Abstract
Phototropins (phot1 and phot2) are blue light (BL) receptors that mediate phototropism, chloroplast movements, and stomatal opening in Arabidopsis thaliana. Physiological studies have suggested that Ca2+ in the cytoplasm plays a pivotal role in these BL-induced responses. A phot1-mediated increase in cytosolic Ca2+ was reported in deetiolated seedlings of A. thaliana; however, the contribution of phot2 remains unknown. We examined a BL-induced transient increase in cytosolic free Ca2+ in leaves of transgenic A. thaliana of WT plants, phot1 and phot2 mutants, and phot1 phot2 double mutants expressing the Ca2+-sensitive luminescent protein aequorin. phot1 and phot2 had different photosensitivities: phot1 increased cytosolic Ca2+ at lower fluence rates (0.1–50 μmol·m-2·s-1) and phot2 increased it at higher fluence rates (1–250 μmol·m-2·s-1). By using Ca2+ channel blockers, Ca2+ chelating agents, and inhibitors of phospholipase C, we further demonstrated that both phot1 and phot2 could induce Ca2+ influx from the apoplast through the Ca2+ channel in the plasma membrane, whereas phot2 alone induced phospholipase C-mediated phosphoinositide signaling, which might result in Ca2+ release from internal Ca2+ stores. These results suggest that phot1 and phot2 mediate the BL-induced increase in cytosolic free Ca2+ differently.
Blue light (BL) induces many physiological responses in plants. Recent genetic studies on Arabidopsis thaliana revealed that the BL receptors phototropin1 (phot1) and phototropin2 (phot2) mediate BL-induced plant movements such as phototropism, chloroplast movements, and stomatal opening (1, 2). Both phot1 and phot2 have a serine/threonine kinase domain located within the C terminus and repeated motifs designated LOV1 (light, oxygen, or voltage-sensing domain 1) and LOV2 in the N terminus (3, 4). phot1 can phosphorylate itself, as can phot2, using two molecules of flavin mononucleotide as the chromophore (5, 6). Autophosphorylation activities of both phot1 and phot2 were detected in a crude microsome (CM) fraction (7, 8). Recently, Sakamoto and Briggs (9) indicated that phot1 is located mainly on the plasma membrane (PM) by using fused phot1-GFP. In addition, phot1 and phot2 have different photosensitivities for inducing BL-dependent movements in plants. phot1 functions over a wide range of fluence rates of BL, mediating phototropism from 0.01 to 100 μmol·m-2·s-1 and chloroplast accumulation from 0.4 to 100 μmol· m-2·s-1 (6). However, phot2 requires higher fluence rates, mediating phototropism from 1 to 100 μmol·m-2·s-1 and chloroplast accumulation from 2 to 16 μmol·m-2·s-1 (6). phot2 by itself can mediate the avoidance response of chloroplasts to strong light in mesophyll cells at fluence rates between 32 and 100 μmol·m-2·s-1 (4, 6, 10). In guard cells, phot1 and phot2 contribute equally to BL-induced stomatal opening only when irradiated at fluence rates higher than 1 μmol·m-2·s-1 (11).
Studies aimed at understanding the steps immediately down-stream from the phototropins have revealed that changes in ion fluxes across the membrane are involved in an early signal-transduction pathway (12, 13). In guard cells, for example, phot1 and phot2 redundantly increase the H+ pump activity of the PM H+-ATPase (11). Cho and Spalding (14) showed BL-dependent activation of an anion channel in cells of Arabidopsis hypocotyls that resulted in membrane depolarization dependent in part on phot1 (15). BL-dependent activation of both the H+ pump and an anion channel in the PM appears to be regulated by Ca2+ (16, 17). A. thaliana transformants expressing the Ca2+-sensitive luminescent protein aequorin were used to show that phot1 regulates a BL-induced transient increase in cytoplasmic Ca2+ concentration ([Ca2+]c) in deetiolated seedlings (18). Babourina et al. (19) used a noninvasive ion-selective microelectrode to detect phot1-dependent Ca2+ uptake into cells from the apoplast in etiolated seedlings of A. thaliana.
Ca2+ is the most versatile intracellular messenger, able to couple a wide range of extracellular signals to specific responses (20). Signals arising from the amplitude, speed, and spatiotemporal patterning of increases in [Ca2+]c are delivered to particular effectors to initiate cellular responses. Ca2+ comes from extracellular space and/or inner Ca2+ stores, such as the endoplasmic reticulum (ER) and vacuoles (20, 21). The different sources of Ca2+ are important in producing the various patterns of [Ca2+]c increases. In studies of BL responses, Babourina et al. (19) reported that BL-induced uptake of Ca2+ from extracellular spaces was completely impaired in phot1 mutants but not in phot2 mutants. This result indicates that phot1 might be solely responsible for mediating Ca2+ influx from extracellular spaces. Sato et al. (22) suggested that external Ca2+ is not essential, but that Ca2+ released from internal stores is possibly responsible for BL-induced chloroplast movements in protonemata cells of Adiantum capillus-veneris. Shimazaki et al. (16) suggested that BL-induced H+ pumping in guard cell protoplasts of Vicia faba might require Ca2+ from intracellular stores, most likely from the ER. Elucidating the contributions of phot1 and phot2 to BL-induced calcium signaling and determining the source of Ca2+ are required to understand the BL-induced signal-transduction pathway.
Here, we made transgenic A. thaliana that expressed cytosolic aequorin from WT plants [ecotypes, Landsberg erecta (Ler) and Wassilewskija (WS)], phot1 and phot2 mutants, and phot1 phot2 double mutants to analyze phot1- and phot2-mediated calcium signaling. Similar to results reported for BL-induced chloroplast relocation and phototropism (6, 23), phot1 and phot2 showed different photosensitivities in inducing an increase in [Ca2+]c.To know the source of Ca2+, we further examined the effects of Ca2+ channel blockers, Ca2+ chelating agents, and inhibitors of phospholipase C (PLC), which might affect Ca2+ release from internal stores, on BL-induced Ca2+ elevation. In addition, immunoblot analysis revealed that phot2, as well as phot1, was located mainly in the PM. We propose a model of the signal-transduction pathway from BL reception to induction of the transient increase in [Ca2+]c.
Materials and Methods
Construction of the Hemagglutinin (HA)-Epitope-Tagged PHOT1 and PHOT2 Genes. HA-epitope-tagged genes were constructed as follows. pUC18-based HA-NotI vector was constructed by using HAFW oligonucleotide (5′-GCAGGATCCACCATGGCATATCCATATGATGTTCCAGATTATGCTGCG-3′) and MRV oligonucleotide (5′-CAAGAGCTCGCGATCACAGATCCTCTTCTGAGATGAGTTTTTGTTCGCGGCCGCAGCATAATCTGG-3′). These were annealed, made into double-stranded DNA with PyroBest DNA polymerase (TaKaRa Bio, Otsu, Japan), and cloned in the pUC18 plasmid. The PHOT1 cDNA gene including the 11th intron and PHOT2 cDNA were cloned into the NotI site on the pHA-NotI plasmid. The HA-PHOT1 gene was fused with a cauliflower mosaic virus (CaMV) 35S promoter and then cloned in a pPZP211 binary vector. The HA-PHOT2 gene was cloned in the pBI121 binary vector.
Plant Materials. The phot1-101, phot2-5 (npl1-1/cav1-5), and phot1-101 phot2-5 Arabidopsis mutants were obtained as described (6) and are null alleles. HA-PHOT1 and HA-PHOT2 genes fused with the CaMV35S promoter were transformed into phot1-101 and phot2-5, respectively, by the vacuum infiltration method (24) mediated by Agrobacterium tumefaciens. Transgenic A. thaliana that expressed cytosolic apoaequorin were obtained by transferring aequorin expression vector pMAQ2 (A-6793; Molecular Probes) to WS and Ler plants phot1-101, phot2-5, and phot1-101 phot2-5 mutants via A. tumefaciens (24). T2 plants were grown at 22°C under 8-h-light/16-h-dark cycles (HA transformants) or continuous light (aequorin transformants) on agar plates containing half-strength Murashige and Skoog medium (Wako Pure Chemical, Osaka), 0.05% 2-morpholinoethanesulfonic acid monohydrate, 300 μg·ml-1 thiamine-HCl, 50 μg·ml-1 pyridoxine-HCl, 500 μg·ml-1 nicotinic acid, 30 μg·ml-1 kanamycin, and 0.8% agar, pH adjusted to 5.7 with 1 M KOH.
Aequorin Reconstitution and [Ca2+]c Measurements. For in vivo reconstitution of aequorin from expressed apoaequorin and coelenterazine, one of the first four leaves was cut from a 3- to 4-wk-old plant and floated on freshly prepared coelenterazine cp (2.5 μM; Molecular Probes) in artificial pond water (APW) containing 0.05 mM KCl, 0.2 mM NaCl, 0.1 mM Ca(NO3)2, 0.1 mM Mg(NO3)2, and 2 mM piperazine-N,N′-bis(2-ethanesulfonic acid)-NaOH at pH 7.0 for 14–20 h in the dark. Leaves treated with drugs were floated on the APW supplemented with each drug before BL irradiation. Luminescence emitted from the leaves was measured with a Lumicounter 2500 (Microtech Nichi-on, Chiba, Japan). The data were stored on a compatible computer by using luminescence curve-analyzing software (Microtech Nichi-on). All measurements were performed in a dark room at 22°C. Green light (550 nm, <0.1 W·m-2) was used for a safe light. In vivo Ca2+ concentrations were estimated according to Baum et al. (18). All results were analyzed by t test, and the responses that were found to be statistically significant are stated.
BL Illumination and Luminescence Measurements. Each leaf whose aequorin was reconstituted with coelenterazine was placed on a 3-cm-diameter plate with 0.8% Phytagel (Sigma) in APW. The plate was set on the plate holder of the Lumicounter in total darkness, avoiding perturbations, which elicit mechanical signaling. The basal luminescence level was measured for at least 15 min. Then the plate holder was pulled away from the photomultiplier tube of the Lumicounter, the leaf was exposed to BL for 10 s, and the holder was put back. In this way, mechanical stimulation and the time required to put the holder back to the photomultiplier tube and commence measurement were minimal. BL was obtained by using light-emitting diode BL lamps with a maximum wavelength of 470 nm and a 30-nm half-bandwidth (Eyela, Tokyo). To estimate concentrations of Ca2+ from the luminescence, all of the aequorin remaining after the experiment was discharged by a series of rapid injections of cold water.
Chemicals. CoCl2, LaCl3 (Nacalai Tesque, Kyoto), and neomycin sulfate (Calbiochem) were diluted in APW from 1 M, 1 M, and 12.5 mM stock solutions in APW, respectively. Nifedipine (Biomol, Plymouth Meeting, PA), 1-{6-[(17β-3-methoxestra-1,3,5 (10)trine-17-yl)amino]hexyl}-1H-pyrrole-2,5-dione (U-73122), and an inactive analogue of U-73122, 1-{6-[(17β-3-methoxestra-1,3,5 (10)trine-17-yl)amino]hexyl}-2,5-pyrrolidine-dione (U-73343; Calbiochem) were diluted from 25 mM, 2.5 mM, and 2.5 mM freshly prepared solutions in DMSO. Each stock solution except CoCl2 and LaCl3 was diluted 250× with APW. APW supplemented with 0.4% DMSO was used as a control for nifedipine.
Isolation of Membrane Fractions and Marker Enzyme Assays. Leaves from T2 plants expressing HA-epitope-tagged PHOT1 and PHOT2 (4 wk old) were homogenized with a Polytron homogenizer (PT 35/2ST OD; Kinematica, Lucerne, Switzerland) in a medium that contained 300 mM sucrose, 25 mM EGTA, 1 mM DTT, 1% (wt/vol) casein, 10 μg·ml-1 aprotinin, 2.5 μg·ml-1 pepstatin, 1 mM phenylmethylsulfonylfluoride, 10 μg·ml-1 leupeptin, and 50 mM Tris·HCl at pH 8.2. The homogenates were filtered through three layers of Miracloth (Calbiochem) and centrifuged at 5,000 × g for 5 min. After the supernatant was centrifuged twice at 156,000 × g for 30 min, the resulting pellet was suspended with buffer A (250 mM sucrose and 10 mM 3-[N-morpholino]propanesulfonic acid-KOH at pH 7.6) and designated the CM fraction. The CM fraction was separated through an aqueous two-phase partitioning system containing 5.6% dextran T500 (Amersham Pharmacia Biotech), 5.6% polyethylene glycol P-3640 (Sigma), 30 mM KCl, 0.25 M sucrose, and 50 mM potassium phosphate buffer (pH 7.8). The resulting upper and lower phases were designated the PM-rich and inner membrane-rich fractions, respectively. Both fractions were suspended in buffer A. All these procedures were carried out at 0–4°C. Marker enzyme activities were assayed according to Harada et al. (ref. 25 and references therein). The protein content was determined by the method of Bradford (26), with BSA as the standard.
Immunoblotting. The extracted proteins were separated by SDS/ PAGE on a 6% polyacrylamide gel (27) and then electrophoretically transferred onto a poly(vinylidene difluoride) membrane. After blocking with skim milk, the poly(vinylidene difluoride) membranes were reacted with a peroxidase-linked mouse monoclonal anti-HA antibody (Roche Molecular Biochemicals). After a rinse, the peroxidase reaction was developed with a chemiluminescent kit according to the instructions of the manufacturer of the ECL Plus system (Amersham Pharmacia Biotech).
Results
BL Transiently Increases [Ca2+]c in Arabidopsis Rosette Leaves. To analyze BL-dependent changes in [Ca2+]c in Arabidopsis leaves, we made transgenic A. thaliana that expressed aequorin in WT plants, phot1 and phot2 mutants, and phot1 phot2 double mutants. Because the phot1 mutant has a Ler background and phot2 has a WS background, we used WT plants of both ecotypes as controls.
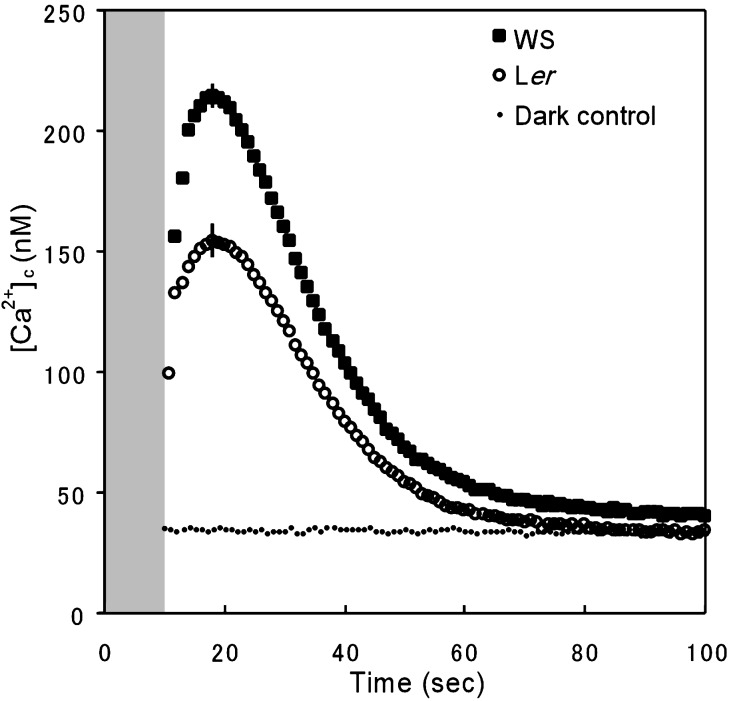
When rosette leaves from 3-wk-old WT plants were irradiated with 100 μmol·m-2·s-1 of BL for 10 s, each leaf exhibited a transient increase in aequorin luminescence lasting 50–70 s (n > 100). Because 95–98% of transgenic aequorin is located in the cytoplasm (28), the [Ca2+]c can be estimated from the aequorin luminescence according to Baum et al. (18). Fig. 1 shows the averaged BL-induced transient increase in [Ca2+]c of leaves from Ler (n = 10) and WS (n = 10). The resting level of [Ca2+]c in the dark was estimated to lie between 25 and 50 nM, with a mean of 34.5 ± 2.5 nM. It took ≈10 s to reach the peak level of [Ca2+]c after the BL source was shut off, then another 40–60 s for the [Ca2+]c to return to the resting level. The kinetics of BL-induced Ca2+ increase is similar to results obtained with deetiolated seedlings (18). The peak [Ca2+]c induced by BL was 214.3 ± 14.2 nM in WS and 154.0 ± 9.3 nM in Ler leaves (Fig. 1). It is not clear whether the difference is due to genetic differences between the ecotypes without further investigation. The kinetics of increase in [Ca2+]c during and immediately after BL irradiation could not be determined in these experiments for the same reasons as in the previous study by Baum et al. (18).
Fig. 1.
BL-induced changes in [Ca2+]c. The averaged increases in [Ca2+]c were obtained from 10 independent experiments in leaves from Ler (circles) and WS (squares) plants. Leaves were illuminated for 10 s (gray zone) with 100 μmol·m-2·s-1 of BL. The vertical bars indicate the standard error. Dots represent dark control ([Ca2+]c in the same plants before illumination in Ler plants).
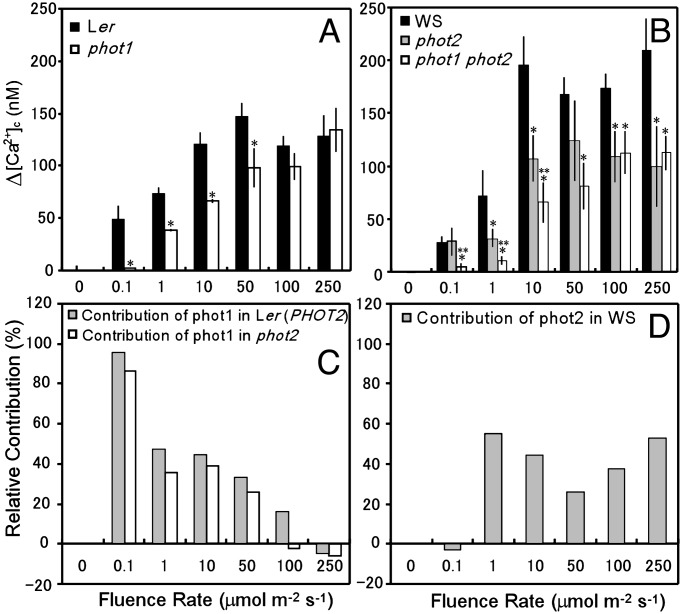
Next, we examined the BL-induced elevation of [Ca2+]c (Δ[Ca2+]c) in the leaves of phot1 and phot2 mutants, phot1 phot2 double mutants, and WT plants at various fluence rates. We examined two lines of transformants, which exhibited high aequorin luminescence, in each mutant and WT plant. Because the results were similar between the two lines, the results of only one are presented. Δ[Ca2+]c was calculated as the difference between [Ca2+]c in the dark before BL irradiation and the BL-induced peak in [Ca2+]c. In leaves of WT plants, BL-induced Δ[Ca2+]c increased in a fluence-rate-dependent manner from 0.1 to 10 μmol·m-2·s-1 and remained constant up to 250 μmol·m-2·s-1 (Fig. 2 A and B, filled bars).
Fig. 2.
BL-induced increase in [Ca2+]c (Δ[Ca2+]c, the difference between [Ca2+]c in the dark before illumination and the BL-induced peak in [Ca2+]c)in leaves of WT plants, phot1 and phot2 mutants, and phot1 phot2 double mutants and relative contributions of phot1 and phot2 to the BL responses. (A and B) Δ[Ca2+]c in leaves of Ler plants and a phot1 mutant (A) and those in leaves of WS plants and phot2 and phot1 phot2 mutants (B) at various fluence rates of BL. (C and D) Relative contributions of phot1 (C) and phot2 (D) to the BL-induced Δ[Ca2+]c. The contributions of phot1 in the presence and absence of phot2 were calculated as the percent difference in Δ[Ca2+]c between phot1 and Ler (i.e., the results in A) and between phot1 phot2 and phot2 (results in B), respectively. The contribution of phot2 was calculated as the percent difference in Δ[Ca2+]c between phot2 and WS. The vertical bars indicate the standard error of 5–20 independent experiments. * and **, significantly different from the data in the WT plant and the phot2 mutant, respectively (P < 0.05).
In leaves of phot1 mutants (Fig. 2 A, open bars), the BL-induced Δ[Ca2+]c was very small at 0.1 μmol·m-2·s-1, remained lower than in the WT at fluence rates between 1 and 50 μmol·m-2·s-1, and reached the same level as in the WT only at fluence rates higher than 100 μmol·m-2·s-1 (Fig. 2 A). The relative contribution of phot1 to the response to BL was calculated from the percent difference between the BL-induced Δ[Ca2+]c values from phot1 and Ler (Fig. 2C, gray bars) and showed that phot1 functions at fluence rates between 0.1 and 100 μmol·m-2·s-1. At 100 μmol·m-2·s-1, however, the Δ[Ca2+]c values in the phot1 mutant and in Ler did not differ significantly (P > 0.05; Fig. 2 A). We thus conclude that phot1 functions at fluence rates between 0.1 and 50 μmol·m-2·s-1.
In the leaves of phot2 mutants (Fig. 2B, gray bars), Δ[Ca2+]c was the same as in the WT at a fluence rate of 0.1 μmol·m-2·s-1; however, between 1 and 250 μmol·m-2·s-1 of BL, Δ[Ca2+]c was less in the phot2 mutant than in the WT. The relative contribution of phot2 (Fig. 2D) showed that phot2 functions between 1 and 250 μmol·m-2·s-1 of BL. These results suggest that, despite their structural similarity, phot1 and phot2 have different photosensitivities with respect to inducing increases in [Ca2+]c, as was also reported for BL-dependent induction of chloroplast movements and phototropism (6, 23).
In leaves of phot1 phot2 double mutants (Fig. 2B, open bars), Δ[Ca2+]c was barely detectable at BL fluence rates of 0.1 and 1 μmol·m-2·s-1, was lower than in the phot2 mutant at fluence rates of 10 μmol·m-2·s-1, and was the same as in the phot2 mutant at 100 and 250 μmol·m-2·s-1 (Fig. 2B), suggesting photoreceptor(s) other than phototropins may induce transient increases in [Ca2+]c at fluence rates between 10 and 250 μmol·m-2·s-1. The relative contribution of phot1 in the absence of phot2, calculated from the differences in Δ[Ca2+]c between the phot2 mutant and the phot1 phot2 double mutant (Fig. 2C, open bars), is similar to its relative contribution in the presence of phot2 (Fig. 2C, gray bars), suggesting that phot1 and phot2 additively contribute to the BL-induced increase in [Ca2+]c.
phot1 and phot2 Mediate Increase in [Ca2+]c in Different Manners. To determine the source of Ca2+ that appeared in the cytoplasm after BL irradiation, we tested the effects of several Ca2+ channel blockers, Ca2+ chelators, and inhibitors of PLC on the BL-induced increase in [Ca2+]c. Because both phot1 and phot2 contribute to the transient increase in [Ca2+]c at 1 μmol·m-2·s-1 (Fig. 2) and no other photoreceptors were functioning (Fig. 2B), we used this fluence rate for the inhibitor tests.
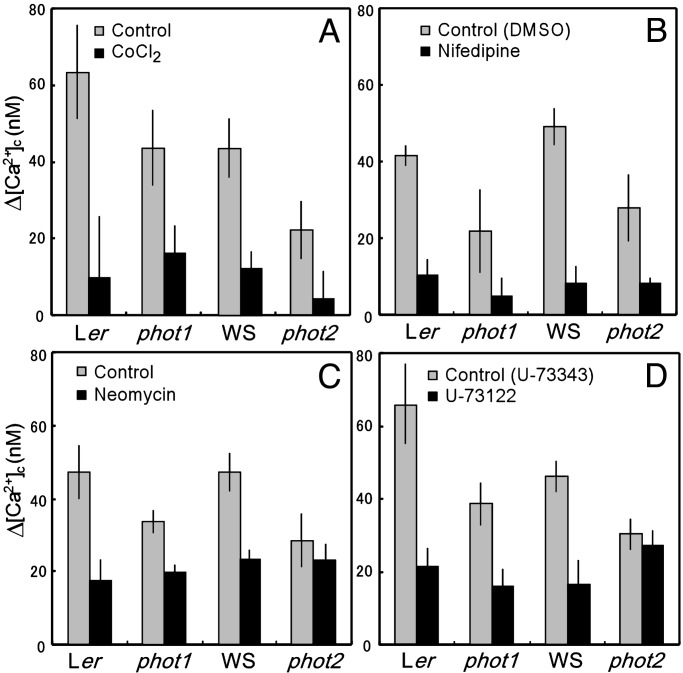
After leaves were treated with the Ca2+ channel blocker CoCl2 (5 mM) for 2 h, the BL-induced increase in [Ca2+]c in WT plants and in phot1 and phot2 mutants was significantly reduced to ≈15–35% of control levels (Fig. 3A and Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Pretreatment with LaCl3 at 0.5 mM (data not shown) for 2 h or a dihydropyridine-type Ca2+ channel blocker, nifedipine, at 100 μM for 4 h (Fig. 3B), similarly inhibited the response in leaves from WT plants and from phot1 and phot2 mutants to 17–30% of control levels. After treatment with Ca2+ chelating agents, EGTA (1 mM) or O,O′-bis(2-aminophenyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid (5 mM), for 30 min, the dark level of [Ca2+]c increased by 10–20 nM compared with the control, and the increase in [Ca2+]c was reduced to 1–10% of control leaves (data not shown). These results suggest that both phot1- and phot2-induced increases in [Ca2+]c require extracellular Ca2+ and that phot1 and phot2 modulate Ca2+ influx through a PM Ca2+ channel.
Fig. 3.
Effects of inhibitors on BL-induced Δ[Ca2+]c in the WT plants and phot1 and phot2 mutants. A leaf, treated with each inhibitor in the dark, was irradiated with 1 μmol·m-2·s-1 of BL. Effects of 5 mM CoCl2 (A), 100 μM nifedipine (B; 0.4% DMSO was used as the control), 10 μM neomycin (C), and 10 μM U-73122 (D; 10 μM U-73343, an inactive analogue, was used as the control) are shown. The vertical bars indicate the standard error of 3–18 independent experiments.
When leaves were treated with an inhibitor of PLC, neomycin (10 μM) or U-73122 (10 μM), for 30 min before BL irradiation, a different pattern of results was obtained. The dark level of [Ca2+]c increased by 5–10 nM compared with the control (Fig. 7, which is published as supporting information on the PNAS web site), and the BL-induced increase in [Ca2+]c was partly suppressed in the Ler and WS plants and in the phot1 mutant (Fig. 3 C and D). However, both inhibitors did not significantly inhibit the BL-induced increase in [Ca2+]c in the phot2 mutant (Fig. 3 C and D). The results suggest that PLC-mediated phosphoinositide signaling is involved in the phot2-mediated increase in [Ca2+]c. Both neomycin and U-73122 have been described as inhibiting PLC activity, thus reducing the amount of inositol 1,4,5-triphosphate (InsP3) released and blocking InsP3-sensitive Ca2+ release from internal stores in plant cells (29–31). It is thus strongly suggested that phot2 induces Ca2+ release from internal stores, such as vacuoles and ER, depending on PLC-mediated InsP3 production. Because the phot2-mediated increase in [Ca2+]c was suppressed in the presence of either Ca2+ chelating agents or Ca2+ channel blockers (Fig. 3 A and B and Fig. 6), Ca2+ release from an internal store occurred only when [Ca2+]c was increased via PM Ca2+ channels. Ca2+-induced Ca2+ release (CICR) was likely involved in the phot2-mediated transient increase in [Ca2+]c.
phot1 and phot2 Are Located on the PM. The location of phot1 on the PM (9) is consistent with the idea that phot1 regulates a Ca2+ channel in the PM. Although the precise location of phot2 remains unknown, autophosphorylation activity of phot2 was detected in a CM fraction (8). To determine whether phot2 locates on the PM and/or on membranes of internal Ca2+ stores, we made transgenic plants expressing HA-epitope-tagged PHOT1 and PHOT2 in phot1 and phot2 mutants, respectively. These transgenic phot1 and phot2 mutants exhibited BL-induced phototropism and chloroplast relocation indistinguishably from WT plants (data not shown), so we refer to them as HA-PHOT1 and -PHOT2 transformants, respectively. We attempted to prepare a PM-rich and an inner membrane-rich fraction by an aqueous two-phase partitioning method.
Assays of marker enzymes showed that the upper and lower phases from the two-phase partitioning system were enriched in PM and inner membranes, respectively (Table 1). The activity of vanadate-sensitive ATPase, which is a marker for PM, was about three times more concentrated in the PM-rich fraction than in the CM fraction, whereas its activity in the inner membrane-rich fraction was only half that in the CM fraction (Table 1). Activities of Triton-stimulated UDPase, antimycin A-resistant NADH–cytochrome c reductase, and antimycin A-sensitive NADH–cytochrome c reductase, which are markers for Golgi, ER, and mitochondrial membranes, respectively, were detected in the inner membrane-rich fractions, with relative activities roughly the same as in the CM fraction. They were not detected or were barely detectable in the PM-rich fraction (Table 1). A marker for vacuole (nitrate-sensitive ATPase) was detected at equal levels in both PM- and inner membrane-rich fractions. These results suggest that our PM-rich fraction is enriched in the PM but contains an amount of vacuolar membranes similar to the inner membrane fraction. The inner membrane fraction contains all kinds of membranes, but the content of the PM was diminished to one-sixth of that in the PM-rich fraction.
Table 1. Distribution of marker enzymes in the CM, inner membrane (IM), and PM fractions isolated from A. thaliana leaves.
| Enzyme | CM | IM (recovery, n-fold) | PM (recovery, n-fold) |
|---|---|---|---|
| Vanadate-sensitive ATPase* | 1.10±0.15 | 0.60±0.04 (0.54) | 3.31±0.58 (3.01) |
| Nitrate-sensitive ATPase* | 0.20±0.05 | 0.27±0.11 (1.35) | 0.31±0.14 (1.55) |
| Triton-stimulated UDPase* | 1.15±0.09 | 1.17±0.27 (1.02) | 0.60±0.19 (0.52) |
| Antimycin A-resistant NADH-Cyt c reductase† | 33.9±2.25 | 22.3±0.21 (0.66) | 0 (0) |
| Antimycin A-sensitive NADH-Cyt c reductase† | 103.9±9.35 | 81.6±1.63 (0.78) | 12.7±1.09 (0.09) |
The activities are the means ± standard error from two independent experiments. n-fold, each enzyme activity in IM or PM fraction relative to that in the CM fraction
μmol inorganic phosphate·mg-1·h-1
nmol cytochrome c (Cyt c)·mg-1·min-1
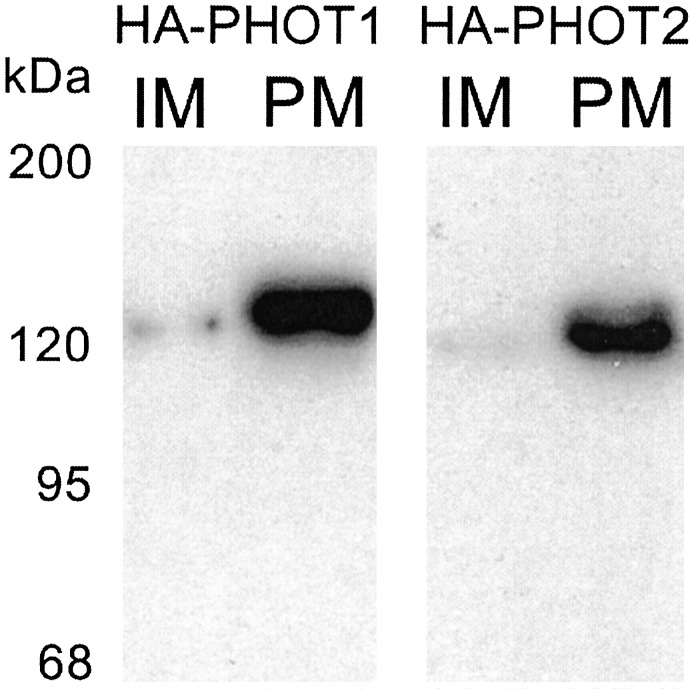
We analyzed both membrane fractions by immunoblotting with an anti-HA antibody. In the HA-PHOT1 transformant, a 137-kDa band was detected in the PM-rich fraction but was barely detected in the inner membrane fraction (Fig. 4). This result is consistent with those of Sakamoto and Briggs (9), indicating that phot1 is localized mainly to the PM. In the HA-PHOT2 transformants, a 129-kDa band was detected in the PM-rich fraction but was only faintly detected in the inner membrane-rich fraction (Fig. 4). These results indicate that both phot2 and phot1 are localized mainly to the PM rather than the inner membranes.
Fig. 4.
Immunodetection of HA-PHOT1 and -PHOT2 from inner membrane-rich (IM) and PM-rich (PM) fractions isolated from leaves of HA-PHOT1 and -PHOT2 transformants, respectively. The proteins (3 μg in each lane) were separated by SDS/PAGE. Epitope-tagged PHOT1 and PHOT2 were detected as slightly larger bands than predicted molecular masses of phot1 (112 kDa) and phot2 (102 kDa), respectively.
Discussion
In this study, we demonstrated that (i) phot2 as well as phot1 is involved in the BL-induced elevation of cytosolic Ca2+;(ii) phot1 and phot2 share partly redundant roles for the Ca2+ elevation (namely, phot1 is responsible for the lower fluence rate of BL and phot2 for the higher fluence rate); (iii) phot1 and phot2 could induce the Ca2+ influx from the apoplast through the Ca2+ channel in the PM; and (iv) phot2 could trigger CICR from internal Ca2+ stores such as the ER or vacuoles, depending on PLC-mediated InsP3 production (Fig. 5). In animal cells, CICR through an InsP3-sensitive Ca2+-release channel in the ER and sarcoplasmic reticulum has been reported (32–34). Similarly, phot2 may activate the CICR via an InsP3-sensitive Ca2+-release channel in membranes of internal Ca2+ stores.
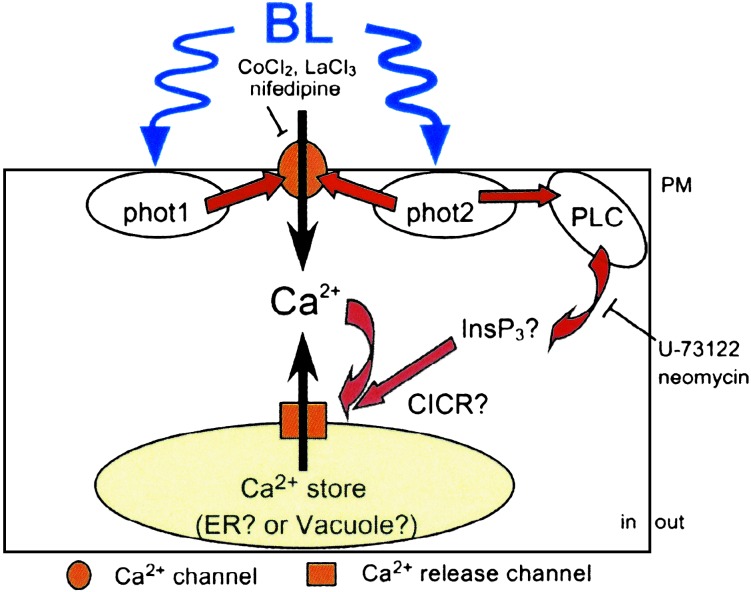
Fig. 5.
A schematic model of the signal pathway of phot1- and phot2-dependent increase in [Ca2+]c in leaves of A. thaliana. phot1 and phot2 in the PM could mediate Ca2+ influx through a Ca2+ channel in the PM. phot2 alone induces CICR from internal Ca2+ stores through PLC-mediated signaling. In WT leaves, phot1 possibly increases the phot2-dependent PLC pathway and suppresses phot2-dependent Ca2+ influx from the apoplast. We describe the possibility in Discussion. See text for details.
We noticed that the inhibitory effects of PLC inhibitors on the BL response in WT leaves were larger than those of the phot1 single mutant (Fig. 3 C and D). Although phot1 was not involved in the PLC pathway in the phot2 mutant (Fig. 3 C and D), phot1 possibly has some relationship to the PLC pathway in WT leaves. Because the PLC pathway mediates the BL-induced elevation of Ca2+ only in the presence of phot2 (Fig. 3 C and D), we propose a model that phot1 increases the phot2-dependent PLC pathway in the WT. Although we should investigate further whether phot1 actually increases the phot2-dependent PLC pathway in WT leaves, this model indicates the possibility that there are some functional interactions between phot1 and phot2 to induce the elevation of cytosolic Ca2+. Because there are no reports that phot1 and phot2 interact in mediating the BL-induced phototropism, chloroplast movement, and stomatal opening, it is important for understanding the phototropin-dependent signaling pathways to clarify whether each phototropin functionally interacts with another phototropin to induce the BL-induced elevation of Ca2+.
We indicated that phot2-dependent Ca2+ influx from the apoplast mediates the BL response in the phot1 mutant (Fig. 3). Babourina et al. (19), on the other hand, indicated that phot2 did not mediate the BL-induced Ca2+ influx from the apoplast in etiolated hypocotyls of A. thaliana by using a noninvasive ion-selective electrode under continuous BL illumination. In this study, we also found that phot2-dependent Ca2+ influx from the apoplast would be extremely low in the WT because PLC inhibitors reduced the BL-induced increase in [Ca2+]c to a level similar to that in the phot2 mutant [Fig. 3 C and D; the increases in Ca2+ in the presence of each drug in WS plants and the phot2 mutant were not significantly different (P > 0.05)]. In the WT, the phot2-dependent PLC pathway increased by phot1 might cover all of the phot2-dependent elevation of [Ca2+]c, and phot2-dependent Ca2+ influx from the apoplast would be equivocally eliminated. It is suggested that phot2 alternatively mediates the Ca2+ influx from the apoplast according to different circumstances. There are differences between our experimental conditions and those of Babourina et al. (19): (i) tissues examined, leaves or hypocotyls; (ii) plants, deetiolated or etiolated; (iii) fluence rates of BL, 1 μmol·m-2·s-1 or 25 μmol·m-2·s-1; and (iv) [Ca2+]c measured after a 10-s pulse of BL irradiation or Ca2+ influx measured during continuous BL irradiation. Further investigation would be required to clarify whether phot2 affects the Ca2+ influx according to the conditions.
PLC-mediated phosphoinositide signaling plays pivotal roles in both animal and plant cells (35, 36). LaBelle et al. (37) revealed that a PLC isoform, PLCβ2, was associated with the PM. In the present study, immunoblot analysis revealed not only phot1 but also phot2 mainly in the PM-rich fraction (Fig. 4). This result is consistent with the idea that the phototropin in the PM regulates PLC activity associated with the same membrane. In mammalian systems, a PLC isoform, PLCβ, is regulated by heterotrimeric G proteins (36). In plant guard cells, abscisic acid activates PLC and can elevate [Ca2+]c via InsP3 (30), and heterotrimeric G protein regulates abscisic acid signaling (38). G protein might be involved in the phototropin-mediated signaling pathway, as was also found for the phytochrome A-dependent signaling pathway (39).
At fluence rates of 10–250 μmol·m-2·s-1, BL induced an elevation in [Ca2+]c in the leaves of the phot1 phot2 double mutants, indicating that some BL photoreceptor(s) other than phototropins are involved in BL-induced changes in [Ca2+]c. In deetiolated seedlings of A. thaliana, it is reported that neither cryptochrome1 nor cryptochrome2 is involved in BL-induced increases in [Ca2+]c (18). However, we observed that red light induced a transient increase in [Ca2+]c in leaves from both WT plants and phot1 phot2 double mutants (data not shown). A phytochrome-mediated elevation of [Ca2+]c was detected in wheat cell protoplasts (40). The possible involvement of phytochrome(s) in BL-induced increases in [Ca2+]c should be examined by using aequorin transformants of these mutants.
This study indicates that the two phototropins are responsible for the BL-dependent increase in cytosolic Ca2+ differently. It should be noted that the two phototropins induce the Ca2+ increase with different photosensitivities, as was also the case in inducing phototropism and chloroplast movements (6, 23); namely, phot1 works at a lower fluence rate of BL, whereas phot2 works at a higher fluence rate of BL. Although it is not clear whether the phototropin-related responses are mediated by the same intracellular signaling pathways, the BL-induced increase in [Ca2+]c could be one of the intermediate signals for the BL-dependent responses. This study shows that the increase in Ca2+ is a common role of phototropins. Furthermore, phot1 and phot2 might increase Ca2+ concentration adjacent to the PM and to the inner compartments, respectively, and these differences in localized changes in [Ca2+]c might function in transferring messages to separate signal transduction pathways. Further examination is required to clarify whether and how phototropin-dependent Ca2+ signaling mediates BL-induced movements in plants, such as chloroplast movement, phototropism, and stomatal opening; how the two phototropin molecules respond to BL of different fluence rates; how the phototropins mediate the light perception signal to each signal-transduction pathway; what the components of the pathways are; and how the signaling system acts on the Ca2+ channels.
Supplementary Material
Acknowledgments
We thank Prof. Shoshi Muto and Dr. Takuya Furuichi of Nagoya University for critical advice on measuring aequorin luminescence, and Dr. Shingo Takagi of Osaka University for allowing us to use the polytron homogenizer.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: APW, artificial pond water; BL, blue light; [Ca2+]c, concentration of Ca2+ in cytoplasm; CICR, Ca2+-induced Ca2+ release; CM, crude microsome; ER, endoplasmic reticulum; HA, hemagglutinin; InsP3, inositol 1,4,5-triphosphate; Ler, Landsberg erecta; PLC, phospholipase C; PM, plasma membrane; WS, Wassilewskija.
References
- 1.Lin, C. (2002) Plant Cell Suppl. 14 S207-S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs, W. R. & Christie, J. M. (2002) Trends Plant Sci. 7 204-210. [DOI] [PubMed] [Google Scholar]
- 3.Huala, E., Oeller, P. W., Liscum, E., Han, I. S., Larsen, E. & Briggs, W. R. (1997) Science 278 2120-2123. [DOI] [PubMed] [Google Scholar]
- 4.Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K. & Wada M. (2001) Science 291 2138-2141. [DOI] [PubMed] [Google Scholar]
- 5.Christie, J. M., Reymond, P., Powell, G. K., Bernasconi, P., Raibekas, A. A., Liscum, E. & Briggs, W. R. (1998) Science 282 1698-1701. [DOI] [PubMed] [Google Scholar]
- 6.Sakai, T., Kagawa, T., Kasahara, M., Swartz, T. E., Christie, J. M., Briggs, W. R., Wada, M. & Okada, K. (2001) Proc. Natl. Acad. Sci. USA 98, 6969-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liscum, E. & Briggs, W. R. (1995) Plant Cell 7 473-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie, J. M., Swartz, T. E., Bogomolni, R. A. & Briggs, W. R. (2002) Plant J. 32 205-219. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto, K. & Briggs, W. R. (2002) Plant Cell 14 1723-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarillo, J. A., Gabrys, H., Capel, J., Alonso, J. M., Ecker, J. R. & Cashmore, A. R. (2001) Nature 410 952-954. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M. & Shimazaki, K. (2001) Nature 414 656-660. [DOI] [PubMed] [Google Scholar]
- 12.Spalding, E. P. (2000) Plant Cell Environ. 23 665-674. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 627-658. [DOI] [PubMed] [Google Scholar]
- 14.Cho, M. H. & Spalding, E. P. (1996) Proc. Natl. Acad. Sci. USA 93 8134-8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folta, K. M. & Spalding, E. P. (2001) Plant J. 26 471-478. [DOI] [PubMed] [Google Scholar]
- 16.Shimazaki, K., Goh, C. H. & Kinoshita, T. (1999) Physiol. Plant. 105 554-561. [Google Scholar]
- 17.Lewis, B. D., Karlin-Neumann, G., Davis, R. W. & Spalding, E. P. (1997) Plant Physiol. 114 1327-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum, G., Long, J. C., Jenkins, G. I. & Trewavas, A. J. (1999) Proc. Natl. Acad. Sci. USA 96 13554-13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babourina, O., Newman, I. & Shabala, S. (2002) Proc. Natl. Acad. Sci. USA 99 2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders, D., Brownlee, C. & Harper, J. F. (1999) Plant Cell 11 691-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders, D., Pelloux, J., Brownlee, C. & Harper, J. F. (2002) Plant Cell Suppl. 14 S401-S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato, Y., Wada, M. & Kadota, A. (2001) Plant Physiol. 127 497-504. [PMC free article] [PubMed] [Google Scholar]
- 23.Kagawa, T. & Wada, M. (2001) Plant Cell Physiol. 41 84-93. [DOI] [PubMed] [Google Scholar]
- 24.Bechtold, N., Ellis, J. & Pelletier, G. (1993) C. R. Acad. Sci. Ser. III 316 1194-1199. [Google Scholar]
- 25.Harada, A., Okazaki, Y. & Takagi, S. (2002) Planta 214 863-869. [DOI] [PubMed] [Google Scholar]
- 26.Bradford, M. M. (1976) Anal. Biochem. 72 248-254. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. (1970) Nature 277 680-685. [DOI] [PubMed] [Google Scholar]
- 28.Knight, H., Trewavas, A. J. & Knight, M. R. (1996) Plant Cell 8 489-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashem, A. M., Itoh, K., Iwabuchi, S., Hori, H. & Mitsui, T. (2000) Plant Cell Physiol. 41 399-407. [DOI] [PubMed] [Google Scholar]
- 30.Staxén, I., Pical C., Montgomery, L. T., Gray, J. E., Hetherington, A. M. & McAinsh, M. R. (1999) Proc. Natl. Acad. Sci. USA 96 1779-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin-Tong, V. E., Drøbak, B. K., Allan, A. C., Watkins, P. A. C. & Trewavas, A. J. (1996) Plant Cell 8 1305-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakui, M., Osipchuk, Y. V. & Petersen, O. H. (1990) Cell 63 1025-1032. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura, T., Barbara, J. G., Nakamura, K. & Ross, W. N. (1999) Neuron 24 727-737. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M.-M. & Kato, K. (2000) Nature 408, 584-588. [DOI] [PubMed] [Google Scholar]
- 35.Drøbak, B. K. (1992) Biochem J. 288 697-712.1335231 [Google Scholar]
- 36.Rebecchi, M. J. & Pentyala, S. N. (2000) Physiol. Rev. 80 1291-1335. [DOI] [PubMed] [Google Scholar]
- 37.LaBelle, E. F., Wilson, K. & Polyák, E. (2002) Biochim. Biophys. Acta 1583 273-278. [DOI] [PubMed] [Google Scholar]
- 38.Wang, X. Q., Ullah, H., Jones, A. M. & Assman, S. M. (2001) Science 292 2070-2072. [DOI] [PubMed] [Google Scholar]
- 39.Neuhaus, G., Bowler, C., Kern, R. & Chua, N. H. (1993) Cell 73 937-952. [DOI] [PubMed] [Google Scholar]
- 40.Shacklock, P. S., Read, N. D. & Trewavas, A. J. (1992) Nature 358 753-755. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.