci. USA 94 1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konig, M., Zimmer, A. M., Steiner, H., Holmes, P. V., Crawley, J. N., Brownstein, M. J. & Zimmer, A. (1996) Nature 383 535-538. [DOI] [PubMed] [Google Scholar]
- 34.Rougeot, C., Rosinski-Chupin, I. & Rougeon, F. (1998) in Biomedical Reviews, eds. Chaldakov, G. N. & Mathison, R. (Bulgarian-American Center, Varna, Bulgaria), Vol. 9, pp. 17-32. [Google Scholar]
- 35.Malfroy, B., Kuang, W. J., Seeburg, P. H., Mason, A. J. & Schofield, P. R. (1988) FEBS Lett. 229 206-210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.