Abstract
Bacterial effector proteins secreted through the type III secretion system (TTSS) play a crucial role in causing plant and human diseases. Although the ability of type III effectors to trigger defense responses in resistant plants is well understood, the disease-promoting functions of type III effectors in susceptible plants are largely enigmatic. Previous microscopic studies suggest that in susceptible plants the TTSS of plant-pathogenic bacteria transports suppressors of a cell wall-based plant defense activated by the TTSS-defective hrp mutant bacteria. However, the identity of such suppressors has remained elusive. We discovered that the Pseudomonas syringae TTSS down-regulated the expression of a set of Arabidopsis genes encoding putatively secreted cell wall and defense proteins in a salicylic acid-independent manner. Transgenic expression of AvrPto repressed a similar set of host genes, compromised defense-related callose deposition in the host cell wall, and permitted substantial multiplication of an hrp mutant. AvrPto is therefore one of the long postulated suppressors of an salicylic acid-independent, cell wall-based defense that is aimed at hrp mutant bacteria.
Many plant pathogenic bacteria, such as Pseudomonas syringae, carry a type III secretion system (TTSS), which delivers effector proteins into the plant cell (1–5). Translocation of these effectors is required for bacterial pathogenesis. The TTSS also plays a crucial virulence role in bacterial diseases of mammals (3, 4, 6, 7). However, mammalian and plant pathogenic bacteria appear to produce largely distinct sets of type III effectors, possibly reflecting their different lifestyles and unique host cellular structures (8–13). For intracellular mammalian pathogenic bacteria, such as Salmonella and Shigella, a key function of type III effectors is the regulation of host cytoskeleton dynamics, which aids the invasion of bacteria into the host cell (6). Most plant pathogenic bacteria, such as P. syringae, however, are noninvasive, extracellular pathogens; they colonize the host intercellular space outside the plant cell wall, a structure absent in animal cells. TTSS-defective bacteria do not usually multiply or cause disease symptoms in otherwise susceptible plants. The inability of TTSS mutants to multiply in the plant intercellular space is similar to that of saprophytic bacteria found in nature.
In plant pathogenic bacteria, the TTSS is encoded by hrp (hypersensitive reaction and pathogenicity) genes (1, 2). We are using P. syringae pv. tomato strain DC3000 (DC3000), which infects Arabidopsis and tomato (14, 15), to elucidate the virulence function of the TTSS in bacterial pathogenesis in plants. In Arabidopsis, DC3000 multiplies aggressively for 2 days before the onset of disease symptoms, which is characterized by water soaking in the apoplast, followed by tissue necrosis and chlorosis (14, 15). We have shown (16, 17) that the ability of DC3000 to infect Arabidopsis depends on the TTSS because hrp mutants [e.g., hrpS and hrcC (formerly hrpH) mutants] of DC3000 do not multiply or cause disease in Arabidopsis. The TTSS of DC3000 is believed to secrete and/or translocate >30 effector proteins into the host cell (8–13). Cumulatively, these effectors alter host cellular processes and promote disease development through largely unknown mechanisms. Although the primary function of type III effectors is to promote plant susceptibility, some effectors may be recognized by the corresponding plant disease resistance proteins in resistant plants and trigger defense responses, including the hypersensitive response (HR) (18, 19). In fact, many type III effector genes in P. syringae were discovered based on their ability to trigger the HR in resistant plants and have been named avr (for avirulence) genes (20). For example, the type III effector, AvrPto, was identified based on its avirulence activity in plants (21–23). Although the ability of type III effectors to trigger defense responses in resistant plants is well understood, the mechanism by which type III effectors, as a group, enable plant pathogenic bacteria to proliferate in the intercellular space of a susceptible plant remains enigmatic. In addition to type III effectors, DC3000 also produces the phytotoxin coronatine (COR), which is required for full virulence in Arabidopsis (24–26).
A decade ago, Jakobek and coworkers (27, 28) showed that in bean, general defense genes encoding phenylalanine ammonialyase, chalcone synthase, and chalcone isomerase, which are involved in the biosynthesis of antimicrobial phytoalexins, are induced by the hrp mutants of a nonhost bacterium, P. syringae pv. tabaci, and saprophytic bacteria, but not by the wild-type virulent P. syringae pv. phaseolicola. Ultrastructural studies have illustrated that hrp mutants of Xanthomonas campestris pv. vesicatoria and P. syringae pv. phaseolicola, as well as a saprophytic bacterium, cause the plant cell wall to thicken, forming a papilla (29, 30, 31). Papillae are cell wall appositions composed of callose, phenolics, hydroxyproline-rich glycoproteins (e.g., extensins), and other materials. The type III secretion-competent wild-type X. campestris pv. vesicatoria, on the other hand, does not induce papillae formation (30). These experiments led to the attractive hypothesis that TTSSs of plant pathogenic bacteria secrete one or more suppressors of this hallmark cell wall-based plant defense response elicited by nonpathogenic bacteria (e.g., hrp mutants and saprophytic bacteria). However, the identity of such a suppressor has remained elusive. Similarly, the plant defense response that is aimed at hrp mutant bacteria, but is overcome by the TTSS, is also poorly defined at the molecular level.
In this article, we used a combination of large-scale host gene expression profiling, transgenic expression of a DC3000 effector, and cytological examination to identify AvrPto as a suppressor of the papillae-associated cell wall defense. Furthermore, we show that the TTSS of DC3000 is involved in highly biased suppression of a set of Arabidopsis genes that encode putatively secreted cell wall and defense proteins in a salicylic acid (SA)-independent manner. This research provides a much needed guide for further progress on the elucidation of the virulence functions of type III effectors in susceptible plants.
Materials and Methods
Plant Growth and Bacteria Enumeration. Arabidopsis thaliana accession Col-0 gl1 plants were grown in soil in growth chambers with a day/night cycle of 12 h/12 h, a light intensity of 100 μE, and a constant temperature of 20°C. Four- to 5-week-old plants were used for experiments. Bacteria were grown in low-salt Luria–Bertani broth (15, 32) to the mid- to late-logarithmic phase at 30°C. Bacterial cultures were centrifuged to recover bacteria, which were resuspended in sterile water to a final OD600 of 0.002 [equivalent to 1 × 106 colony-forming units (cfu)/ml]. Fully expanded leaves were infiltrated with bacterial suspensions, and bacteria were enumerated as described by Katagiri et al. (15). The mean values of the bacterial populations are plotted with the SD displayed as error. Plants analyzed in Fig. 4 were sprayed daily with a 30-μM dexamethasone solution containing 0.02% Silwet L-77 (Osi Specialties, Friendship, WV). Bacterial suspensions were infiltrated into leaves 1 day after the first dexamethasone treatment. The regulation-defective hrpS mutant and the secretion-defective hrcC mutant used in this article were described (16).
Construction of the COR- hrpS Double Mutant. The COR- hrpS double mutant was generated by introducing a reported (24) Tn5Sp-disrupted hrpS gene into the chromosome of DC3118 (COR- mutant) through marker exchange mutagenesis. The COR- mutant causes a normal HR in tobacco, but slightly reduced and delayed disease symptoms in Arabidopsis, suggesting a virulence role of COR in Pst DC3000–Arabidopsis interaction. The COR- hrpS mutant does not elicit an HR in tobacco or cause disease in Arabidopsis. The wild-type hrpS gene carried on pHRPRS2 (33) restored the ability of the COR- hrpS mutant to elicit an HR in tobacco and cause disease symptoms in Arabidopsis.
Production of AvrPto Transgenic Plants. avrPto was amplified by PCR from DC3000 (not strain JL1065) genomic DNA using the following primers: sense primer 5′-CCGCTCGAGACCATGGGAAATATATGTGTC-3′ and antisense primer 5′-GACTAGTTCATTGCCAGTTACGGTACG-3′. The avrPto fragment was cloned into pTA7002 under the control of the dexamethasone-inducible promoter (34, 35) and confirmed by sequencing. AvrPto transgenic plants were produced after a protocol that was described (36). Seven independent avrPto transformants were analyzed and all exhibited characteristics similar to those of lines 76 and 129 reported here.
Microarray Experiments. Four- to 5-week-old A. thaliana accession Col-0 gl1 leaves were vacuum-infiltrated with bacterial suspensions containing 1 × 106 cfu/ml bacteria (15). For microarray analysis, infiltrated leaves were collected at 12, 24, and 36 h postinoculation, before the appearance of water-soaking symptoms (at ≈48 h) and necrosis and chlorosis (at ≈72 h). Total RNA was isolated from each leaf sample and equal amounts of RNA from different time points were pooled for DNA microarray analysis according to the protocol described (37). The first two microarray experiments were performed by using the Arabidopsis Functional Genomic Consortium's (Michigan State University) microarray slides, each containing ≈7,200 unique genes (37). Subsequent experiments were performed by using a subarray enriched for DC3000-regulated genes (R.T. and S.Y.H., unpublished data).
Genes with a ≥2-fold expression difference (a ratio of ≤0.5 for repressed genes or a ratio of ≥2.0 for induced genes) in at least two of the three biological replicates of the DC3000/hrpS mutant comparison in Col-0 Arabidopsis plants (I-A, I-B, and I-C) are described in Table 2 and Supporting Methods, which are published as supporting information on the PNAS web site, www.pnas.org). Gene clustering analysis shown in Fig. 2B was performed by using the CLUSTER and TREEVIEW programs (38). The predicted protein locations were determined using TARGETP analysis conducted on the Arabidopsis genome by the Munich Information Center for Protein Sequences (Neuherberg, Germany), which can be accessed at http://mips.gsf.de/proj/thal/db/tables/tables_menu.html (ref. 39).
Callose Staining. Arabidopsis leaves were sprayed with 30 μM dexamethasone and then infiltrated 24 h later with a bacterial suspension of OD600 = 0.2 (1 × 108 cfu/ml). Leaves were harvested 12 h after bacterial infiltration, cleared, and stained with aniline blue for callose as described (40). Leaves were examined with a Leica DM RA2 microscope with an A4 fluorescence cube. The number of callose depositions was determined with QUANTITY ONE software (Bio-Rad). More than 10 adjacent fields of view along the length of the leaf (not including the midvein or leaf edge) were analyzed and averaged. The values in Fig. 3B are the average and SD of more than five independent leaves for each treatment.
Results
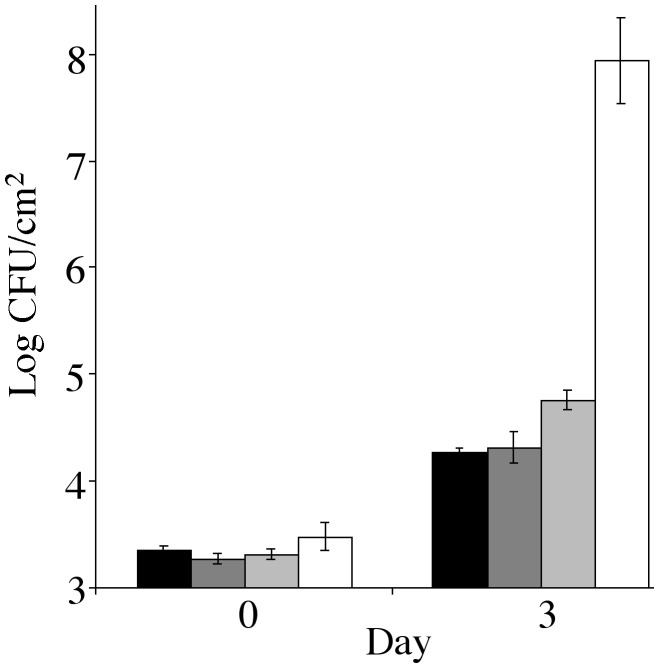
Roles of SA- and Ethylene-Mediated Defense Pathways in Resistance to hrp Mutants. Recently, several P. syringae type III effectors, most notably AvrPtoB, VirPphA, VirPphF, AvrRpt2, and AvrRpm1 have been shown or suggested to modulate the HR or SA defense (41–45). To test the hypothesis that it is host defense that prevents efficient multiplication of the TTSS-defective mutants in the intercellular space, we examined the multiplication of the DC3000 hrcC mutant in nahG (46) and ein2 (47) plants, which are defective in two major defense pathways effective against avirulent and/or virulent strains of P. syringae: the SA-mediated pathway and the ethylene-mediated pathway, respectively (48). We found that the hrcC mutant reached a slightly higher population in nahG plants, compared with wild-type control plants (Fig. 1). However, the 5-fold population increase was small compared with the >10,000-fold increase of the DC3000 population in wild-type leaves (Fig. 1). No significant increase in multiplication was observed for the hrcC mutant population in the ein2 plants, compared with that in wild-type plants (Fig. 1). Thus, abrogation of the SA- or ethylene-mediated defense pathway is not sufficient for a TTSS-defective mutant to multiply efficiently in the Arabidopsis intercellular space. These observations argue against a primary role of the SA- or ethylene-mediated resistance in preventing the growth of the nonpathogenic hrp mutants in Arabidopsis.
Fig. 1.
Bacterial populations in wild-type Col-0, ein2, and nahG transgenic plants. hrcC mutant growth in Col-0 (black bars), ein2 (dark gray bars), and nahG leaves (light gray bars) are shown. DC3000 growth in Col-0 (white bars) is shown for comparison.
Biased Suppression of Arabidopsis Genes Encoding Putatively Secreted Cell Wall and Defense Proteins. To date, a host gene expression signature that marks the virulence function of the TTSS has not been identified in any plant pathogenic bacterium. To gain molecular insight into the enigmatic virulence functions of DC3000 type III effectors, we used a cDNA microarray to examine the expression of ≈7,200 randomly chosen Arabidopsis genes in presymptomatic leaves inoculated with DC3000 or hrp mutants (Table 2). We identified a subset of 117 genes whose expression was associated with the functions of the DC3000 TTSS (Table 2). Of the 117 genes, 53 were repressed and 64 were induced.
Examination of the Arabidopsis genes repressed by the DC3000 TTSS revealed that a surprisingly large percentage of the genes encode putatively secreted proteins. In fact, 42% of repressed genes are predicted to encode proteins that enter the plant secretory pathway, compared with only 17% of the whole genome and 16% of genes on the microarray used in this article (Table 1). On the other hand, the proteins encoded by the TTSS-induced genes exhibited no obvious bias toward secreted proteins. This result is in contrast to the moderately enriched chloroplast-targeted proteins in both TTSS-repressed and TTSS-induced gene sets (Table 1). Interestingly, we observed relatively little type III effector-mediated repression of genes involved in primary metabolic pathways in the cytoplasm, nucleus, or mitochondria, suggesting that in the first 36 h postinfection, host cells had not yet undergone global, nonspecific deterioration. This result is expected because we used presymptomatic tissues for RNA isolation.
Table 1. Predicted locations of proteins encoded by TTSS-regulated Arabidopsis genes.
| Predicted location | Repressed genes, % | Induced genes, % | Microarray, % | Genome wide, % |
|---|---|---|---|---|
| Secreted | 42 | 20 | 16 | 17 |
| Chloroplast | 28 | 23 | 18 | 14 |
| Mitochondria | 2 | 9 | 10 | 11 |
| Others | 28 | 47 | 56 | 58 |
Predicted locations of proteins encoded by the 53 TTSS-repressed and 64 TTSS-induced Arabidopsis genes were analyzed by targetp (www.cbs.dtu.dk/services/targetp) and compared to those of the 25,534 genes in the Arabidopsis genome and the 7,155 genes present on the microarray used in this article
The strong bias of TTSS-repressed genes toward those encoding secreted proteins can best be explained by suppression of extracellular plant defense. Indeed, we found that the majority of TTSS-repressed genes are apparently associated with plant cell wall functions including hydroxyproline-rich proteins or extensins, which are known components of papillae; and at least four genes which share sequence similarities with genes encoding known extracellular defense-associated proteins: a germin-like protein (49, 50), a nonspecific lipid transfer protein (51, 52), and two acid phosphatases (ref. 53; see Table 3, which is published as supporting information on the PNAS web site). Interestingly, germin-like proteins have also been shown to be associated with papillae (49, 50). Overall, the biased repression of genes encoding secreted proteins appears to provide a molecular explanation for the type III secretion-dependent suppression of papillae formation observed using microscopic analysis (29, 30) as well as additional extracellular host responses that are not microscopically visible.
The TTSS of DC3000 induced the expression of several SA-dependent putative defense genes, including PR1 (Table 2). This finding supports earlier observations (54, 55) that virulent P. syringae strains induce these genes in susceptible Arabidopsis plants, albeit with slower kinetics and at lower levels compared with those in resistant plants. Because our comparison was made between bacteria that differed in the ability to secrete type III effector proteins, we can now conclude that type III effectors are responsible for the induction of these genes in Arabidopsis. Yet, DC3000 multiplies aggressively under these conditions, suggesting that this level of SA-dependent defense is not effective at limiting DC3000 multiplication or symptom development.
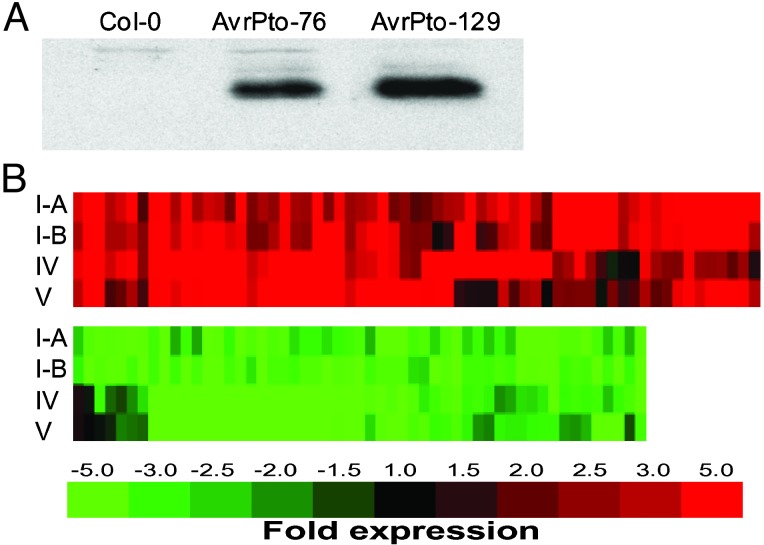
Transgenic Expression of a Single Effector, AvrPto, Regulates Host Genes in a Manner Similar to That of the DC3000 TTSS. To provide further evidence that the regulation of TTSS-associated genes was caused by the action of type III effectors, we decided to examine host gene expression in response to type III effectors expressed in plants. We expressed AvrPto, a type III effector well known for its avirulence activity in plants (21–23), in susceptible Arabidopsis under the control of the glucocorticoid-inducible promoter (34, 35). In these transgenic plants, the expression of AvrPto was induced to a level detectable by Western blotting 24 h after spraying with 30 μM dexamethasone (Fig. 2A). Leaves became chlorotic after 4 days of daily induction with dexamethasone. However, no disease-associated water soaking or necrosis developed. Two independent lines of AvrPto transgenic Arabidopsis plants, AvrPto-76 and AvrPto-129, were further analyzed by microarray. Remarkably, AvrPto alone regulated ≈80% of the TTSS-regulated genes, including those that encode putatively secreted cell wall and defense protein genes, in the same manner as DC3000 (Fig. 2B). These results confirm that type III effector-associated genes are indeed regulated directly by at least the type III effector AvrPto. The striking similarity between the TTSS- and AvrPto-regulated host gene expression profiles demonstrates that AvrPto expression in transgenic Arabidopsis globally mimicked the DC3000 TTSS functions at the molecular level.
Fig. 2.
(A) Western blot analysis of AvrPto expression in leaves of wild-type and AvrPto transgenic plants 24 h after spraying with 30 μM dexamethasone. (B) Cluster analysis of the expression profiles of 117 TTSS-regulated genes (colored bars) after DC3000 infection and transgenic expression of AvrPto. Rows I-A and I-B represent DC3000 TTSS-regulated genes from two independent biological replicates (see columns I-A and I-B in Table 2). Rows IV and V represent gene expression in AvrPto-129 and AvrPto-76 transgenic plants, respectively, 24 h after dexamethasone induction (see columns IV and V in Table 2).
We also found that the repression of TTSS/AvrPto-regulated Arabidopsis secreted cell wall and defense protein genes in nahG plants was not reproducibly different from that in wild-type plants (see columns III-A and III-B in Table 2). Thus, the TTSS- and AvrPto-targeted cell wall-based defense is largely SA-independent. This result suggests that the AvrPto-suppressed cell wall-based defense is fundamentally different from that suppressed by AvrPtoB, VirPphA, AvrPphF, AvrRpt2, or AvrRpm1, which target HR cell death or SA-mediated defenses (41–45). Consistent with this conclusion, AvrPto-expressing plants still responded to DC3000 (avrRpt2) with an HR (data not shown).
The TTSS of DC3000 secretes >30 effector proteins (10, 11). Because mutations in individual effector genes often give only a subtle virulence phenotype or none at all, it is widely believed that the virulence functions of individual effector proteins, at the concentrations delivered by bacteria, are redundant or additive (56, 57). Consistent with this hypothesis, we show that AvrPto is only one of the DC3000 effectors that modulate TTSS-associated Arabidopsis genes because an AvrPto deletion mutant (58) still regulated Arabidopsis gene expression (see columns VI-A and VI-B in Table 2) in a manner similar to DC3000. This result provides molecular evidence from the host side for the functional redundancy of DC3000 type III effectors.
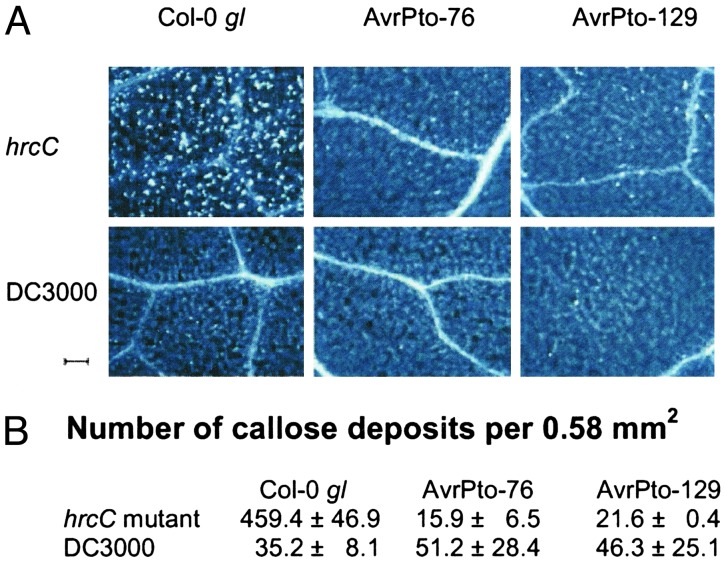
The Cell Wall-Based Extracellular Defense Is Compromised in DC3000-Infected and AvrPto Transgenic Plants. Because the germin-like proteins and hydroxyproline-rich cell wall proteins repressed by the DC3000 TTSS and AvrPto are associated with the papillae-associated cell wall defense, we suspected that AvrPto is one of the long postulated suppressors of extracellular defense elicited by hrp mutant bacteria (30). We examined this possibility by treating leaves with Aniline blue to stain callose, a major component of papillae (29, 30). Indeed, we found that the hrcC mutant (positive control) induced a large number of highly localized callose deposits in leaves of wild-type plants (Fig. 3). A significantly lower level of callose deposition was found in DC3000-infected wild-type leaves (Fig. 3), clearly demonstrating that the TTSS of DC3000 is involved in the suppression of callose-associated cell wall modifications in Arabidopsis. This result establishes that the Arabidopsis-DC3000 system can be used to identify the elusive suppressor of the papillae-associated plant defense.
Fig. 3.
(A) Portions of wild-type and AvrPto transgenic leaves stained with Aniline blue for callose (white dots in these images) after inoculation with the hrcC mutant or DC3000. (Scale bar, 100 μm.) (B) Average number of callose depositions per field of view (0.58 mm2) with SD displayed as error.
We next examined the ability of the hrcC mutant to induce callose deposition in AvrPto-expressing plants. We found that AvrPto-expressing plants were severely compromised in mounting an active papillae-based response to the hrcC mutant (Fig. 3). The number of callose deposits in hrcC-inoculated AvrPto leaves was only ≈5% of that in hrcC-inoculated wild-type leaves. As expected, DC3000 also did not induce a significant level of callose deposition in AvrPto-expressing leaves (Fig. 3). Thus, transgenic expression of AvrPto functionally mimicked the TTSS of DC3000 not only in regulating Arabidopsis gene expression, but also in effectively suppressing the papillae-associated plant cell wall defense.
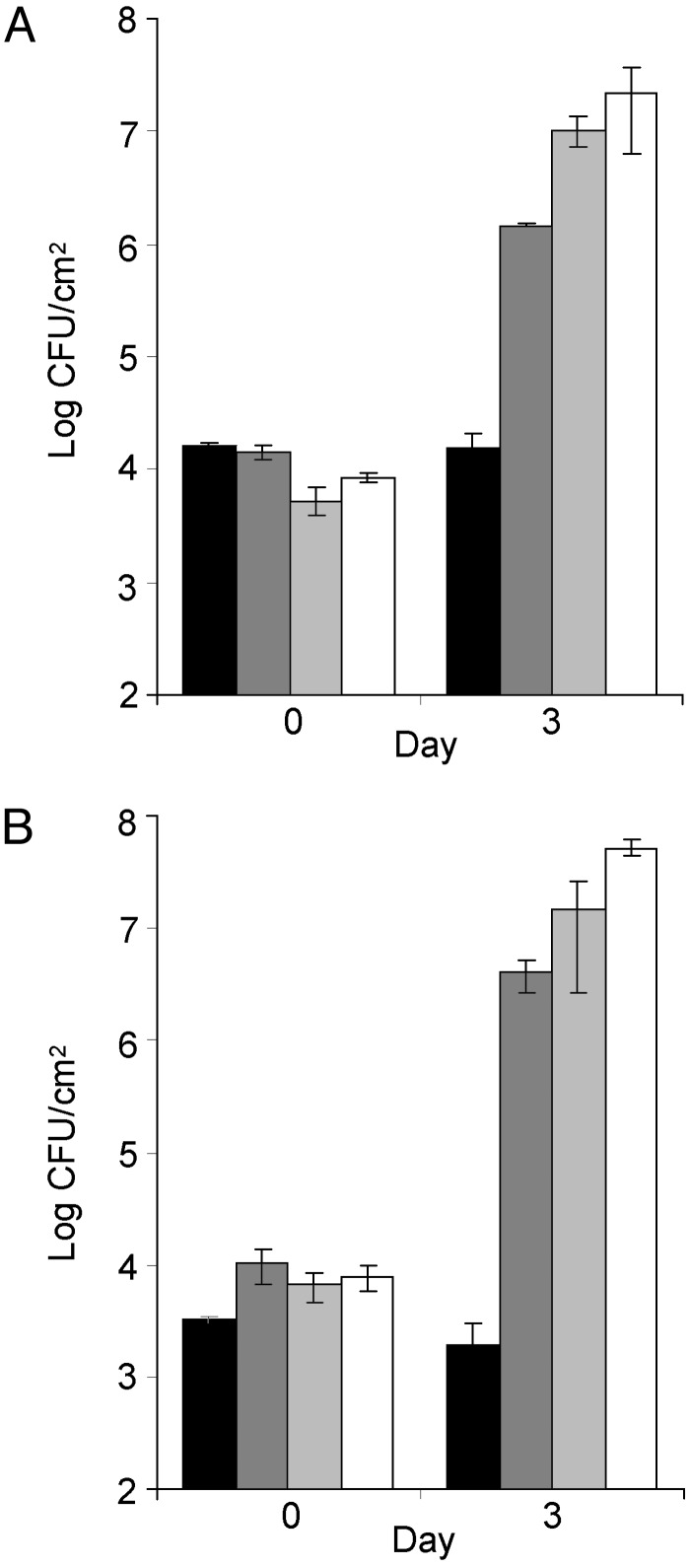
Enhanced Growth of the hrcC Mutant in AvrPto Transgenic Plants. The remarkable ability of AvrPto to mimic DC3000 in the regulation of host gene expression and the suppression of callose deposition prompted us to examine the susceptibility of the AvrPto transgenic plants to the hrcC mutant. We found that expression of AvrPto alone was sufficient to allow substantial multiplication of the hrcC mutant in the transgenic plants (up to 500-fold, which was ≈10-fold lower than the levels reached by DC3000 in these experiments; Fig. 4). Unlike DC3000, however, hrcC-inoculated leaves did not exhibit typical water soaking or extensive necrosis, suggesting a requirement of other effectors for wild-type levels of bacterial multiplication and symptom production. Transgenic expression of AvrPto did not significantly affect DC3000 multiplication because DC3000 multiplied similarly in the AvrPto plants and wild-type Columbia plants (Fig. 4).
Fig. 4.
(A) hrcC mutant growth in wild-type (black bars) and AvrPto-76 plants (dark gray bars). DC3000 growth in wild-type (light gray bars) and AvrPto-76 (white bars) plants. (B) hrcC mutant growth in wild-type (black bars) and AvrPto-129 plants (dark gray bars). DC3000 growth in wild-type (light gray bars) and AvrPto-129 plants (white bars).
Discussion
The hypothesis of a TTSS-dependent suppressor of a hrp mutant-elicited, HR-independent, and cell wall-based plant defense was formulated almost a decade ago. However, the identity of such a suppressor remained elusive. Using a combination of large-scale host gene expression profiling, transgenic expression of AvrPto, and cytological examination, we have now demonstrated that AvrPto is one of suppressors of this defense response in susceptible Arabidopsis. In addition, our TTSS-specific host gene expression analysis provided global insight into the collective virulence functions of DC3000 type III effectors in Arabidopsis, revealing an SA-independent, plant cell wall-based extracellular defense as a major target for DC3000 type III effectors.
The ability of AvrPto to globally mimic the TTSS modulation of host gene expression, to effectively suppress the papillae-associated cell wall response, and to substantially enhance multiplication of nonpathogenic hrp mutant bacteria provides important clues into two long-standing questions in plant-microbe interactions: First, why do the vast majority of nonpathogenic microbes (e.g., saprophytic bacteria) in nature fail to colonize plants? Second, what is the role of the TTSS in the evolution of bacterial pathogenicity? One possibility is that the SA-independent papillae-associated cell wall defense is a critical part of the still poorly defined plant basal defense system that prevents multiplication of saprophytic bacteria to which plants are exposed in nature. In this scenario, acquisition of the TTSS and the AvrPto class of type III effectors, which may vary in different bacteria, by a saprophytic ancestor may have enabled it to down regulate this cell wall-based defense, allowing it to multiply substantially in the plant intercellular space. This acquisition could therefore represent a milestone in the evolution of P. syringae as a virulent pathogen of higher plants. Effector interference with the plant cell wall-based defense also provides a possible explanation for the production of a largely distinct set of type III effectors by extracellular plant pathogenic bacteria, compared with intracellular mammalian pathogenic bacteria (10–12). Down-regulation of the coordinated extracellular host defenses may be especially important for plant pathogenic bacteria (such as P. syringae) and reflects the need for this group of bacteria to overcome the unique host cell wall-based defense of plants. Future research is needed to further define the exact extracellular defense compounds and structures that are modulated by P. syringae type III effectors to overcome plant resistance. Such research will provide critical information for comparative studies of the common and unique functions of type III effectors produced by plant pathogenic bacteria and mammalian pathogenic bacteria, some of which also inhibit host defense (59, 60).
Our identification of AvrPto as a suppressor of papillae-associated extracellular responses is intriguing because in tomato, AvrPto interacts with two Ras-related small G proteins, Rab proteins, which are involved in vesicular trafficking (61). Previous ultrastructural studies (29, 30) showed that papilla formation was accompanied by accelerated extracellular trafficking, as illustrated by an increased abundance of host endoplasmic reticulum and membrane vesicles. One of the AvrPto-interacting Rab proteins shows sequence similarity with Rab8, which in mammalian systems is involved specifically in extracellular secretion (62). Therefore, one mechanism by which AvrPto could act to suppress cell wall-based plant defense would be to inhibit an extracellular vesicle trafficking pathway (Fig. 2). This inhibition may indirectly lead to feedback repression of genes encoding secreted proteins that are transported through this particular trafficking pathway. It is also possible that AvrPto interacts with a component of a signal transduction pathway to inhibit the expression of the cell wall-based extracellular defense. A recent proposal hypothesizes that the tomato Pto kinase, with which AvrPto interacts to trigger resistance responses in tomato, may be a virulence target of AvrPto guarded by the resistance protein Prf (48, 63). If this hypothesis is true, AvrPto could interact with a Pto-like kinase in Arabidopsis to directly down-regulate a signal transduction pathway leading to the activation of a SA-independent, host cell wall-based defense and other associated genes (see Fig. 5, which is published as supporting information on the PNAS web site).
Whereas the AvrPto class of effectors appears to play a key role in overcoming a largely SA-independent cell wall-based extracellular defense, we hypothesize that an additional class of effectors in DC3000 could have evolved to optimize bacterial virulence in specific plant genotypes by blocking gene-for-gene resistance, HR-type programmed cell death, and/or SA-dependent responses. The gene-for-gene resistance and/or SA-dependent responses could result either from plant recognition of certain effectors as Avr proteins, or from cellular perturbation caused by the virulence action of other effectors. Effectors modulating these particular defense responses are exemplified by AvrPtoB, VirPphA, AvrPphF, AvrRpt2, and AvrRpm1 (41–45, 64, 65). This class of effectors would be especially relevant to battling the ever-evolving host recognition system and may account for the presence of a large number of effector genes in the P. syringae genome. It is apparent that plants use type III effectors as a main source of recognition to activate innate defense and turn virulence-intended effector proteins into avirulence proteins. To remain a successful pathogen, P. syringae must evade recognition by mutating these avr genes or evolve additional effector genes that mask avr gene recognition. It is possible that various defense mechanisms, as well as the actions of various effectors, may be interconnected at some level. However, the two classes of effectors appear to target different plant defenses. Therefore, elucidating the functions of both of these classes will be essential to our understanding of P. syringae pathogenesis and the different stages of virulence evolution in P. syringae.
The study of the functions of the 30 or more DC3000 effectors has been thwarted by the typically weak contributions they individually make to virulence. Deletion of a single effector gene does not often lead to a noticeable loss of virulence. In most cases, the virulence contribution, as measured by attenuation of symptoms and bacterial growth, is subtle, which supports the concept that type III effectors, at the concentrations delivered by bacteria, contribute to virulence in a subtle or partially redundant manner (56, 57). Therefore, to efficiently study the functions of most type III effectors in P. syringae and other plant pathogenic bacteria, methods other than the traditional ones that measure bacterial populations or assess disease symptoms must be developed. Despite the apparent functional subtlety and redundancy of type III effectors when delivered by bacteria, we show here that transgenic expression of AvrPto alone, which likely results in a higher level of AvrPto in the plant cell than that delivered by bacteria during infection, could effectively substitute for the redundant/additive functions of a class of effectors in DC3000 to modulate host gene expression, to effectively suppress the papillae-associated cell wall response, and to substantially enhance multiplication of nonpathogenic hrp mutant bacteria. Because TTSS suppression of cell wall-based defense is likely to be a common feature in plant pathogenic bacteria (29, 30), we believe that the global host gene expression, cytological examination, and transgenic expression methods used to identify AvrPto as a suppressor of this host defense will facilitate the functional study of type III effectors not only in P. syringae but also in other plant pathogenic bacteria.
Supplementary Material
Acknowledgments
We thank J. Walton and members of our laboratory for critical reading of the manuscript; R. Schaffer, J. Landgraf, M. Larson, C. Wilkerson, and E. Wisman for help with microarray and bioinformatic analyses; K. Osteryoung and T. Sanderfoot for assistance with callose analysis; and K. Bird for assistance in preparation of this paper. This work was supported by grants from the U.S. Department of Energy (to S.Y.H.), the U.S. National Science Foundation (to S.Y.H.), and the U.S. Department of Agriculture (to R.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TTSS, type III secretion system; SA, salicylic acid; HR, hypersensitive response; DC3000, Pseudomonas syringae pv. tomato strain DC3000; cfu, colony-forming unit; COR, coronatine.
References
- 1.Alfano, J. R. & Collmer, A. (1997) J. Bacteriol. 179 5655-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindgren, P. B. (1997) Annu. Rev. Phytopathol. 35 129-152. [DOI] [PubMed] [Google Scholar]
- 3.He, S. Y. (1998) Annu. Rev. Phytopathol. 36 363-392. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis, G. R. & Van Gijsegem, F. (2000) Annu. Rev. Microbiol. 54 735-774. [DOI] [PubMed] [Google Scholar]
- 5.Buttner, D. & Bonas, U. (2002) EMBO J. 21 5313-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galán, J. E. & Collmer, A. (1999) Science 284 1322-1328. [DOI] [PubMed] [Google Scholar]
- 7.Staskawicz, B. J., Mudgett, M. B., Dangl, J. L. & Galán, J. E. (2001) Science 292 2285-2289. [DOI] [PubMed] [Google Scholar]
- 8.Boch, J., Joardar, V., Gao, L., Robertson, T. L., Lim, M. & Kunkel, B. N. (2002) Mol. Microbiol. 44 73-88. [DOI] [PubMed] [Google Scholar]
- 9.Fouts, D. E., Abramovitch, R. B., Alfano, J. R., Baldo, A. M., Buell, C. R., Cartinhour, S., Chatterjee, A. K., D'Ascenzo, M., Gwinn, M. L., Lazarowitz, S. G., et al. (2002) Proc. Natl. Acad. Sci. USA 99 2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman, D. S., Vinatzer, B. A., Sarkar, S. F., Ranall, M. V., Kettler, G. & Greenberg, J. T. (2002) Science 295 1722-1726. [DOI] [PubMed] [Google Scholar]
- 11.Petnicki-Ocwieja, T., Schneider, D. J., Tam, V. C., Chancey, S. T., Shan, L., Jamir, Y., Schechter, L. M., Janes, M. D., Buell, C. R., Tang, X., et al. (2002) Proc. Natl. Acad. Sci. USA 99 7652-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salanoubat, M., Genin, S., Artiguenave, F., Gouzy, J., Mangenot, S., Arlat, M., Billault, A., Brottier, P., Camus, J. C., Cattolico, L., et al. (2002) Nature 415 497-502. [DOI] [PubMed] [Google Scholar]
- 13.Zwiesler-Vollick, J., Plovanich-Jones, A. E., Nomura, K., Bandyopadhyay, S., Joardar, V., Kunkel, B. N. & He, S. Y. (2002) Mol. Microbiol. 45 1207-1218. [DOI] [PubMed] [Google Scholar]
- 14.Whalen, M. C., Innes, R. W., Bent, A. F. & Staskawicz, B. J. (1991) Plant Cell 3 49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katagiri, F., Thilmony, R. & He, S. Y. (2002) in The Arabidopsis Book, eds. Somerville, C. R. & Meyerowitz, E. M. (Am. Soc. Plant Biologists, Rockville, MD) doi/10.1199/tab. 0039.
- 16.Yuan, J. & He, S. Y. (1996) J. Bacteriol. 178 6399-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roine, E., Wei, W., Yuan, J., Nurmiaho-Lassila, E. L., Kalkkinen, N., Romantschuk, M. & He, S. Y. (1997) Proc. Natl. Acad. Sci. USA 94 3459-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman, R. N. & Novacky, A. J. (1994) The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon (Am. Phytopathol. Soc., St. Paul).
- 19.Greenberg, J. T. (1997) Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 525-545. [DOI] [PubMed] [Google Scholar]
- 20.Leach, J. E. & White, F. F. (1996) Annu. Rev. Phytopathol. 34 153-179. [DOI] [PubMed] [Google Scholar]
- 21.Ronald, P. C., Salmeron, J. M., Carland, F. M. & Staskawicz, B. J. (1992) J. Bacteriol. 174 1604-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scofield, S. R., Tobias, C. M., Rathjen, J. P., Chang, J. H., Lavelle, D. T., Michelmore, R. W. & Staskawicz, B. J (1996) Science, 274 2063-2065. [DOI] [PubMed] [Google Scholar]
- 23.Tang, X., Xie, M., Kim, Y. J., Zhou, J., Klessig, D. F. & Martin, G. B. (1999) Plant Cell 11 15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma, S. W., Morris, V. L. & Cuppels, D. A. (1991) Mol. Plant–Microbe Interact. 4 69-74. [Google Scholar]
- 25.Mittal, S. & Davis, K. R. (1995) Mol. Plant–Microbe Interact. 8 165-171. [DOI] [PubMed] [Google Scholar]
- 26.Bender, C. L., Alarcon-Chaidez, F. & Gross, D. C. (1999) Microbiol. Mol. Biol. Rev. 63 266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakobek, J. L., Smith, J. A. & Lindgren, P. B. (1993) Plant Cell 5 57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobek, J. L. & Lindgren, P. B. (1993) Plant Cell 5 49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bestwick, C. S., Bennett, M. H. & Mansfield, J. W. (1995) Plant Physiol. 108 503-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown, I., Mansfield, J. & Bonas, U. (1995) Mol. Plant–Microbe Interact. 8 825-836. [Google Scholar]
- 31.Brown, I., Trethowan, J., Kerry, M., Mansfield, J. & Bolwell, G. P. (1998) Plant J. 15 333-344. [Google Scholar]
- 32.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 2nd. Ed.
- 33.Wei, W., Plovanich-Jones, A., Deng, W. L., Jin, Q. L., Collmer, A., Huang, H. C. & He, S. Y. (2000) Proc. Natl. Acad. Sci. USA 97 2247-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoyama, T. & Chua, N.-H. (1997) Plant J. 11 605-612. [DOI] [PubMed] [Google Scholar]
- 35.McNellis, T. W., Mudgett, M. B., Li, K., Aoyama, T., Horvath, D., Chua, N. H. & Staskawicz, B. J. (1998) Plant J. 14 247-257. [DOI] [PubMed] [Google Scholar]
- 36.Gopalan, S., Bauer, D. W., Alfano, J. R., Loniello, A. O., He, S. Y. & Collmer, A. (1996) Plant Cell 8 1095-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaffer, R., Landgraf, J., Accerbi, M., Simon, V., Larson, M. & Wisman, E. (2001) Plant Cell 13 113-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. (1998) Proc. Natl. Acad. Sci. USA 95 14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emanuelsson, O., Nielsen, H., Brunak, S. & von Heijne, G. (2000) J. Mol. Biol. 300 1005-1016. [DOI] [PubMed] [Google Scholar]
- 40.Adam, L. & Somerville, S. C. (1996) Plant J. 9 341-356. [DOI] [PubMed] [Google Scholar]
- 41.Abramovitch, R. B., Kim, Y. J., Chen, S., Dickman, M. B. & Martin, G. B. (2003) EMBO J. 22 60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, Z., Kloek, A. P., Boch, J., Katagiri, F. & Kunkel, B. N. (2000) Mol. Plant–Microbe Interact. 13 1312-1321. [DOI] [PubMed] [Google Scholar]
- 43.Jackson, R. W., Athanassopoulos, E., Tsiamis, G., Mansfield, J. W., Sesma, A., Arnold, D. L., Gibbon, M. J., Murillo, J., Taylor, J. D. & Vivian, A. (1999) Proc. Natl. Acad. Sci. USA 96 10875-10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackey, D., Holt, B. F., Wiig, A. & Dangl, J. L. (2002) Cell 108 743-754. [DOI] [PubMed] [Google Scholar]
- 45.Tsiamis, G., Mansfield, J. W., Hockenhull, R., Jackson, R. W., Sesma, A., Athanassopoulos, E., Bennett, M. A., Stevens, C., Vivian, A., Taylor, J. D. & Murillo J. (2000) EMBO J. 19 3204-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaney, T. P., Uknes, S., Vernooij, B., Friedrich, L., Weymann, K., Negrotto, D., Graffney, T., Gut-Rella, M., Kessmann, H., Wards, E., et al. (1994) Science 266 1247-1250. [DOI] [PubMed] [Google Scholar]
- 47.Guzman, P. & Ecker, J. R. (1990) Plant Cell 2 513-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dangl, J. L. & Jones, J. D. (2001) Nature 411 826-833. [DOI] [PubMed] [Google Scholar]
- 49.Wei, Y., Zhang, Z., Andersen, C. H., Schmelzer, E., Gregersen, P. L., Collinge, D. B., Smedegaard-Petersen, V. & Thordal-Christensen, H. (1998) Plant Mol. Biol. 36 101-112. [DOI] [PubMed] [Google Scholar]
- 50.Schweizer, P., Christoffel, A. & Dudler, R. (1999) Plant J. 20 541-552. [DOI] [PubMed] [Google Scholar]
- 51.Maldorado, A., Doener, P., Dixon, R. A., Lamb, C. & Cameron, R. K. (2002) Nature 419 399-403. [DOI] [PubMed] [Google Scholar]
- 52.Molina, A. & Garcia-Olmedo, F. (1997) Plant J. 12 669-675. [DOI] [PubMed] [Google Scholar]
- 53.Jakobek, J. L. & Lindgren, P. B. (2002) J. Exp. Bot. 53 387-389. [DOI] [PubMed] [Google Scholar]
- 54.Nawrath, C., Heck, S., Parinthawong, N. & Metraux, J. P. (2002) Plant Cell 14 275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao, Y., Xie, Z., Chen, W., Glazebrook, J., Chang, H. S., Han, B., Zhu, T., Zou, G. & Katagiri, F. (2003) Plant Cell 15 317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collmer, A., Lindeberg, M., Petnicki-Ocwieja, T., Schneider, D. & Alfano, J. (2002) Trends Microbiol. 10 462-469. [DOI] [PubMed] [Google Scholar]
- 57.Kjemtrup, S., Nimchuk, Z. & Dangl, J. L. (2000) Curr. Opin. Microbiol. 3 73-78. [DOI] [PubMed] [Google Scholar]
- 58.Jin, Q. L. & He, S. Y. (2001) Science 294 2556-2558. [DOI] [PubMed] [Google Scholar]
- 59.Juris, S. J., Shao, F. & Dixon, J. E. (2002) Cell Microbiol. 4 201-211. [DOI] [PubMed] [Google Scholar]
- 60.Fang, F. & Vazquez-Torres, A. (2002) Trends Microbiol. 10 391-392. [DOI] [PubMed] [Google Scholar]
- 61.Bogdanove, A. J. & Martin, G. B. (2000) Proc. Natl. Acad. Sci. USA 97 8836-8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peranen, J., Auvinen, P., Virta, H., Wepf, R. & Simons, K. (1996) J. Cell Biol. 135 153-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Biezen, E. A. & Jones, J. D. (1998) Trends Biochem. Sci. 23 454-456. [DOI] [PubMed] [Google Scholar]
- 64.Axtell, M. J. & Staskawicz, B. J. (2003) Cell 112 369-377. [DOI] [PubMed] [Google Scholar]
- 65.Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R. & Dangl, J. L. (2003) Cell 112 379-389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.