Abstract
Background
Oxidative stress may play a critical role in the vascular disease of end stage renal failure and hemodialysis patients. Studies, analyzing either discrete analytes and antioxidant substances, or the integrated total antioxidant activity of human plasma during hemodialysis, give contradictory results.
Methods
Recently, we have introduced a new automated method for the determination of Total Antioxidant Capacity (TAC) of human plasma. We have serially measured TAC and corrected TAC (cTAC: after subtraction of the interactions due to endogenous uric acid, bilirubin and albumin) in 10 patients before the onset of the dialysis session, 10 min, 30 min, 1 h, 2 h and 3 h into the procedure and after completion of the session.
Results
Our results indicate that TAC decreases, reaching minimum levels at 2 h. However, corrected TAC increases with t1/2 of about 30 min. We then repeated the measurements in 65 patients undergoing dialysis with different filters (36 patients with ethylene vinyl alcohol copolymer resin filter -Eval-, 23 patients with two polysulfone filters -10 with F6 and 13 with PSN140-, and 6 patients with hemophan filters). Three specimens were collected (0, 30, 240 min). The results of this second group confirm our initial results, while no significant difference was observed using either filter.
Conclusions
Our results are discussed under the point of view of possible mechanisms of modification of endogenous antioxidants, and the interaction of lipid- and water-soluble antioxidants.
Background
Hemodialysis represents a chronic stress status for its recipients [1-3]. Although life salvaging, this procedure, by the application of a modified circulation and the forced passage of blood through a number of filters, activates endogenous inflammatory mechanisms and induces chronic release of molecules resulting in an increased production of reactive oxygen species [reviewed in [4,5]]. In addition, uric acid, an endogenous metabolite eliminated by hemodialysis, possesses significant antioxidant activity [6], while fluctuations in other endogenous antioxidant systems (plasma proteins, vitamins, etc) may lead to major variations of the internal redox state [1,3,6-8].
Circulation of oxidative molecules has been incriminated in protein, carbohydrate and lipoprotein oxidation and the generation of an increased arterial deposit, leading ultimately to atherosclerosis [9,10]. Indeed, accelerated development of atherogenesis and a number of vascular episodes characterize patients with chronic renal failure subjected to hemodialysis. In these patients oxidative stress relies on three major components: (1) The dialysis membrane, (2) the microbial contamination or pyrogen content of the dialysate, (3) the possible prooxidant effect of a number of metabolites, found at high concentrations in the patients' plasma, including uric acid [11].
Cross-sectional studies of dialysis patients reveal that, while traditional cardiovascular risk factors (hypertension, hypercholesterolemia) do not discriminate as well as in the general population, markers of inflammation and protein-calories malnutrition are highly correlated with cardiovascular mortality. Interesting hypotheses have been advanced, linked to the presence of oxidant stress and its sequelae as a unifying concept of cardiovascular disease in uremia [12]. A number of preventive strategies have been recently introduced, during and after hemodialysis, in order to counteract vascular disease. They include administration of antioxidant vitamins, the use of new biocompatible filters, presumably less immunogenic, and the addition of vitamin, hormone or trace metals in the patients' diet [1,2,13-23]. Nevertheless, although it is generally accepted that oxidative stress may result from dialysis therapy, no direct evidence exists confirming this hypothesis. A number of reports, either measuring specific analytes or enzymes [8,15,22,24-30], or estimating the total antioxidant activity of the plasma [1-3,6,7,9,26,29,31,32] give contradictory and non-conclusive results.
Recently, we have introduced a new automated method for the estimation of the plasma total antioxidant capacity [33]. This method is based on the inhibition of oxidation by plasma of an exogenously added marker (crocin) by an added pro-oxidant (ABAP). In this respect, it integrates the totality of circulating pro- and antioxidants, and gives a rough (although accurate) estimate of the antioxidant status of plasma at a given moment. In addition, we have also corrected these results for a number of analytes, directly affecting redox potential, thus introducing the concept of "corrected antioxidant capacity". We have used this assay in order to evaluate changes of the antioxidant capacity of patients during a cycle of hemodialysis. Our results indicate that, although the total antioxidant capacity of hemodialyzed patients shows a decrease during the procedure, the corrected antioxidant capacity increases, indicating that counterbalancing mechanisms might occur in human plasma, equilibrating the loss of uric acid and other antioxidant metabolites.
Methods
Patients and controls
Ten patients dialyzed with an ethylene vinyl alcohol copolymer resin (Eval) filter were analyzed. Seven samples were obtained from each patient, before the initiation of dialysis, 30 min, 1, 2 and 3 hours into the session and upon its completion. Sixty-five additional patients were examined (32 males [age range 15–91 years, mean 62.7, median 54.3 years] and 34 females [age range 26–88 years, mean 60.9, median 55.7 years]) and 56 volunteer blood donors on a normal diet (38 males and 18 females, age range 21–52 years, median 43.5 years). Polysulphone dialysis membranes were used on 23 patients (F6: 10 patients and PSN140: 13 patients), hemophan membranes on 6 (GFS 12 Plus) and ethylene vinyl alcohol copolymer resin filters (Eval 1.6 or 1.3) on 36. Dialysis age varied from 1 to 13.3 years (mean ± SE 6.0 ± 0.4 years, median 5.2 years). No significant changes among the different groups were found. Renal failure was due to diabetes (4 cases), polycystic kidney disease (8 cases), vasculitis (4 cases), tuberculosis (1 case), chronic pyelonephritis (4 cases), interstitial nephritis (2 cases), chronic glomerulonephritis (1 case), glomerulosclerosis (2 cases), hypertension (2 cases), while 37 patients were refered to the hospital with renal failure of unknown cause. All patients received non-steroid antiinflammatory, Vitamin B, and erythropoietin treatment. An informed consent was obtained from all participants in the study. Three samples (times 0, 30 and 240 min) were withdrawn from each patient, while a single sample was obtained from each blood donor on K3-EDTA. Plasma was immediately separated by centrifugation (2000 g, 4°C), aliquoted and stored at -80°C until assayed.
Determination of TAC
Plasma total antioxidant capacity (TAC) was measured on an Olympus AU-600 chemistry analyzer using the TAC kit, described previously [33] (Medicon SA, Gerakas, Greece). Briefly, antioxidants in the sample inhibit the oxidation (bleaching) of crocin from ABAP [2,2-Azobis-(2-amidinopropane) dihydrochloride] to a degree that is proportional to their concentration. The assay was performed at 37°C in the following steps: 2 μl of sample, calibrator or control were mixed with 250 μl of crocin reagent (R1) and incubated for 160 s. Subsequently, 250 μl of ABAP (R2) were added and the decrease in absorbance at 450 nm was measured 256 s later. Values of TAC were expressed as mmol/l of Trolox (a hydrophilic Vitamin E derivative) equivalents. During the initial validation of the TAC assay [33] we found that uric acid, bilirubin, and albumin accounted for 0.11, 0.11, and 0.01 mmol/mg of the antioxidant capacity, respectively. Subtraction of these interferences from the TAC value results in the calculation of corrected TAC (cTAC), an estimate of the redox state attributed, mainly to circulating exogenous antioxidants.
Routine clinical chemistry
Plasma uric acid, albumin, total and direct bilirubin, cholesterol, HDL-cholesterol and triglycerides were determined on an Olympus AU-600 chemistry analyzer using Olympus reagents provided by Medicon SA (Gerakas, Greece), as follows: uric acid OSR6136, albumin OSR6102, total bilirubin OSR6112, direct bilirubin OSR6111, cholesterol OSR6116, HDL-cholesterol OSR6187 and triglycerides OSR6133. LDL-cholesterol was estimated by the Friedewald equation [LDL-cholesterol = total cholesterol - (HDL-cholesterol + triglycerides/5)], when triglyceride values were <400 mg/dl. For the group of ten patients dialyzed with Eval membranes, a full blood count (white blood cells, polymorphonuclear cells, hemoglobin concentration, hematocrit value) accompanied all serial measurements.
Statistics
Statistical analysis of data was performed by the use of the SyStat v 10.0 program (SPSS Inc, Chicago, IL). Group differences were compared at each data point using ANOVA with the Bonferroni correction for small data sets. The residual variance was used as a common estimate of the Standard Error, and group means were therefore compared by the Student's t-test. The Origin v 5.0 program (MicroCal, Northampton, MA) was used for curve fitting.
Results
Metabolite variation during dialysis
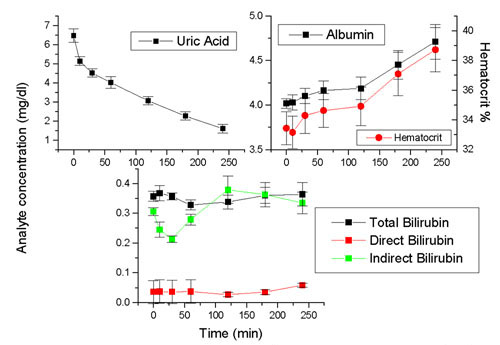
Kinetics of a number of analytes during dialysis is presented in Figure 1. Uric acid concentrations decrease during dialysis, following an exponential decay curve with t1/2 of 104 min. Albumin, on the other hand presents a gradual increase during dialysis, following an exponential growth curve, with t1/2 of 101 ± 12.8 min, due to hemoconcentration, as reflected by the hematocrit count. In contrast, minor changes of bilirubin were found during the dialysis cycle. Cholesterol on the other hand, as well as HDL and LDL cholesterol present minor changes during the dialysis cycle. A slight increase of triglycerides was observed, due probably to the feeding of subjects during hemodialysis.
Figure 1.
Variation of uric acid, albumin, hematocrit, bilirubin and lipids during dialysis Data obtained from 10 dialysis patients. Mean ± SEM are depicted.
Total and corrected plasma antioxidant capacity during dialysis
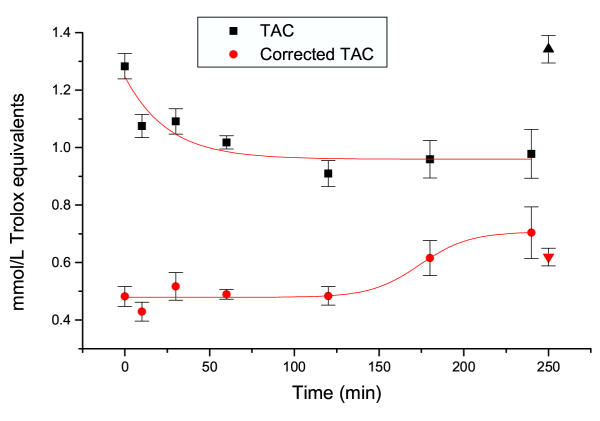
Figure 2 presents the variation of TAC during dialysis, in the ten hemodialyzed patients. As expected, due to the presence of a number of endogenous metabolites dotted with antioxidant activity (for example uric acid) initial TAC values of hemodialyzed patients are high, as compared to those of control individuals. During dialysis however, these elevated values decrease, according to an exponential decay model, with t1/2 of 24.8 min. Thereafter, they remain constant during the whole time of dialysis.
Figure 2.
Variations of TAC and corrected TAC during dialysis Up and down arrows depict values obtained in normal blood donors. Mean ± SEM of 10 patients and 56 blood donors
Corrected TAC is also depicted in Figure 2. As stated in our previous work [33], this calculated parameter represents the fraction of circulating antioxidants, after the elimination of interference of endogenous metabolites. Our previous work has shown that uric acid and bilirubin, and to a lesser degree albumin, are the major analytes interfering linearly with coefficients of 0.11, 0.11 and 0.01 mmol/L of TAC per mg/dL of each analyte respectively. We have therefore calculated the corrected TAC values in the same patients. As shown, corrected TAC increases during the dialysis procedure, following a sigmoidal curve, with t1/2 of 174 min. It is interesting to note that this value is slightly higher from the t1/2 of uric acid decay (174 ± 14.1 min, as compared to 101.2 ± 12.8 min respectively).
Comparing TAC and corrected TAC values with those obtained in normal blood donors (depicted in Figure 2 as up and down triangles respectively), it is observed that total TAC values are significantly decreased (t = 3.75, p < 0.001) in hemodialyzed patients as compared to controls. In contrast, while initial corrected TAC values are significantly lower than those of controls (t = 2.97, p < 0.01), they reach normal values at the end of dialysis.
Effect of different dialysis filters on metabolites and TAC values
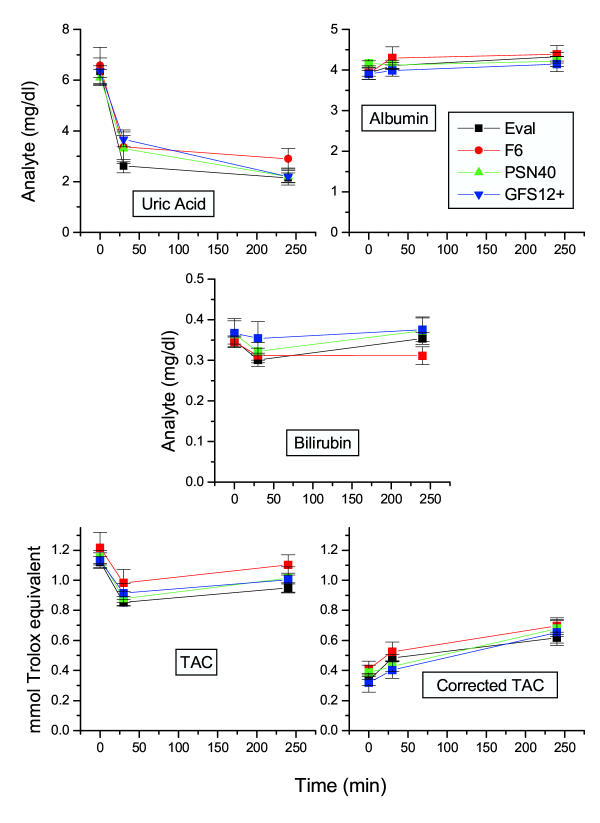
Previous works have suggested that hemodialysis-related oxidative burden relies greatly on the dialysis membrane used. In this respect, as antioxidants might be consumed during a surge of oxidative molecules, TAC (as well as corrected TAC) might be decreased with the use of different membranes. We therefore attempted to investigate the effect of filters on TAC and corrected TAC values, in 65 patients (33 males and 32 females). Thirty-six patients were dialyzed using an ethylene vinyl alcohol copolymer resin (Eval) filter. In 10 and 13 patients, F6 and PSN140 polysulfone filters were used, respectively. Finally, 6 patients were dialyzed using a GFS12+ hemophan filter. The obtained results are presented in Figure 3. As shown, no significant differences were observed in any group (Kruskal-Wallis test statistics with p > 0.05 in any case), indicating that at least the filters used in the present study do not modify drastically the redox state of patients, during the dialysis procedure.
Figure 3.
Effect of different filters used in dialysis on the concentration of different analytes and TAC levels Parameter variation in 65 patients under dialysis. 23 patients were dialyzed with polysulphone dialysis membranes (F6: 10 patients and PSN140: 13 patients), 6 patients with hemophan membranes (GFS 12 Plus) and in 36 patients ethylene vinyl alcohol copolymer resin filters (Eval 1.6 or 1.3) were used. Mean ± SEM of vaues.
Discussion
The primary defense against oxidative stress in extracellular fluids results from a number of low molecular weight antioxidant molecules being either water- (ex. ascorbic acid) or lipid-soluble (ex. Vitamin E). These antioxidants are either generated during normal metabolism (ex. uric acid, bilirubin, albumin, thiols) or introduced in the body by the consumption of dietary products rich in antioxidants (olive oil, fruits and vegetables, tea, wine, etc) [34]. The sum of endogenous plus exogenous (food-derived) antioxidants represents the total antioxidant capacity of extracellular fluids. Changes of these antioxidants reflect their consumption during acute oxidative stress states. It should be noted that cooperation between different antioxidant pathways provides greater protection against attack by reactive oxygen or nitrogen radicals, compared to any single compound. Thus, the overall antioxidant capacity may give more relevant biological information compared to that obtained by the measurement of individual biomarkers, as it considers the cumulative effect of all antioxidants present in plasma and body fluids [35]. A theory has recently been proposed, taking into account the redox potentials of exogenous and endogenous antioxidants. It postulates a cascade of reactions, in which following an oxidative stress, a lesser antioxidant is generated from a more potent one. Through this cascade, interactions among the lipid and the aqueous phases could be established [36].
A great variety of methods have been proposed for the assay of total antioxidant activity or capacity of serum or plasma [reviewed extensively and critically in [34,35]]. They stress the fine distinction between antioxidant activity and antioxidant capacity: Antioxidant activity corresponds to the rate constant of a single antioxidant against a given free radical; a ntioxidant capacity, on the other hand, is the number of moles of a given free radical scavenged by a test solution, independently of the capacity of any one antioxidant present in the mixture [35]. In the case of plasma, being a heterogeneous solution of diverse antioxidants, the antioxidant status is better reflected by antioxidant capacity rather than activity alone. This capacity is a combination of all redox chain antioxidants, including several analytes such as thiol bearing proteins, and uric acid. It thus appears that plasma antioxidant capacity is rather a concept than a simple analytical determination. Indeed, an increase of antioxidant capacity of plasma indicates absorption of antioxidants and improved in vivo antioxidant status [37], or the result of the activation of an adaptation mechanism to oxidative stress. It should be noted that, due to the contribution of diverse metabolites to the antioxidant capacity of human plasma, its increase may not necessarily be a desirable condition. Indeed, in some cases, such as renal failure (uric acid), icteric status (bilirubin), hepatic damage (hypoalbuminemia) the increase or decrease of several metabolites modifies plasma antioxidant capacity, a situation returning to physiological values after correction of the underlying disease [38]. In addition, high concentration of a number of metabolites, including uric acid, can lead to prooxidant effects, introducing a further decrease of the plasma antioxidant capacity [11]. Recently, we have introduced a new automated method for the assay of the plasma antioxidant capacity, based on the bleaching of crocin. This method (the TAC assay) gives an estimation of the integrated plasma antioxidant capacity. Furthermore, we also determined the interference of a number of endogenous analytes, such as uric acid, and bilirubin, which have been found to produce a major interference of TAC, while albumin results in a smaller interference [33]. The subtraction of these interferences in TAC assay resulted in the calculation of corrected TAC, cTAC representing the amount of antioxidant capacity due to the action of (mainly) exogenous antioxidants.
In the present work, we have assayed simultaneously TAC and the concentrations of these analytes during a single episode of hemodialysis. Although uremic plasma is almost unique in its concentration of numberless metabolites and toxins, identified or unknown, measurable or not, the concept of the TAC assay, measuring the inhibition of an exogenously added oxidant makes the assay suitable for the identification of the antioxidant capacity of uremic plasma. Concerning cTAC, we are aware that the subtraction of the interaction of albumin, bilirubin and uric acid only, may not take into account other (known or unknown) endogenous metabolites and toxins with a proper antioxidant or prooxidant activity. Therefore, the results concerning the cTAC measurements must be considered as indicative. In addition, a number of medications may influence the plasma antioxidant capacity. Nevertheless, an almost similar medication regime was followed by the parients' group. Therefore, the effect of medication (if any) on the results of the TAC assay, may be considered as constant.
Major modifications of a number of endogenous metabolites were observed during dialysis. Namely, uric acid is rapidly eliminated, with t1/2 of 101 min (Figure 1), while albumin plasma concentration is increased (t1/2 21 min) probably due to the hemoconcentration during dialysis. Bilirubin, on the other hand, follows a biphasic pattern with an initial decrease (possibly due to elimination) followed by an increase due to hemoconcentration. Lipids do not present major variations during the dialysis episode studied, with the exception of triglycerides due to the feeding of patients. As significant changes were not observed, the implication of lipids on the TAC assay was omitted. Total TAC measurements present equally major changes, following those of the above analytes. Indeed, results presented in Figure 2 show that the plasma antioxidant capacity of patients is higher before than during or after a session of dialysis. This can be due to the elimination of a number of metabolites, such as uric acid and bilirubin (Figure 1). In this respect our results are similar to those presented in previous reports, in which a comparable decrease of plasma antioxidant capacity was observed during renal dialysis [2,3,8-10,15,26,32,39,40]. Total TAC was found to increase later in hemodialysis, most probably due to either hemoconcentration [31], adaptation [41], or to a possible exchange of antioxidants between the lipid and aqueous phases [36]. Whether urate by itself and other analytes, at the concentrations encountered in dialysis patients and in the milieu of uremic plasma, exert a pro-oxidant [11] or antioxidant effect remains a matter of debate.
Calculation of the corrected TAC appears to provide a better estimate of the actual antioxidant activity of the organism, especially in cases such as renal dialysis, in which major fluctuations of endogenous metabolites and the elimination of a number of toxins occur. Indeed, as shown in Figure 2, the curve of corrected TAC is different from that of TAC. Specifically, a gradual increase of plasma antioxidant capacity is observed, with t1/2 of about 30 min. Various explanations for these results could be proposed: (1) Water elimination during dialysis causes increased concentration of endogenous antioxidant substances [31]. (2) Elimination of uric acid modifies the equilibrium between oxidized and reduced states of endogenous and exogenous antioxidants [41,42]. (3) It has been recently proposed that elimination of water-soluble metabolic antioxidants (bilirubin, uric acid) modifies the equilibrium of lipid- and water-soluble antioxidants [36]. (4) The presumed "antioxidant effect" of hemodialysis, detected here by cTAC, has also been attributed to the plasma glutathione increase by hemodialysis [25,43,44].
Redox state in uremic patients undergoing dialysis is rather confused. Several reports provide possible pathophysiological explanations of the observed changes in redox state and antioxidant status. It appears that patients with malnutrition and a low plasma albumin concentration have significantly reduced plasma antioxidant capacity due to the diminished availability of thiol groups [45]. Serologic evidence of an activated inflammatory response has been reported [46], as well as the contribution of phagocytes and cytokines to increased production of ROS [47-50]. Several lines of evidence indicate that further oxidative modification of retained solutes in the uremic milieu (ex. β2 microglobulin, homocysteine, cysteine) may potentiate their pathogenicity [51-53]. Dialytic therapy, which acts to reduce the concentration of oxidized substrates, improves the redox balance [12,54]. However, processes related to repetitive extracorporeal dialytic therapies (prolonged use of catheters for vascular access, use of bioincompatible dialysis membranes) can incite further inflammatory and oxidative stimuli (via complement and leukocyte activation), thus contributing to a pro-atherogenic state [55,56].
Our data, presented in Figure 3 indicate that no major changes in both analytes and TAC are observed with the use of modern filters. It has already been reported that hemodialysis decreases the oxidation levels of plasma protein-associated thiol groups [51]. It would be of interest to measure TAC and cTAC in patients dialyzed with vitamin E-modified membranes. It should be noted, however, that even with the use of vitamin E-modified filters, results on oxidative stress markers can be confounding.
In conclusion, our data suggest that although during hemodialysis several factors contribute to the generation of oxidative radicals, the organism is able to successfully resist the flood of oxidative substances. Oxidation and peroxidation reactions of renal failure patients must be reevaluated under this point of view, taking into account the auto-oxidation of excess antioxidants, as was recently reported for vitamin C and tocopherols [57-60], rather than a decrease of the plasma antioxidant capacity. Nevertheless, more extensive studies must be performed, taking into acoount the possible abrupt change of the plasma oxidative status at the end of dialysis, or the hours following it [12].
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Niki Malliaraki, Email: nikim@acn.gr.
Dimitris Mpliamplias, Email: mpdim@medscape.com.
Marilena Kampa, Email: kampa@med.uoc.gr.
Kostas Perakis, Email: perkost@her.forthnet.gr.
Andrew N Margioris, Email: andym@med.uoc.gr.
Elias Castanas, Email: castanas@med.uoc.gr.
References
- Bonnefont-Rousselot D, Lehmann E, Jaudon MC, Delattre J, Perrone B, Rechke JP. Blood oxidative stress and lipoprotein oxidizability in haemodialysis patients: effect of the use of a vitamin E-coated dialysis membrane. Nephrol Dial Transplant. 2000;15:2020–2028. doi: 10.1093/ndt/15.12.2020. [DOI] [PubMed] [Google Scholar]
- Eiselt J, Racek J, Trefil L, Opatrny K., Jr Effects of a vitamin E-modified dialysis membrane and vitamin C infusion on oxidative stress in hemodialysis patients. Artif Organs. 2001;25:430–436. doi: 10.1046/j.1525-1594.2001.025006430.x. [DOI] [PubMed] [Google Scholar]
- Fiorillo C, Oliviero C, Rizzuti G, Nediani C, Pacini A, Nassi P. Oxidative stress and antioxidant defenses in renal patients receiving regular haemodialysis. Clin Chem Lab Med. 1998;36:149–153. doi: 10.1515/CCLM.1998.028. [DOI] [PubMed] [Google Scholar]
- Morena M, Cristol JP, Canaud B. Why hemodialysis patients are in a prooxidant state? What could be done to correct the pro/antioxidant imbalance? Blood Purif. 2000;18:191–199. doi: 10.1159/000014418. [DOI] [PubMed] [Google Scholar]
- Tetta C, Biasioli S, Schiavon R, Inguaggiato P, David S, Panichi V, Wratten ML. An overview of haemodialysis and oxidant stress. Blood Purif. 1999;17:118–126. doi: 10.1159/000014383. [DOI] [PubMed] [Google Scholar]
- Roselaar SE, Nazhat NB, Winyard PG, Jones P, Cunningham J, Blake DR. Detection of oxidants in uremic plasma by electron spin resonance spectroscopy. Kidney Int. 1995;48:199–206. doi: 10.1038/ki.1995.285. [DOI] [PubMed] [Google Scholar]
- Weinstein T, Chagnac A, Korzets A, Boaz M, Ori Y, Herman M, Malachi T, Gafter U. Haemolysis in haemodialysis patients: evidence for impaired defence mechanisms against oxidative stress. Nephrol Dial Transplant. 2000;15:883–887. doi: 10.1093/ndt/15.6.883. [DOI] [PubMed] [Google Scholar]
- Kim SB, Yang WS, Min WK, Lee SK, Park JS. Reduced oxidative stress in hypoalbuminemic CAPD patients. Perit Dial Int. 2000;20:290–294. [PubMed] [Google Scholar]
- Eiselt J, Racek J, Holecek V, Krejcova I, Opatrny K. [Antioxidants and malondialdehyde during hemodialysis with cellulose diacetate and polysulfone membranes] Cas Lek Cesk. 1996;135:691–694. [PubMed] [Google Scholar]
- Schettler V, Methe H, Staschinsky D, Schuff-Werner P, Muller GA, Wieland E. Review: the oxidant/antioxidant balance during regular low density lipoprotein apheresis. Ther Apher. 1999;3:219–226. doi: 10.1111/j.1091-6660.1999.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- Abuja PM. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett. 1999;446:305–308. doi: 10.1016/S0014-5793(99)00231-8. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kindey Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- Carbonneau MA, Leger CL, Monnier L, Bonnet C, Michel F, Fouret G, Dedieu F, Descomps B. Supplementation with wine phenolic compounds increases the antioxidant capacity of plasma and vitamin E of low-density lipoprotein without changing the lipoprotein Cu(2+)-oxidizability: possible explanation by phenolic location. Eur J Clin Nutr. 1997;51:682–690. doi: 10.1038/sj.ejcn.1600464. [DOI] [PubMed] [Google Scholar]
- Eiselt J, Racek J, Opatrny K., Jr The effect of hemodialysis and acetate-free biofiltration on anemia. Int J Artif Organs. 2000;23:173–180. [PubMed] [Google Scholar]
- Koenig JS, Fischer M, Bulant E, Tiran B, Elmadfa I, Druml W. Antioxidant status in patients on chronic hemodialysis therapy: impact of parenteral selenium supplementation. Wien Klin Wochenschr. 1997;109:13–19. [PubMed] [Google Scholar]
- Mydlik M, Derzsiova K, Racz O, Sipulova A, Lovasova E, Petrovicova J. A modified dialyzer with vitamin E and antioxidant defense parameters. Kidney Int Suppl. 2001;78:S144–147. doi: 10.1046/j.1523-1755.2001.59780144.x. [DOI] [PubMed] [Google Scholar]
- Vesela E, Racek J, Trefil L, Jankovy'ch V, Pojer M. Effect of L-carnitine supplementation in hemodialysis patients. Nephron. 2001;88:218–223. doi: 10.1159/000045993. [DOI] [PubMed] [Google Scholar]
- Allman MA, Truswell AS, Tiller DJ, Stewart PM, Yau DF, Horvath JS, Duggin GG. Vitamin supplementation of patients receiving haemodialysis. Med J Aust. 1989;150:130–133. doi: 10.5694/j.1326-5377.1989.tb136390.x. [DOI] [PubMed] [Google Scholar]
- Boran M, Kucukaksu C, Balk M, Cetin S. Red cell lipid peroxidation and antioxidant system in haemodialysed patients: influence of recombinant human erythropoietin (r-HuEPO) treatment. Int Urol Nephrol. 1998;30:507–512. doi: 10.1007/BF02550233. [DOI] [PubMed] [Google Scholar]
- Cavdar C, Camsari T, Semin I, Gonenc S, Acikgoz O. Lipid peroxidation and antioxidant activity in chronic haemodialysis patients treated with recombinant human erythropoietin. Scand J Urol Nephrol. 1997;31:371–375. doi: 10.3109/00365599709030622. [DOI] [PubMed] [Google Scholar]
- Descombes E, Hanck AB, Fellay G. Water soluble vitamins in chronic hemodialysis patients and need for supplementation. Kidney Int. 1993;43:1319–1328. doi: 10.1038/ki.1993.185. [DOI] [PubMed] [Google Scholar]
- Girelli D, Lupo A, Trevisan MT, Olivieri O, Bernich P, Zorzan P, Bassi A, Stanzial AM, Ferrari S, Corrocher R. Red blood cell susceptibility to lipid peroxidation, membrane lipid composition, and antioxidant enzymes in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1992;12:205–210. [PubMed] [Google Scholar]
- Kamata K, Okubo M, Marumo F. Water soluble vitamins in patients with chronic renal failure and effect of B6 administration of immunological activity. Proc Clin Dial Transplant Forum. 1979;9:194–196. [PubMed] [Google Scholar]
- Biasioli S, Schiavon R, De Fanti E, Cavalcanti G, Giavarina D. The role of erythrocytes in the deperoxidative processes in people on hemodialysis. Asaio J. 1996;42:M890–894. doi: 10.1097/00002480-199609000-00120. [DOI] [PubMed] [Google Scholar]
- Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thevenin M, Jaudon MC, Zingraff J, Verger C, Jungers P, Descamps-Latscha B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. 1996;21:845–853. doi: 10.1016/0891-5849(96)00233-X. [DOI] [PubMed] [Google Scholar]
- Mimic-Oka J, Simic T, Djukanovic L, Reljic Z, Davicevic Z. Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin Nephrol. 1999;51:233–241. [PubMed] [Google Scholar]
- Mohora M, Mircescu G, Cirjan C, Mihailescu I, Girneata L, Ursea N, Dinu V. Effect of hemodialysis on lipid peroxidation and antioxidant system in patients with chronic renal failure. Rom J Intern Med. 1995;33:237–242. [PubMed] [Google Scholar]
- Richard MJ, Arnaud J, Jurkovitz C, Hachache T, Meftahi H, Laporte F, Foret M, Favier A, Cordonnier D. Trace elements and lipid peroxidation abnormalities in patients with chronic renal failure. Nephron. 1991;57:10–15. doi: 10.1159/000186208. [DOI] [PubMed] [Google Scholar]
- Soriani M, Pietraforte D, Minetti M. Antioxidant potential of anaerobic human plasma: role of serum albumin and thiols as scavengers of carbon radicals. Arch Biochem Biophys. 1994;312:180–188. doi: 10.1006/abbi.1994.1297. [DOI] [PubMed] [Google Scholar]
- Toborek M, Wasik T, Drozdz M, Klin M, Magner-Wrobel K, Kopieczna-Grzebieniak E. Effect of hemodialysis on lipid peroxidation and antioxidant system in patients with chronic renal failure. Metabolism. 1992;41:1229–1232. doi: 10.1016/0026-0495(92)90014-2. [DOI] [PubMed] [Google Scholar]
- Meucci E, Littarru C, Deli G, Luciani G, Tazza L, Littarru GP. Antioxidant status and dialysis: plasma and saliva antioxidant activity in patients with fluctuating urate levels. Free Radic Res. 1998;29:367–376. doi: 10.1080/10715769800300411. [DOI] [PubMed] [Google Scholar]
- Nguyen-Khoa T, Massy ZA, Witko-Sarsat V, Thevenin M, Touam M, Lambrey G, Lacour B, Drueke TB, Descamps-Latscha B. Critical evaluation of plasma and LDL oxidant-trapping potential in hemodialysis patients. Kidney Int. 1999;56:747–753. doi: 10.1046/j.1523-1755.1999.00565.x. [DOI] [PubMed] [Google Scholar]
- Kampa M, Nistikaki A, Tsaoussis V, Maliaraki N, Notas G, Castanas E. A new automated method for the determination of the Total Antioxidant Capacity (TAC) of human plasma, based on the crocin bleaching assay. BMC Clin Pathol. 2002. accepted. [DOI] [PMC free article] [PubMed]
- Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med. 1999;27:1173–1181. doi: 10.1016/S0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29:1106–1114. doi: 10.1016/S0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Barbaste M, Verge S, Dumas M, Soulet S, Nay B, Arnaudinaud V, Delaunay J-C, Castagnino C, Cheze C, Vercauteren J. Dietary antioxidants, peroxidation and cardiovascular risks. J Nutr Health Aging. 2002;6:138–152. [PubMed] [Google Scholar]
- Cao G, Booth SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity following consumption of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1998;68:1081–1087. doi: 10.1093/ajcn/68.5.1081. [DOI] [PubMed] [Google Scholar]
- Jackson P, Loughrey CM, Lightbody JH, McNamee PT, Young IS. Effect of hemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin Chem. 1995;41:1135–1138. [PubMed] [Google Scholar]
- Gunduz Z, Dusunsel R, Kose K, Utas C, Dogan P. The effects of dialyzer reuse on plasma antioxidative mechanisms in patients on regular hemodialysis treatment. Free Radic Biol Med. 1996;21:225–231. doi: 10.1016/0891-5849(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Lim PS, Wei YH, Yu YL, Kho B. Enhanced oxidative stress in haemodialysis patients receiving intravenous iron therapy. Nephrol Dial Transplant. 1999;14:2680–2687. doi: 10.1093/ndt/14.11.2680. [DOI] [PubMed] [Google Scholar]
- Canestrari F, Buoncristiani U, Galli F, Giorgini A, Albertini MC, Carobi C, Pascucci M, Bossu M. Redox state, antioxidative activity and lipid peroxidation in erythrocytes and plasma of chronic ambulatory peritoneal dialysis patients. Clin Chim Acta. 1995;234:127–136. doi: 10.1016/0009-8981(94)05990-A. [DOI] [PubMed] [Google Scholar]
- Hultqvist M, Hegbrant J, Nilsson-Thorell C, Lindholm T, Nilsson P, Linden T, Hultqvist-Bengtsson U. Plasma concentrations of vitamin C, vitamin E and/or malondialdehyde as markers of oxygen free radical production during hemodialysis. Clin Nephrol. 1997;47:37–46. [PubMed] [Google Scholar]
- Ross EA, Koo LC, Moberly JB. Low whole blood and erythrocyte levels of glutathione in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 1997;30:489–494. doi: 10.1016/s0272-6386(97)90306-1. [DOI] [PubMed] [Google Scholar]
- Usberti M, Gerardi G, Micheli A, Tira P, Bufano G, Gaggia P, Movilli E, Cancarini GC, De Marinis S, D'Avolio G, Broccoli R, Manganoni A, Albertin A, Di Lorenzo D. Effects of a vitamin E-bonded membrane and of glutathione on anemia and erythropoietin requirements in hemodialysis patients. J Nephrol. 2002;15:558–564. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001;19:53–61. doi: 10.1159/000014479. [DOI] [PubMed] [Google Scholar]
- Ward RA, McLeish KR. Polymorphonuclear leukocyte oxidative burst is enhanced in patients with chronic renal insufficiency. J Am Soc Nephrol. 1995;5:1697–1702. doi: 10.1681/ASN.V591697. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW, Blumberg JB. Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 2001;59:1960–1966. doi: 10.1046/j.1523-1755.2001.0590051960.x. [DOI] [PubMed] [Google Scholar]
- Ikizler TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol. 2002;58:190–197. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- Nguyen-Khoa T, Massy ZA, De Bandt JP, Kebede M, Salama L, Lambrey G, Witko-Sarsat V, Drueke TB, Lacour B, Thevenin M. Oxidative stress and haemodialysis: role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant. 2001;16:335–340. doi: 10.1093/ndt/16.2.335. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, McMenamin E, McMonagle E. Plasma aminothiol oxidation in chronic hemodialysis patients. Kidney Int. 2002;61:705–716. doi: 10.1046/j.1523-1755.2002.00151.x. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- Odani H, Oyama R, Titani K, Ogawa H, Saito A. Purification and complete amino acid sequence of novel beta 2-microglobulin. Biochem Biophys Res Commun. 1990;168:1223–1229. doi: 10.1016/0006-291x(90)91159-p. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- Parker TF, 3rd, Wingard RL, Husni L, Ikizler TA, Parker RA, Hakim RM. Effect of the membrane biocompatibility on nutritional parameters in chronic hemodialysis patients. Kidney Int. 1996;49:551–556. doi: 10.1038/ki.1996.78. [DOI] [PubMed] [Google Scholar]
- Tayeb JS, Provenzano R, El-Ghoroury M, Bellovich K, Khairullah Q, Pieper D, Morrison L, Calleja Y. Effect of biocompatibility of hemodialysis membranes on serum albumin levels. Am J Kidney Dis. 2000;35:606–610. doi: 10.1016/s0272-6386(00)70005-9. [DOI] [PubMed] [Google Scholar]
- Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- Paolini M, Pozzetti L, Pedulli GF, Marchesi E, Cantelli-Forti G. The nature of prooxidant activity of vitamin C. Life Sci. 1999;64:273–278. doi: 10.1016/S0024-3205(99)00167-8. [DOI] [PubMed] [Google Scholar]
- Edge R, Truscott TG. Prooxidant and antioxidant reaction mechanisms of carotene and radical interactions with vitamins E and C. Nutrition. 1997;13:992–994. doi: 10.1016/S0899-9007(97)00346-8. [DOI] [PubMed] [Google Scholar]
- Otero P, Viana M, Herrera E, Bonet B. Antioxidant and prooxidant effects of ascorbic acid, dehydroascorbic acid and flavonoids on LDL submitted to different degrees of oxidation. Free Radic Res. 1997;27:619–626. doi: 10.3109/10715769709097865. [DOI] [PubMed] [Google Scholar]