Abstract
Carbon monoxide (CO) is proposed as a physiological messenger. CO activates cGMP and has a direct effect on potassium channels. Both actions of CO lead to hyperpolarization of a cell's resting membrane potential, suggesting that CO may function as a hyperpolarizing factor, although direct evidence is still lacking. Here we take advantage of the known membrane potential gradient that exists in the muscle layers of the gastrointestinal tract to determine whether CO is an endogenous hyperpolarizing factor. We find that heme oxygenase-2-null mice have depolarized smooth muscle cells and that the membrane potential gradient in the gut is abolished. Exogenous CO hyperpolarizes the membrane potential. Regions of the canine gastrointestinal tract that are more hyperpolarized generate more CO and have higher heme oxygenase activity than more depolarized regions. Our results suggest that CO is a critical hyperpolarizing factor required for the maintenance of intestinal smooth muscle membrane potential and gradient.
Arole for carbon monoxide (CO) is proposed in cell signaling and neurotransmission (1, 2), hormone release (3, 4), as a cytoprotective agent (5), and in regulation of vascular tone (6, 7). The major source of CO is from the breakdown of heme by heme oxygenase (HO); the absence of HO-2, the constitutive HO isoform, significantly impairs the production of CO in gut and other tissues (8). HO-2 is widely expressed in tissues containing smooth muscle, including blood vessels (6), the pulmonary system (9), and the gastrointestinal tract (10), suggesting that CO is continually produced in these tissues. Multiple mechanisms of action for CO have been proposed, including activation of guanylyl cyclase and direct activation of K+ channels (11). Both mechanisms can result in membrane hyperpolarization, raising the possibility that CO can act as an endogenous hyperpolarizing factor. To determine whether CO is an endogenous hyperpolarizing factor, we took advantage of the known membrane potential gradients that exist in the muscle layers of the gastrointestinal tract. We chose the gastrointestinal tract as our model because our previous studies in the HO-2-null mouse demonstrate that intestinal smooth muscle is depolarized in the HO-2-null mouse (2), and because of the presence of a measurable smooth muscle membrane potential gradient that has not been reported in vascular smooth muscle. We hypothesized that if CO acts as a physiologically relevant hyperpolarizing factor, then CO production should mirror the membrane potential gradient along the long axis of the stomach as well as across the gut wall and that these resting membrane potential gradients would be diminished or abolished in HO-2-null mice. In the gastrointestinal tract of all species studied, there is a large gradient (≈30 mV) in membrane potential along the long axis of the stomach from the fundus (proximal stomach) to the pylorus (distal stomach) (12). There is also a membrane potential gradient across the thickness of circular muscle layer (12, 13). In the distal stomach and small intestine the membrane potential in the outer circular smooth muscle region (next to the longitudinal muscle) is ≈10 mV hyperpolarized compared with circular smooth muscle cells at the inner circular smooth muscle region (next to the submucosa) (12, 13). In the large intestine, the gradient is present but reversed (14). Circular smooth muscle cells in the outer circular smooth muscle region of the colon have a membrane potential 30 mV more depolarized compared with smooth muscle cells in the inner region (14, 15). The smooth muscle gradient allows a graded contractile response to a stimulus, with a weak stimulus recruiting only the more depolarized smooth muscle and a stronger stimulus recruiting more hyperpolarized smooth muscle.
Methods
Gas Chromatography to Measure CO Production. Endogenous production of CO was measured from muscle strips dissected from dog intestine. Canine tissue was obtained from adult mongrel dogs (25 kg) of either sex. Dogs were killed with thiopental (20 mg/kg i.v.) followed by exsanguination. A total of 12 dogs were used for this and the HO experiments. The use of canine tissue was approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. Half-wall thickness preparations (300 mg wet weight) were used to include the hyperpolarized region in one sample and the depolarized region in the other sample. Each tissue was placed in its own 10-ml glass vial containing 5 ml of normal Krebs solution, and the vial was placed in a 50-ml gas syringe with the vial resting on the plunger and the syringe placed upright. CO was purged from the syringe at time 0 by connecting the nozzle of the syringe to a 97% O2/3% CO2 gas cylinder through a catalytic combustion filter (Trace Analytical, Sparks, MD) CO scrubber to remove all ambient CO, and the gas mixture was drawn into the syringe by withdrawing the plunger. The gas was then expelled by detaching the gas cylinder from the nozzle and pushing the plunger up until the glass vial hit the end of the syringe; the nozzle was then closed and the process was repeated 12 times. On the 12th time, the plunger was not advanced all of the way in but left at the 50-cm3 mark. In each set of experiments, a vial in two gas syringes contained Krebs solution without tissue to act as a control, and another two syringes contained no vials but a known amount of CO. As further positive controls, one gas syringe contained ≈300 mg wet weight of spleen and another contained ≈300 mg wet weight of liver. All gas syringes were placed upright in an incubator at 37°C for 12 h and then removed. An aliquot of gas (25 cm3) was drawn out by advancing the plunger and the amount of CO was determined by gas chromatography (16). Results were expressed in nanomoles of CO generated per milligram of tissue, wet weight.
Bilirubin Measurement to Measure HO Activity. Bilirubin production was measured in preparations similar to those described above. Immediately after dissecting, the preparations were snap frozen for analysis of bilirubin production, and HO activity was measured by the rate of generation of bilirubin in microsomes isolated from the strips of gut tissue (17, 18). The gut tissue was homogenized in 5 vol of 0.1 M potassium phosphate (pH 7.4) buffer by using a PowerGen homogenizer (Fisher Scientific) followed by sonication on ice for 30 s. The homogenate was centrifuged at 3,000 × g at 4°C for 10 min, and the supernatant subsequently was centrifuged at 12,000 × g at 4°C for 20 min. Microsomes were pelleted from the resulting supernatant by centrifugation at 105,000 × g at 4°C for 60 min. The microsomal pellet was resuspended in 0.1 M potassium phosphate buffer (pH 7.4) containing 2 mM MgCl2. An aliquot of the microsomal suspension (0.2 mg of protein) was added to a reaction mixture (0.4 ml total volume) containing rat liver cytosol (2 mg of cytosolic protein), hemin (20 μM), glucose 6-phosphate (2 mM), glucose-6-phosphate dehydrogenase (0.2 unit), and NADPH (0.8 mM) and incubated for 1 h at 37°C in the dark. The formed bilirubin was extracted with chloroform (0.5 ml) and Δ(A464 - A530) was measured (extinction coefficient, 40 mM-1·cm-1 for bilirubin). HO activity is expressed as picomoles of bilirubin formed per 60 min per milligram of protein, determined by the Lowry method.
Electrophysiology to Measure Membrane Potential. After dissection,
murine muscle strips were placed in a recording chamber containing Krebs
solution. The use of murine tissue was approved by the Institutional Animal
Care and Use Committee of the Mayo Clinic. The chamber (3 ml total volume) was
perfused with warmed (37°C) and oxygenated Krebs solution at a constant
flow rate of 3 ml/min. Krebs solution had the following ionic composition (in
mM): Na+ 127.4, K+ 5.9, Ca2+ 2.5,
Mg2+ 1.2, Cl- 134,
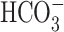 15.5,
15.5,
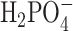 1.2, and
glucose 11.5 and was freshly prepared daily. The solution was continuously
bubbled with 97% O2/3% CO2 and maintained at pH 7.4.
After an equilibration period of at least 2 h, the muscle strips were
stretched to an initial tension of 50–100 mg above the baseline tension.
Recordings of intracellular electrical activity from smooth muscle cells were
obtained by using glass capillary microelectrodes filled with 3 M KCl and with
resistances ranging from 30 to 80 MΩ. A mechanized stage was used to
record circular smooth muscle membrane potential at 5-μm intervals across
the thickness of the circular muscle layer of the gut wall. Unless otherwise
indicated, all chemicals were obtained from Sigma. CO was obtained from Scott
Specialty Gasses (Troy, MI).
1.2, and
glucose 11.5 and was freshly prepared daily. The solution was continuously
bubbled with 97% O2/3% CO2 and maintained at pH 7.4.
After an equilibration period of at least 2 h, the muscle strips were
stretched to an initial tension of 50–100 mg above the baseline tension.
Recordings of intracellular electrical activity from smooth muscle cells were
obtained by using glass capillary microelectrodes filled with 3 M KCl and with
resistances ranging from 30 to 80 MΩ. A mechanized stage was used to
record circular smooth muscle membrane potential at 5-μm intervals across
the thickness of the circular muscle layer of the gut wall. Unless otherwise
indicated, all chemicals were obtained from Sigma. CO was obtained from Scott
Specialty Gasses (Troy, MI).
Immunostaining to Detect HO. The mucosa-free dissected muscle layers were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4), by incubation at 4°C for 12 h. Next, tissues were rinsed with PBS before immunostaining by using a rabbit polyclonal Ab to HO-2 (1:1,000 dilution, StressGen Biotechnologies, Victoria, BC, Canada) or a rabbit polyclonal Ab to an HO-2-α-GST fusion protein (1:1,200 dilution). The secondary Ab (donkey anti-rabbit IgG, Chemicon, 1:100 dilution) was conjugated to FITC. Staining was analyzed by examining the tissues by using an epifluorescence light microscope (Zeiss Axiophot) or laser scanning confocal microscope (Zeiss LSM510).
Statistical Analysis. All observed values are expressed as the mean ± SEM. The number of cells recorded is designated (n) in Results. Statistical significance was determined by using paired or nonpaired Student's t tests as appropriate. A P value of <0.05 was considered significant.
Results
The smooth muscle membrane potential in the fundus, the most depolarized region of the gastrointestinal tract (12), was -53 ± 0.3 mV in WT mice and was not different (-51 ± 0.6 mV, n = 56, P > 0.05) in HO-2-null mice (Table 1). In contrast, as reported (2), the outer small intestinal circular smooth muscle membrane potential was -66 ± 0.4 mV in the WT mouse and was depolarized to -57 ± 0.9 mV in HO-2-null mice (n = 150, P < 0.05). Addition of CO (1.6 μM) to fundic smooth muscle strips in WT mice hyperpolarized the membrane potential by 5 ± 1 mV (n = 4, P < 0.05), suggesting that the depolarized membrane potential in fundic muscle was not due to a defect at the smooth muscle layer but was possibly due to decreased CO production when compared with the small intestine. Bilirubin had no effect on fundic smooth muscle membrane potential.
Table 1. Membrane potential in mice.
|
Potential, mV
|
||||
|---|---|---|---|---|
| Tissue | WT | HO-2-null | HO-2 immunoreactivity | |
| Gastrointestinal muscle | ||||
| Fundus | -53 | -51 | ICC | - |
| Antrum | -70 | -58* | ICC | + |
| Intestine | -66 | -57* | ICC | + |
| Skeletal muscle | ||||
| Abdominal transversalis | -64 | -61 | - | |
| Superior mesenteric ganglia | ||||
| Neurons | -46 | -47 | + | |
ICC, interstitial cells of Cajal
, P < 0.05
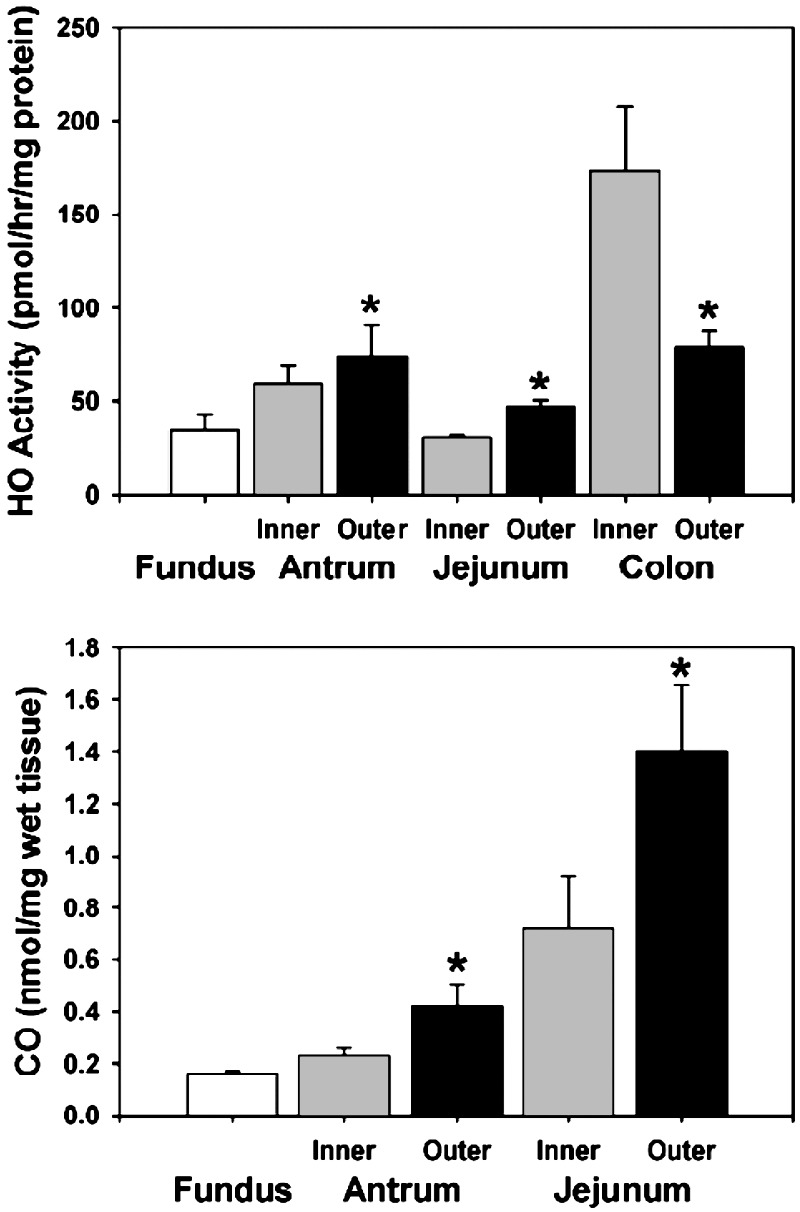
The thickness of the murine antrum, small intestine, and colon circular muscle layers is <100 μm, making dissection of the layer into different regions difficult. In contrast, the corresponding smooth muscle layers in the canine gastrointestinal tract are much thicker, allowing dissection into two regions, a depolarized region and a hyperpolarized region. After dissection, muscle strips (300 mg) from the dog intestine were immediately frozen in dry ice, and HO activity was measured by bilirubin production (18). HO activity closely mirrored membrane potential, with the lowest HO activity in the fundus and the highest values in the more hyperpolarized regions of the gastrointestinal tract (n = 6 animals, Fig. 1). Interestingly, HO activity was higher in the outer half than in the inner half of antral and small intestinal circular muscle layers, but in the colon, in contrast, activity was higher in the inner half of the circular muscle layer, again mirroring the membrane potential (Fig. 1). Moreover, the difference in HO activity between the inner and outer regions was highest in the colon, which has the largest membrane potential gradient. CO production in the canine tissues was also directly measured by gas chromatography (16). CO production also mirrored membrane potential, with higher production in the hyperpolarized regions compared with the depolarized regions (n = 6 animals, Fig. 1). Reproducible measurements of CO production were not possible in the colon, likely reflecting a large and variable bacterial load.
Fig. 1.
HO activity and CO production. (Upper) HO activity in canine gastric fundus and in the inner and outer gastric antrum, jejunum, and colon. (Lower) CO production in canine gastric fundus and in the inner and outer gastric antrum and jejunum. Both HO activity and CO production paralleled smooth muscle membrane potential. *, P < 0.05 compared with same region of the gastrointestinal tract.
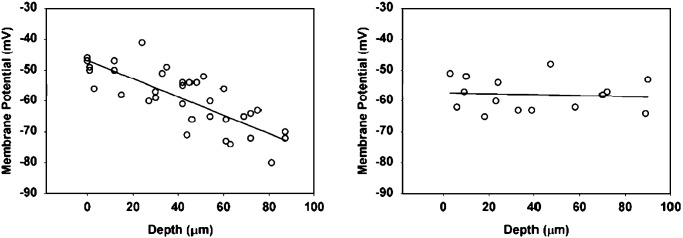
Although a smooth muscle membrane potential gradient has been established for several species, it has not been reported in the murine intestine. To take advantage of murine knockout models, we used a mechanized stage to directly record smooth muscle membrane potential across the thickness of murine antrum to determine whether a smooth membrane potential gradient was present. Recordings were made at 5-μm intervals across the thickness of the circular muscle layer. The results showed that, similar to that in larger species, a membrane potential gradient was present in mice with a potential of approximately -70 mV at the myenteric border decreasing to -49 mV at the mucosal border (n = 6 animals). In HO-2-null mice, the smooth muscle membrane potential was more depolarized, averaging a depolarized -58 mV across the whole muscle layer thickness, and the gradient seen in WT mice was abolished (Fig. 2).
Fig. 2.
Loss of the membrane potential gradient and depolarization in HO-2-null mice. (Left) Membrane potential gradient across the thickness of antral circular smooth muscle of WT mice. (Right) Depolarization of smooth muscle and loss of the membrane potential gradient in HO-2-null mice.
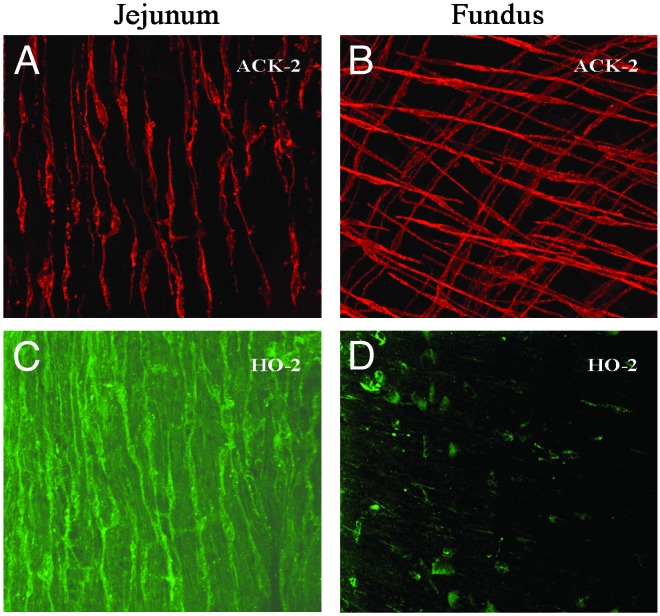
We next determined the cellular origin of CO. HO-2 immunoreactivity was not detected in smooth muscle cells. Enteric ganglion neurons express HO-2, suggesting that the source of CO may be from neuronal tissue. However, the membrane potential of intestinal smooth muscle in c-RET-null mice that lack an enteric nervous system (19) is not depolarized compared to WT controls. Furthermore, tetrodotoxin (1 μM), a blocker of nerve transmission, had no effect on the membrane potential of outer murine or outer canine small intestinal smooth muscle [-68 ± 4 mV (before) and -69 ± 4 mV, n = 4, P > 0.05]. These observations suggest that the hyperpolarizing CO was in fact not of neuronal origin. We have observed that the resting membrane potential of smooth muscle cells from the antrum and jejunum of HO-2-null mice is similar to the resting membrane potential of the fundus, which is unchanged in HO-2-null mice compared with WT. These data raised the possibility that hyperpolarizing CO is produced by a cell type that is absent or altered in the fundus. Intestinal circular smooth muscle is depolarized in W/Wv mice, which lack small intestinal myenteric networks of interstitial cells of Cajal (20). Therefore, we hypothesized that interstitial cells of Cajal might be the source of the hyperpolarizing CO. Interstitial cells of Cajal generate rhythmic changes in membrane potential and are essential for gastrointestinal motility (20–22). Interstitial cells of Cajal in the murine small intestine were found to express HO-2, suggesting that they may provide a source for CO (10). Interestingly, interstitial cells of Cajal in the fundus are morphologically different from interstitial cells of Cajal in the rest of the gastrointestinal tract and may have a different function (23). Staining for HO-2 in colonic and small intestinal tissues in WT mice showed strong immunoreactivity for HO-2 in interstitial cells of Cajal, whereas interstitial cells of Cajal in the fundus did not express significant amounts of HO-2 (Fig. 3). These results suggest that HO-2 expressed by interstitial cells of Cajal is likely the source for CO in the small intestine and colon and that CO released from these cells acts as a smooth muscle hyperpolarizing factor.
Fig. 3.
Confocal images of murine interstitial cells of Cajal immunolabeled with ACK-2, an antibody directed against c-kit, a tyrosine kinase receptor expressed in interstitial cells of Cajal, to visualize interstitial cells of Cajal and HO-2. (A and B) Interstitial cells of Cajal within the muscle layers of the jejunum and fundus. (C and D) HO-2 immunolabeling of the same sections. Interstitial cells of Cajal of the jejunum contained HO-2 immunoreactivity, whereas HO-2 immunoreactivity was only in enteric neurons (at a plane just beneath the interstitial cells of Cajal) and not in interstitial cells of Cajal of the fundus.
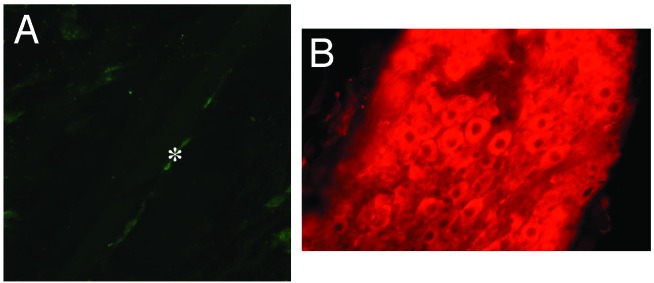
To determine whether the hyperpolarizing effect of CO was limited to the smooth muscle of the gastrointestinal tract or was more widespread to other tissues, membrane potentials were measured in skeletal muscle and in peripheral autonomic ganglion neurons in WT and HO-2-null mice (Table 1). Previous reports have suggested that HO-2 may be expressed in skeletal muscle (24). In the abdominal transversalis muscle, no difference was seen in membrane potential recorded from transversalis myocytes from HO-2-null mice (-61 ± 1 mV, n = 32) and WT mice (-64 ± 3 mV, n = 14, P > 0.05). Interstitial cells of Cajal have not been described in skeletal muscle and none were found in our experiments. The tissue was also immunolabeled for HO-2, and no immunoreactivity was noted except in nerve fibers (Fig. 4). Peripheral autonomic ganglion neurons express HO-2 (25, 26). Membrane potential was measured in superior mesenteric ganglion neurons of mice to determine whether CO acts as a hyperpolarizing factor in these postganglionic sympathetic neurons. In WT mice, neuronal membrane potential was -46 ± 1 mV (n = 139) and was unchanged in HO-2-null mice (-47 ± 1 mV, n = 16, P > 0.05). HO-2 immunopositivity in the superior mesenteric ganglion was confirmed by using Abs to HO-2 (Fig. 4).
Fig. 4.
Confocal images of murine abdominal skeletal muscle and the superior mesenteric ganglion immunolabeled with an Ab to HO-2. (A) Abdominal transversalis skeletal muscle cells lacked immunoreactivity for HO-2; *, HO-2-immunopositive nerve fiber. (B) The superior mesenteric ganglion contained HO-2-immunopositive nerve cell bodies.
Discussion
This study on CO as a hyperpolarizing factor has important implications for the control of gastrointestinal contractile activity. Smooth muscle membrane potential is tightly regulated because it is the primary determinant of the contractile state of visceral smooth muscle cells. Hyperpolarization of the membrane potential, usually through activation of potassium channel, results in relaxation and, conversely, depolarization activates voltage-gated L-type calcium channels, calcium entry, and contraction (27). In the gastrointestinal tract, the response to a given stimulus is determined by the degree of hyperpolarization of the membrane potential of gastrointestinal smooth muscle from the threshold required to activate L-type calcium channels. The voltage gradient may be central to the ability of circular smooth muscle to produce weak contractions that barely indent the wall of the gut to strong, mixing, propulsive, and occlusive contractions that produce a deep ring indentation in the gut wall, with gradations in the strength of contraction ranging between these two extremes. The gradient in resting membrane potential across the wall may be considered to function as a biological rheostat regulating how much the thickness of the circular muscle layer contracts during each slow wave. If there were no gradient in resting membrane potential, the entire thickness of the circular muscle wall would contract in an all-or-none manner with each passing electrical event. The genesis of this membrane potential gradient was unknown.
The main finding of this study is that HO activity and CO production coincide quite remarkably with the membrane potential gradient across both the length and thickness of the gastrointestinal tract; furthermore, we validated this relationship by demonstrating that the loss of HO-2 enzyme leads to depolarization of gastrointestinal smooth muscle to values normally seen in the gastric fundus and leads to loss of the intestinal membrane potential gradient across the thickness of the circular smooth muscle layer. Additionally, we demonstrate that this phenomenon is cell and tissue-type specific and that just the presence of HO-2 does not predict a hyperpolarizing role for CO. Superior mesenteric neurons as well as other peripheral autonomic ganglion neurons are strongly immunopositive for HO-2 (25, 26) yet are not depolarized in HO-2-null mice. In these neurons, HO-2 and subsequently CO may play a different role, perhaps as a neurotransmitter or cellular messenger (2). Rather, the presence of a cell type immunopositive for HO-2 adjacent to a target cell that does not contain HO-2 may predict a hyperpolarizing role for CO in that tissue. In intestinal smooth muscle, muscle cells themselves are not immunopositive for HO-2, but other adjacent cells are. In the gastrointestinal tract these cells are interstitial cells of Cajal, specialized cells of mesenchymal origin that are required for normal intestinal motility. The main roles for interstitial cells of Cajal are thought to be to generate an electrical signal that paces intestinal smooth muscle (13, 20, 21, 28) and to modulate enteric neurotransmission (29). Our data suggest that an additional role for interstitial cells of Cajal is to generate CO and hyperpolarize intestinal smooth muscle.
In summary, CO appears to be a hyperpolarizing factor that in the gastrointestinal tract is required to maintain a hyperpolarized membrane potential and to help set the intestinal smooth muscle membrane potential gradient. It remains to be determined whether the graded hyperpolarization produced by CO is limited to the gastrointestinal tract or is a more widespread function of CO.
Acknowledgments
We thank Anthony Croatt for HO activity measurements and Kristy Zodrow for secretarial assistance. This work was supported by National Institutes of Health Grants DK17238 (to J.H.S.) and DK52766 (to G.F.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: HO, heme oxygenase.
References
- 1.Jaffrey, S. R. & Snyder, S. H. (1995) Annu. Rev. Cell Dev. Biol. 11 417-440. [DOI] [PubMed] [Google Scholar]
- 2.Xue, L., Farrugia, G., Miller, S. M., Ferris, C. D., Snyder, S. H. & Szurszewski, J. H. (2000) Proc. Natl. Acad. Sci. USA 97 1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamar, C. A., Mahesh, V. B. & Brann, D. W. (1996) Endocrinology 137 790-793. [DOI] [PubMed] [Google Scholar]
- 4.Mancuso, C., Kostoglou-Athanassiou, I., Forsling, M. L., Grossman, A. B., Preziosi, P., Navarra, P. & Minotti, G. (1997) Brain Res. Mol. Brain Res. 50 267-276. [DOI] [PubMed] [Google Scholar]
- 5.Otterbein, L. E., Bach, F. H., Alam, J., Soares, M., Tao Lu, H., Wysk, M., Davis, R. J., Flavell, R. A. & Choi, A. M. (2000) Nat. Med. 6 422-428. [DOI] [PubMed] [Google Scholar]
- 6.Zakhary, R., Gaine, S. P., Dinerman, J. L., Ruat, M., Flavahan, N. A. & Snyder, S. H. (1996) Proc. Natl. Acad. Sci. USA 93 795-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christodoulides, N., Durante, W., Kroll, M. H. & Schafer, A. I. (1995) Circulation 91 2306-2309. [DOI] [PubMed] [Google Scholar]
- 8.Maines, M. D. (1988) FASEB J. 2 2557-2568. [PubMed] [Google Scholar]
- 9.Marks, G. S., McLaughlin, B. E., Vreman, H. J., Stevenson, D. K., Nakatsu, K., Brien, J. F. & Pang, S. C. (1997) J. Cardiovasc. Pharmacol. 30 1-6. [DOI] [PubMed] [Google Scholar]
- 10.Miller, S. M., Farrugia, G., Schmalz, P. F., Ermilov, L. G., Maines, M. D. & Szurszewski, J. H. (1998) Gastroenterology 114 239-244. [DOI] [PubMed] [Google Scholar]
- 11.Wang, R., Wu, L. & Wang, Z. (1997) Pflügers Arch. 434 285-291. [DOI] [PubMed] [Google Scholar]
- 12.Szurszewski, J. H. (1987) in Physiology of the Gastrointestinal Tract, ed. Johnson, L. R. (Raven, New York), Vol. 2, pp. 435-466. [Google Scholar]
- 13.Hara, Y., Kubota, M. & Szurszewski, J. H. (1986) J. Physiol. (London) 372 501-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders, K. M. (1989) in The Gastrointestinal System, eds. Schultz, S. G. & Wood, J. D. (Oxford Univ. Press, New York), Vol. 1, pp. 251-271. [Google Scholar]
- 15.Smith, T. K., Reed, J. B. & Sanders, K. M. (1987) Am. J. Physiol. 252 C215-C224. [DOI] [PubMed] [Google Scholar]
- 16.Levitt, M. D., Ellis, C., Springfield, J. & Engel, R. R. (1995) J. Chromatogr. A 695 324-328. [DOI] [PubMed] [Google Scholar]
- 17.Liang, M., Croatt, A. J. & Nath, K. A. (2000) Am. J. Physiol. 279 F728-F735. [DOI] [PubMed] [Google Scholar]
- 18.Nath, K. A., Balla, G., Vercellotti, G. M., Balla, J., Jacob, H. S., Levitt, M. D. & Rosenberg, M. E. (1992) J. Clin. Invest. 90 267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pachnis, V., Durbec, P., Taraviras, S., Grigoriou, M. & Natarajan, D. (1998) Am. J. Physiol. 275 G183-G186. [DOI] [PubMed] [Google Scholar]
- 20.Ward, S. M., Burns, A. J., Torihashi, S. & Sanders, K. M. (1994) J. Physiol. (London) 480 91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huizinga, J. D., Thuneberg, L., Kluppel, M., Malysz, J., Mikkelsen, H. B. & Bernstein, A. (1995) Nature 373 347-349. [DOI] [PubMed] [Google Scholar]
- 22.Der-Silaphet, T., Malysz, J., Hagel, S. L., Arsenault, A. & Huizinga, J. D. (1998) Gastroenterology 114 724-736. [DOI] [PubMed] [Google Scholar]
- 23.Burns, A. J., Lomax, A. E., Torihashi, S., Sanders, K. M. & Ward, S. M. (1996) Proc. Natl. Acad. Sci. USA 93 12008-12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum, O., Feussner, M., Richter, H. & Gossrau, R. (2000) Acta Histochem. 102 281-298. [DOI] [PubMed] [Google Scholar]
- 25.Vollerthun, R., Hohler, B. & Kummer, W. (1995) NeuroReport 7 173-176. [PubMed] [Google Scholar]
- 26.Magnusson, S., Ekstrom, T. J., Elmer, E., Kanje, M., Ny, L. & Alm, P. (2000) Neuroscience 95 821-829. [DOI] [PubMed] [Google Scholar]
- 27.Holm, A. N., Rich, A., Sarr, M. G. & Farrugia, G. (2000) Am. J. Physiol. 279 G1155-G1161. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, N., Prosser, C. L. & Dahms, V. (1986) Am. J. Physiol. 250 G287-G294. [DOI] [PubMed] [Google Scholar]
- 29.Ward, S. M., Beckett, E. A., Wang, X., Baker, F., Khoyi, M. & Sanders, K. M. (2000) J. Neurosci. 20 1393-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]