Abstract
Ectopic calcification is a frequent complication of many degenerative diseases. Here we identify the serum protein α2–Heremans-Schmid glycoprotein (Ahsg, also known as fetuin-A) as an important inhibitor of ectopic calcification acting on the systemic level. Ahsg-deficient mice are phenotypically normal, but develop severe calcification of various organs on a mineral and vitamin D–rich diet and on a normal diet when the deficiency is combined with a DBA/2 genetic background. This phenotype is not associated with apparent changes in calcium and phosphate homeostasis, but with a decreased inhibitory activity of the Ahsg-deficient extracellular fluid on mineral formation. The same underlying principle may contribute to many calcifying disorders including calciphylaxis, a syndrome of severe systemic calcification in patients with chronic renal failure. Taken together, our data demonstrate a critical role of Ahsg as an inhibitor of unwanted mineralization and provide a novel therapeutic concept to prevent ectopic calcification accompanying various diseases.
Introduction
Physiological mineralization is restricted to bones and teeth. This is in fact surprising given the high extracellular concentrations of calcium and phosphate, the two major components of the mineral phase (1). Pathological mineralization (i.e., ectopic calcification) can be found in many organs. Recent genetic evidence has revealed the necessity of inhibitory mechanisms to prevent ectopic calcification. This is most obvious in mice lacking matrix GLA protein, which display a lethal calcification of arteries and cartilage (2). Other examples include mice lacking osteopontin, which worsens the effect of matrix GLA protein deficiency (3) and mice carrying mutations in Ank or Npps that have a mild calcification in articular cartilage and spinal ligaments, respectively (4, 5). Undesirable calcification can also result from the induction of osteogenic signaling outside the skeleton, for instance in arterial calcification (6, 7). Thus ectopic calcification may result from an imbalance of positive and negative regulatory factors.
In humans there is also a genetic component of ectopic calcification (8–10). However, in most cases it is associated with other primary diseases such as atherosclerosis, cancer, or chronic renal failure, where calcification can be widespread and affect several organs (11–14). The molecular basis of calcification is still poorly understood, but it is generally assumed that certain pathological conditions favor ectopic calcification. This can be caused by local or systemic changes in the availability of calcium, phosphate, or calcifiable surfaces or by ectopic activation of osteogenic signaling, but also by the downregulation of inhibitory mechanisms. Thus, the identification of inhibitors is one important goal in an attempt to prevent disease-associated calcification.
Most protein inhibitors of calcification identified to date act locally and are cellular proteins. Although matrix GLA protein, the most powerful among these inhibitors, is a secreted protein, it is embedded in ECM and has low solubility in physiological buffers (15). Therefore, it is important to identify additional inhibitors of ectopic calcification, if possible circulating molecules that act systemically. α2–Heremans-Schmid glycoprotein/fetuin-A (genetic symbol Ahsg) is an excellent candidate for such a function. The serum protein fetuin was originally described as the major globulin in fetal and newborn calf serum (16). The human homologue was named α2–Heremans-Schmid glycoprotein after two codiscoverers (17). Ahsg is a member of the cystatin superfamily of cysteine protease inhibitors. Additional members of this superfamily sharing cystatin-like domains are kininogen and histidine-rich glycoprotein (18). A closely related protein, fetuin-B (genetic symbol Fetub) was recently detected by searching human, mouse, and rat gene expression databases (19).
Ahsg is an abundant serum protein in mammalians, and related proteins exist in birds and reptiles. (For an excellent monograph see ref. 20.) Ahsg is expressed in the adult liver and in many organs during embryogenesis (21). It accumulates in the skeleton due to a high affinity to hydroxyapatite, the major mineral found in vertebrates (22, 23). Our mechanistic studies suggest that Ahsg inhibits the de novo formation and precipitation of the apatite precursor mineral, basic calcium phosphate (BCP), only transiently (for several hours), and does not dissolve BCP once it is formed (23, 24). Therefore, Ahsg can inhibit undesirable calcification in circulation without inhibiting bone mineralization. The inhibitory activity of serum proteins on apatite formation was largely reduced after the specific depletion of Ahsg from the serum. Recently we showed that the inhibition is mediated by the transient formation of “calciprotein particles,” soluble colloidal spheres containing Ahsg and BCP (24).
To analyze a possible function of Ahsg as an inhibitor of systemic calcification in vivo, we generated Ahsg-deficient mice by targeted deletion of the Ahsg gene (25). These mice in the untreated state have no obvious abnormalities, but alizarin red staining of skeletal preparations revealed the existence of soft-tissue calcification in some female Ahsg-deficient ex-breeders. At that point we reasoned that a pregnancy-associated phenomenon known as physiological maternal hyperparathyroidism (26) increased the calcium load during pregnancy to meet the developing fetus’s high requirement for skeletal mineral. We hypothesized that Ahsg may act as a systemic inhibitor of ectopic calcification under certain conditions favoring calcification. Here we experimentally tested this hypothesis by a feeding experiment and by a genetic approach combining the Ahsg null mutation with the calcification-sensitive mouse strain DBA/2 (27). We provide evidence that both treatments changed the mild calcification phenotype of the original Ahsg null mutants into severe, systemic calcification of vital organs. We report phenotypic similarities of Ahsg-deficient DBA/2 mice with calciphylaxis, a particularly severe calcifying disorder.
Methods
Animals and diets.
The original Ahsg-deficient C57BL/6-129/Sv hybrid mice (25) were backcrossed for at least ten successive generations to pure C57BL/6 and DBA/2 genetic background mice obtained from a commercial breeder (Charles River Wiga GmbH, Sulzfeld, Germany). Thus the original mutant mouse strain named B6;129-Ahsgtm1Mbl was derived into B6-Ahsgtm1Wja and D2-Ahsgtm1Wja according to Institute of Laboratory Animal Resources (ILAR, http://dels.nas.edu/ilar/) terminology. Control mice for all experiments were littermate Ahsg WT mice obtained from heterozygous Ahsg+/– matings. Mice were kept in a climate-controlled room (22°C; 45–54% relative humidity) with a 12-hour light/12-hour dark cycle. Food and water were allowed ad libitum.
Genotyping was performed by Southern blotting as described (25) or by PCR. PCR mixtures contained 0.2 μM WT forward primer (5′-ACTCTTCATTCTCCTAAGGTGG-3′), 0.2 μM knockout forward primer (5′-TTGAATGGAAGGATTGGAGC-3′), 0.4 μM of WT reverse primer (5′-TATGCCTTGTCACAGCACCG-3′), 200 μM of each dNTP, 1 M betaine, 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl, and 2.5 U of Taq DNA polymerase in a total volume of 25 μl. Diagnostic amplicons were 2.0 kb for WT and 0.6 kb for the Ahsg–/– allele, respectively. Mice were fed a standard pelleted rodent diet (Altromin 1324) with the following composition: 0.9% calcium, 0.7% phosphate, 600 IU/kg vitamin D3, 19% crude protein, 4% crude fat, 6% crude fiber, and 7% crude ash. A cohort of ten WT mice (five male and five female) and nine Ahsg-deficient mice (five male and four female, strain B6;129-Ahsgtm1Mbl) were fed for 4 months with a mineral/vitamin D–rich purified diet (Altromin C1032; Altromin GmbH, Lage, Germany) modified with 5% calcium, 3.5% phosphate, and 5,000 IU/kg vitamin D3. In addition, ten mice of each genotype (five male and five female) were fed the standard diet. Mice were 12 weeks of age at the start of the feeding experiment. For all other studies, the age of mice at time of analysis is indicated in the figure legends. Animal experiments were conducted in agreement with German Animal Protection Law under counseling of the University of Mainz and the University Clinics Aachen animal welfare and protection officers.
Determination of ectopic calcification.
For skeletal analysis, mice were eviscerated, fixed in ethanol, cleared in KOH, and stained with alizarin red. For histology, tissues were dissected, fixed in formalin, and processed for paraffin or methacrylate embedding. Sections were stained by von Kossa staining and counterstained with H&E or with safranin O as indicated. Bone histomorphometry analysis was performed using the Osteomeasure Analysis System (Osteometrics Inc., Atlanta, Georgia, USA). The bone parameters of bone volume per total volume, osteoblast number, and osteoclast number were determined according to standard protocols (28). For radiographic analysis, mice were anesthetized with isoflurane and x-rayed using a Senographe DMR x-ray system (GE Medical Systems, Solingen, Germany) with a magnification of ×1.9 at 25 kV and 35 mA. Samples for electron microscopy were fixed in glutaraldehyde and postfixed with osmium tetroxide. Sections were contrasted with uranyl acetate/lead citrate and viewed with a Philips EM400T electron microscope fitted with an EDAX 9100 energy dispersal x-ray elemental analyzer.
Physiological analysis.
Systolic and diastolic blood pressure was recorded in conscious animals using a tail cuff pressure transducer (BP-98A; Softron Co. Ltd., Tokyo, Japan). Values for each individual animal comprised ten successive, averaged measurements within about 5 minutes. Measurements were taken from untrained, conscious mice at the beginning of their active cycle, after the mice had become accustomed to a temperature-controlled restrainer within about 10 minutes. To measure albuminuria, 5 μl of urine was analyzed by SDS-PAGE and Coomassie staining. Gels were computer scanned and the urinary albumin content was analyzed on a Macintosh computer using the public domain NIH Image program (http://rsb.info.nih.gov/nih-image/) with BSA as an internal standard. Intact parathyroid hormone measurements were performed using a commercial RIA kit (Immutopics International LLC, San Clemente, California, USA).
Serum analysis.
Serum for clinical biochemical analysis was obtained from spontaneously clotted blood drawn by retro-orbital bleeding after isoflurane anesthesia. Ionized calcium levels were determined using a Chiron 634 Ca2+/pH analyzer (Chiron Diagnostics GmbH, Fernwald, Germany). Total serum calcium, magnesium, phosphate, and serum creatinine were measured using a Vitros 250 autoanalyzer, Vitros slides, and the Vitros Calibrator Kit 1 (Ortho-Clinical Diagnostics GmbH, Neckargemünd, Germany). To estimate the inhibition of BCP precipitation by serum, precipitation assays were performed using 100,000 cpm of [45Ca]Cl2 dissolved in 500 μl buffer (50 mM Tris-HCl, pH 7.4, 4.8 mM CaCl2, and 1.6 mM Na2HPO4) with and without added protein or increasing amounts of dialyzed serum as described (23, 24). After a 90-minute incubation period, BCP was quantified by measuring the amount of coprecipitated [45Ca]. Assays were done in triplicates. Mouse Ahsg protein for serum reconstitution experiments was purified from pooled serum by ammonium sulfate fractionation, followed by gel filtration and cation exchange chromatography. Fractions were analyzed by ELISA, and mouse serum Ahsg purity was estimated to be greater than 95% using gel electrophoresis, silver staining, and densitometry scanning. Human serum Ahsg protein was from Dade-Behring Marburg GmbH (Marburg, Germany). Ahsg concentration in both mouse and human serum was measured using ELISA and species-specific antisera as described (25). The amount of Ahsg protein added back to the precipitation reactions was estimated by densitometry and immunoblot analysis of Ahsg-deficient and reconstituted precipitation reactions and is given as absolute amounts (human samples) or as Ahsg serum equivalents (mouse samples).
Statistical differences between groups were assessed by the unpaired Student t test.
Results
Ectopic calcification in Ahsg-deficient mice on a diet rich in mineral and vitamin D.
To analyze whether Ahsg is acting as a systemic inhibitor of ectopic calcification in a situation of increased mineral/vitamin D intake, we fed a normal diet or a diet rich in calcium, phosphate, and vitamin D to male and female Ahsg-deficient (Ahsg–/–) and WT littermate mice with a mixed C57BL/6-129/Sv genetic background. These animals represent the original Ahsg gene knockout, which showed a mild calcification phenotype (25). After 4 months of continuous mineral/vitamin D–rich feeding, we sacrificed the animals and determined that serum calcium and phosphate were not elevated regardless of diet or genotype. We performed alizarin red staining of skeletal preparations. In comparison with the mild and rare calcification previously observed in a few Ahsg–/– female ex-breeders (25), calcification was increased both in frequency and extent (Figure 1). We observed calcification in muscles along the vertebrae in five mice in the cohort of nine Ahsg–/– mice (five male, four female) on a mineral/vitamin D–rich diet (Figure 1a). Three of the affected mice were female, two were male. Such a strong calcification phenotype was not observed in ten WT littermates fed the mineral/vitamin D–rich diet or in ten WT or ten Ahsg-deficient mice fed the normal diet. In histological preparations, we observed extracellular calcified lesions and calcification within small blood vessels in several tissues such as kidney and lung (Figure 1b). No quantitative assessment of soft tissue calcification (e.g., by serial sectioning and histomorphometry or by tissue extraction and mineral ash analysis) was made to detect subtle calcification in WT mice on a high mineral/vitamin D diet or in Ahsg-deficient mice on a normal diet. Nevertheless, the major qualitative differences illustrated in Figure 1 demonstrate that Ahsg can act directly or indirectly as an inhibitor of ectopic calcification in vivo in a specialized situation such as high mineral/vitamin D intake.
Figure 1.
A mineral/vitamin D–rich diet leads to ectopic calcification in Ahsg–/– mice. Ahsg+/+ and Ahsg–/– mice (mixed genetic background C57BL/6-129/Sv) were fed a mineral/vitamin D–rich diet for 4 months and analyzed for the presence of ectopic calcification. (a) Alizarin red staining of skeletal preparations. Note that calcified deposits depict small vessels in muscles along the vertebra in Ahsg–/– mice, but not in Ahsg+/+ mice. (b) von Kossa staining and counterstaining with safranin O of kidney and lung. Calcified lesions are stained black in renal tubules and interstitium and pulmonary alveoli of Ahsg–/– mice, but not in Ahsg+/+ mice.
Ectopic calcification in Ahsg–/– mice on a DBA/2 genetic background.
To analyze whether Ahsg has a similar function in a situation of normal mineral intake, we took advantage of the mouse strain DBA/2. This inbred strain is prone to microsurgery-induced calcification in heart and tongue (29); the molecular basis of this inducible phenotype has not yet been uncovered (30). When we combined the Ahsg deficiency with this genetic background, we observed that the breeding performance of homozygous DBA/2-Ahsg–/– mice was significantly reduced compared with that of heterozygous DBA/2-Ahsg+/–, DBA/2-Ahsg+/+, or C57BL/6 mice (data not shown). In addition, the viability of the DBA/2-Ahsg–/– mice was decreased, with a 6-month survival rate of 74% in females and 76% in males. We reasoned that the reduced breeding performance and the increased mortality was caused by an exacerbated calcification phenotype, so we performed high-resolution radiography. Virtually all DBA/2-Ahsg–/– mice examined at age 4 months and older (n = 95; 65 male, 30 female) displayed a severe systemic calcification phenotype (Figure 2a). Calcification was punctate and affected almost every organ, although radiographically it appeared most prominent in skin, kidney, and testis. Such a severe calcification phenotype was never observed in DBA/2-Ahsg+/+ mice (n = 37; 21 male, 16 female), in C57BL/6-Ahsg–/– mice (n = 42; 27 male, 15 female), or in mice kept on a mineral/vitamin D–rich diet (Figure 2a and data not shown). DBA/2-Ahsg+/– heterozygous mice were also not affected by this severe calcification phenotype and appeared normal in radiography (n = 17; 10 male, 7 female). Thus, we focused our subsequent analysis on the DBA/2-Ahsg–/– mice.
Figure 2.
Ahsg deficiency in DBA/2 mice leads to severe ectopic calcification on a normal diet. (a) Radiological analysis of 9-month-old male DBA/2-Ahsg+/+ and DBA/2-Ahsg–/– littermate mice. Note the punctuate soft tissue calcification of the thorax, kidneys, and testes in DBA/2-Ahsg–/– mice. (b) Histological analysis of 7-month-old DBA/2-Ahsg+/+ and DBA/2-Ahsg–/– mice. Plastic-embedded tissues were sectioned and prepared with von Kossa staining and H&E counterstaining. Note the positive staining of calcified lesions in DBA/2-Ahsg–/–, but not in littermate DBA/2-Ahsg+/+ mice. Lesions were extracellular in dilated tubules of kidney cortex tissue. Lesions were often associated with atrophied glomeruli. The myocardium (myocard) contained lesions with a fibrosis capsule. Lung tissue contained numerous spherical, scale-like, calcified deposits blocking alveoli. Subdermal adipose tissue contained large calcified nodules. (c) Electron micrograph of kidney tubule interstitium (top). The electron-dense mineral deposits were first detected in foam cell–like phagocytes infiltrating the extracellular space between tubular cells and the basement membrane. Energy dispersive x-ray analysis of the electron dark mineral deposit in these cells identified Ca and P as the major elements present in this sample (bottom).
We analyzed various organs of the DBA/2-Ahsg–/– mice by undecalcified histology and observed a similar picture in all tissues, to varying degrees (Figure 2b). Generally, calcification was extracellular and most prominent in organs involved in the secretion or transport of mineral-rich fluids or in the generation of local pH changes. In the kidney, the calcification of renal arterioles was associated with atrophy of the downstream glomeruli. Furthermore, obstructed renal collecting ducts were observed in combination with marked dilatation of upstream renal tubules. The myocardium of untreated DBA/2-Ahsg+/+ mice rarely contained calcified lesions. In contrast, all sections from DBA/2-Ahsg–/– mice contained numerous and widespread calcified lesions. Larger lesions were embedded in a fibrosis capsule. In the lung of DBA/2-Ahsg–/– mice, we found a large number of alveoli that were obstructed by globular, scale-like calcified deposits. In skin, we found numerous areas where severe calcification was present in subcutaneous fat.
Next we performed transmission electron microscopy and observed a large number of smaller calcified deposits in addition to the larger ones seen by light microscopy. Figure 2c shows one example of the kidney revealing nascent calcified plaques within the remnants of a phagocytic cell next to the basement membrane in the intercellular space between two tubular cells. To determine the type of mineral formed in the absence of Ahsg, we performed energy dispersive x-ray analysis of several mineral deposits. In all cases the analysis identified calcium and phosphorus as the major components of the mineral phase (Figure 2c).
Pathological consequences of the ectopic calcification in DBA/2-Ahsg–/– mice.
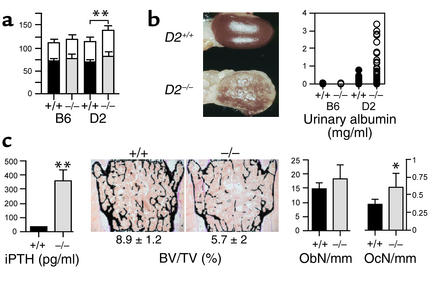
The ectopic calcification phenotype of the DBA/2-Ahsg–/– mice led to several pathological consequences. Systolic and diastolic blood pressure were significantly elevated in DBA/2-Ahsg–/– mice compared with their DBA/2-Ahsg+/+ littermates (Figure 3a). This elevation was not observed in C57BL/6-Ahsg–/– mice, demonstrating that it is a consequence of vascular calcification and renal failure and not an additional function of Ahsg.
Figure 3.
The ectopic calcification in DBA/2-Ahsg–/– mice leads to high blood pressure, renal failure, and secondary hyperparathyroidism. (a) Blood pressure was recorded in 4- to 5-month-old Ahsg+/+ and Ahsg–/– mice on a C57BL/6 (B6) and a DBA/2 (D2) genetic background. Values are presented as mean ± SE in mmHg. Note that systolic blood pressure (white bars) and diastolic blood pressure (black bars) are significantly elevated (**P < 0.0001) in DBA/2-Ahsg–/– mice (n = 12) compared with pressures in DBA2/Ahsg+/+ (n = 13), C57BL/6-Ahsg–/– (n = 20), and C57BL/6-Ahsg+/+ mice (n = 20). (b) Macroscopic view of the kidney at 9 months of age shows severe calcification and hydronephrosis in DBA/2-Ahsg–/– mice but not in DBA2/Ahsg+/+ littermates (left). Urinary albumin was measured in Ahsg+/+ and Ahsg–/– mice on a C57BL/6 and a DBA/2 genetic background (right). Severe albuminuria was observed in DBA/2-Ahsg–/– mice. (c) Determination of serum concentrations of intact parathyroid hormone (iPTH) and the bone parameters bone volume per total volume (BV/TV), osteoblast number (ObN), and osteoclast number (OcN) in 9-month-old DBA/2-Ahsg–/– and DBA/2-Ahsg+/+ littermates (*P < 0.05). Note the presence of hyperparathyroidism, osteopenia, and an increased number of osteoclasts in DBA/2-Ahsg–/– mice (n = 6 for all parameters).
Severe nephrocalcinosis and hydronephrosis were easily observed macroscopically (Figure 3b). This calcification phenotype and the ensuing renal damage was associated with increased serum creatinine levels in DBA/2-Ahsg–/– mice compared with DBA/2-Ahsg+/+ mice (31.2 ± 11.28 μM, n = 12 vs. 19.7 ± 3.13 μM, respectively, n = 10, P = 0.025). In addition, heavy proteinuria was found. Urinary albumin concentrations were below 0.15 mg/ml in urine of DBA/2-Ahsg+/+ mice (n = 36), and up to 3.5 mg/ml in DBA/2-Ahsg–/– mice (n = 38), equivalent to almost 10% of the serum concentration (Figure 3b). These data indicate that both glomerular integrity and retention of proteins with high molecular weight were severely compromised in DBA/2-Ahsg–/– mice. Again, this phenotype is a consequence of ectopic calcification, since we did not observe a difference between WT and Ahsg-deficient mice on a C57BL/6 genetic background.
DBA/2-Ahsg–/– mice developed a secondary hyperparathyroidism that may have been a result of renal damage (31), although we cannot exclude the possibility that Ahsg deficiency, per se, influenced parathyroid function. Serum parathyroid hormone levels were elevated tenfold in DBA/2-Ahsg–/– mice compared with their DBA/2-Ahsg+/+ littermates (Figure 3c). Accordingly, the absence of Ahsg in the DBA/2 genetic background led to a marked osteopenia characterized by an increase in osteoclast number (Figure 3c). The fact that bone volume and serum intact parathyroid hormone in C57BL/6-Ahsg–/– mice was normal (data not shown) indicates that the osteopenia in DBA/2-Ahsg–/– mice was a consequence of renal damage and not a direct effect of the Ahsg deficiency on bone cells. Taken together, the DBA/2-Ahsg–/– mice display important clinical hallmarks of human patients suffering from chronic renal failure (12, 32).
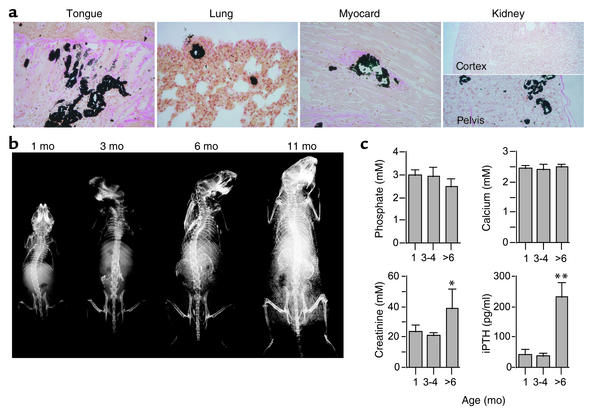
Renal failure is associated with calcification and therefore it is important to determine whether the calcification in the DBA/2-Ahsg–/– mice developed before or after renal failure. Figure 4 shows that calcification was detectable in histological sections using von Kossa staining at 1 month of age. Calcified lesions were present in tongue, lung, myocardium, and kidney tissue. In the kidney only the pelvis, not the medulla or the cortex, was affected at this young age. In contrast, WT DBA/2 mice showed no calcification even at 9 months of age (see Figure 2b). Next we analyzed the time course of calcification by radiography. Figure 4b illustrates that calcification was detectable in the renal pelvis starting at 2 months of age and progressed into calcification of both entire kidneys at 6 months of age. Note that the mice depicted in Figure 4b were alive at the time of radiography, and therefore calcified heart and lung tissue (Figure 2 and Figure 4a) was blurred due to movement. Importantly, we measured normal serum calcium, phosphate, creatinine, and intact parathyroid hormone in Ahsg-deficient mice until 4 months of age (Figure 4c). Hence, the calcification preceded the severe kidney damage observed in mice of 6 months age and older. Therefore, calcification was primarily a consequence of the lack of Ahsg and not of renal damage. Kidney damage may, however, further exacerbate the calcification phenotype.
Figure 4.
Calcification of Ahsg-deficient DBA/2 mice precedes kidney failure. (a) Plastic-embedded tissues of 1-month-old mice were sectioned and stained by von Kossa staining and counterstained with H&E. Calcification was detected in soft tissues including tongue, lung, myocardium, and kidney pelvis, but not yet in the kidney cortex. Note that even at 9 months of age, WT DBA/2 mice showed no calcification (see Figure 2b). (b) Radiological analysis detected progressive calcification of the kidneys and the skin. Note that the mice were alive at the time of radiography and therefore calcified heart and lung tissue was blurred due to movement. (c) Determination of serum phosphate, calcium, creatinine, and intact parathyroid hormone indicated that kidney failure in DBA/2-Ahsg–/– mice occurred after 4 months of age and therefore after the onset of soft tissue calcification (1 month, n = 4; 3–4 months, n = 6; over 6 months, n = 4). *P < 0.05, **P < 0.001.
Explaining the phenotype of DBA/2-Ahsg–/– mice.
To find a molecular explanation for the severe calcification phenotype of the DBA/2-Ahsg–/– mice, we first measured ion concentrations in the serum of WT and Ahsg-deficient mice on a C57BL/6 and DBA/2 genetic background, respectively. All the animals analyzed had calcium and phosphate concentrations within the normal range, thus ruling out hypercalcemia or hyperphosphatemia as a cause of the ectopic calcification phenotype in DBA/2-Ahsg–/– mice (Figure 4c and Figure 5a). Magnesium concentrations were lower in DBA/2 mice regardless of the presence or absence of Ahsg (Figure 5a). Magnesium is a potent inhibitor of calcification, because Mg2+ ions readily substitute for Ca2+ in the apatite crystal lattice and cause crystal poisoning (33). Accordingly, low serum magnesium has previously been identified as one possible reason for the predisposition of DBA/2 mice to experience calcification of their tongue and heart tissue (27). It may therefore contribute to the background differences between C57BL/6 and DBA/2 mice. Thus we tentatively conclude that the phenotype of DBA/2-Ahsg–/– mice is most likely a consequence of the combination of the DBA/2 strain–dependent propensity to calcify and the deficiency of Ahsg as a serum-borne inhibitor of calcification.
Figure 5.
Serum chemistry of Ahsg-deficient mice. (a) Serum electrolytes are given as the mean ± SE of at least six mice for each measurement (**P < 0.01). Where there are no error bars, the SEs were too small to be visible. (b) Sera from C57BL/6-Ahsg+/+ (filled circles) and DBA/2-Ahsg+/+ mice (filled squares) both inhibited the de novo formation of BCP from supersaturated solutions of calcium and phosphate with an IC50 of 0.8% serum. Serum from C57BL/6-Ahsg–/– (open circles) and DBA/2-Ahsg–/– (open squares) mice inhibited at a much reduced rate (IC50 5.2% and 6.8% serum, respectively). (c) Reconstitution of BCP precipitation inhibition. Note that the reduced inhibition of BCP precipitation by sera from Ahsg-deficient mice could be restored to the WT level by adding back purified mouse serum Ahsg. (d) Serum electrolyte concentrations in normal and calciphylaxis patients (*P < 0.05). (e) Inhibition of BCP precipitation by sera from three healthy subjects (filled circles) and eight calciphylaxis patients (open circles). Note that the inhibition of BCP precipitation is greatly reduced in all calciphylaxis patients. (f) Serum reconstitution by purified human Ahsg in serum of a calciphylaxis patient. Note that the reduced inhibition of BCP precipitation by serum from the calciphylaxis patient could be restored to normal levels by adding back purified human serum Ahsg.
To analyze the serum inhibition of calcification, we performed an in vitro precipitation inhibition assay. The chemical and crystallographic nature of the mineral formed in this assay is complex and comprises a mixture of amorphous calcium phosphate, octacalcium phosphate, and apatite, most aptly described as basic calcium phosphate (BCP). Serum from DBA/2-Ahsg+/+ mice inhibited BCP precipitation in a dose-dependent manner, with an IC50 of 0.8% serum (Figure 5b). In contrast, serum from DBA/2-Ahsg–/– littermates did not influence the formation of BCP at a final concentration of up to 4% and had an IC50 of 6.8%. Similar values were obtained when serum from Ahsg–/– mice (IC50, 5.2%) or Ahsg+/+ mice (IC50, 0.8%) on a C57BL/6 background were compared. These results suggest that Ahsg acts as a systemic inhibitor and that Ahsg deficiency in serum elicits the formation of insoluble BCP. As expected, adding purified Ahsg to the Ahsg–/– serum restored the inhibition of BCP precipitation to the same extent as the serum from Ahsg+/+ mice (Figure 5c). This experiment indicates that the absence of Ahsg is associated with a lack of inhibitory activity of serum proteins on BCP precipitation. Low-molecular-weight serum inhibitors like magnesium and pyrophosphate are not measured in the precipitation inhibition assay, which employs dilute, dialyzed serum. Likewise, tissue-bound inhibitors or activators of calcification are not contained in this assay, but do all contribute to the final outcome in intact animals. This may explain why DBA/2-Ahsg–/– mice do, but C57BL/6-Ahsg–/– mice do not, spontaneously calcify to the same extent detectable by radiography, although both strains show similarly reduced serum inhibition capacity.
Ahsg, calcium, and phosphate in calciphylaxis patients.
We reasoned that the lack of inhibition of BCP precipitation, and not only an elevated calcium phosphate product, could be a contributing factor for ectopic calcification in humans. We analyzed calciphylaxis patients, who suffer a particularly severe calcification disorder. Calciphylaxis is found in 1–4% of dialysis patients with end-stage renal failure (34–36), and according to a recent report, may in fact be clinically silent in many patients (37). Concerning human pathology, calciphylaxis shares similarities with DBA/2-Ahsg–/– mice, as the patients are characterized by chronic renal failure, severe ectopic calcification of various organs, and a high rate of mortality. We therefore collected sera from eight calciphylaxis patients and three control subjects and assayed for inhibition of BCP precipitation in vitro in the same way as described above for mouse sera. Figure 5d shows that total serum calcium and serum magnesium were similar in calciphylaxis patients and control subjects (Ca, 2.4 ± 0.2 mM calciphylaxis patients vs. 2.4 ± 0.3 mM control; Mg, 1.0 ± 0.19 mM calciphylaxis patients vs. 0.9 ± 0.2 mM control), and serum phosphate was mildly elevated in calciphylaxis patients compared with control subjects (1.8 ± 0.2 mM vs. 1.2 ± 0.3 mM, respectively). It is worth noting that half the patients had a calcium phosphate product in the normal range, consistent with other literature reports (38). Thus, it is not possible to explain the severe systemic calcification of calciphylaxis patients by increased levels of calcium and phosphate alone. When we measured the serum potential to inhibit spontaneous BCP formation in a precipitation assay, we determined that the sera from the control group inhibited with an IC50 of 2.2% ± 0.2% serum, and all sera derived from calciphylaxis patients had a largely decreased inhibitory potential on BCP precipitation (Figure 5e). In fact the IC50 values of these sera were almost twofold higher (4.3% ± 0.7%, P < 0.001) on average, ranging from 3.75% to more than 6% serum. These elevated IC50 values correlated with a low serum Ahsg concentration in all patients (0.26 ± 0.08 mg/ml vs. control, 0.59 ± 0.07 mg/ml, P = 0.0017), and reconstitution experiments demonstrated that the elevated IC50 values were paralleled by relative Ahsg deficiency (Figure 5f and data not shown). Taken together, these data suggest that decreased inhibitory activity of serum proteins, notably associated with low Ahsg levels, contributes to the systemic calcification seen in these patients.
Discussion
The existence of protein inhibitors of ectopic calcification has long been suggested, given the fact that all extracellular fluids contain calcium and phosphate concentrations exceeding the solubility product for spontaneous precipitation (33, 39). A few such inhibitors have already been identified. They include, among others, mineral-binding ECM proteins such as matrix GLA protein and cellular proteins such as Npps or Ank that act by increasing extracellular concentrations of the calcification inhibitor pyrophosphate (2–5). In this study we demonstrate that the serum protein Ahsg may act as a systemic inhibitor of ectopic calcification. This activity is unique to Ahsg in that proteins of comparable molecular weight or similar negative charge like ovalbumin, albumin, lysozyme, glutathione S–transferase, mannose-binding protein, and the established calcium-binding protein calmodulin all failed to inhibit calcification at the micromolar concentrations sufficient for effective inhibition by Ahsg (23, 40). Structure-function studies in our laboratory showed that Ahsg solubilizes BCP as a colloid by forming “calciprotein particles” (24). The underlying mechanism involved a direct interaction with the mineral phase and the prevention of large crystal formation. This was confirmed in vivo by the discovery of Ahsg as the major protein of a high-molecular-weight complex also containing calcium, phosphate, and matrix GLA protein in serum of etidronate-treated rats (41–44).
Such a mechanism is much reminiscent of the way in which apolipoproteins keep insoluble lipids in solution by covering them with a soluble sheet. Alternative biological activities of Ahsg could also be responsible for the observed effects.
Apart from direct inhibition of calcification on a chemical level, Ahsg could also indirectly influence calcification by regulating energy metabolism (45) or bone metabolism (46). In particular, Ahsg has been shown to antagonize TGFs and bone morphogenetic proteins (47–48), which are potent osteogenic growth and differentiation factors possibly involved in atherosclerotic calcification (49) and muscle calcification (50). In addition, Ahsg could aid the removal of BCP precipitates by phagocytosis. Several reports suggest that Ahsg may play a more general role in phagocytosis regulation and innate immunity. Ahsg can act as an opsonin (51), it quenches the oxidative burst associated with the uptake of apatite crystals by neutrophils (52), and it forms antibody complexes involved in marking and removal of apoptotic neutrophils (53). Low Ahsg serum concentrations have been found to be associated with depressed cellular immunity (54) and nonspecific host defense (55). Recent clinical studies suggest that low Ahsg serum levels are an outstanding predictor of short-term mortality in patients with liver cirrhosis and liver cancer (56) and correlate with short-term mortality in uremic patients (57). These reports do not necessarily infer causality, but they are fully compatible with a role of Ahsg in clearing insoluble remnants and cellular debris. It is interesting that a similar function in innate immunity has been recently demonstrated for a structurally related member of the cystatin superfamily of serum proteins, histidine-rich glycoprotein (58).
Regardless of the precise molecular mechanism of the inhibition of undesirable calcification by Ahsg, it is important to state that, in contrast to other inhibitors of ectopic calcification, Ahsg acts in all extracellular fluids and is not locally restricted. Therefore, any situation that lowers Ahsg serum concentration will increase the risk of systemic calcification. It is important in this context to recall that many calcification disorders, including arteriosclerosis and calciphylaxis, have an inflammatory component, and that human Ahsg is a negative acute-phase protein that is downregulated after infection or trauma (59). We analyzed calciphylaxis patients because they suffer a particularly severe form of soft tissue calcification. This includes the precipitation of calcium phosphate in the microvasculature, which also occurs in Ahsg-deficient DBA/2 mice. However, the etiology of calciphylaxis is complex and we do not suggest that the Ahsg-deficient mice are a model for human calciphylaxis. Our ongoing analysis of patients demonstrates that calcification is often associated with depressed serum Ahsg. It is quite possible that in some cases Ahsg was decreased to start with, as it was in the Ahsg-deficient mice. In other cases, probably including most human calcification diseases, Ahsg could be reduced due to inflammation or consumption in the progression of the calcification disease. Both situations are fully compatible with the hypothesis that prolonged deficiency of Ahsg as a major serum inhibitor of calcification may increase the risk of calcification.
The function of Ahsg as an inhibitor of ectopic calcification, at least in mice, is dispensable in the physiological situation, but is essential in conditions favoring calcification. For now it is impossible to draw the same conclusions for human pathology, because no Ahsg deficiency has been described yet. Moreover, there is experimental evidence that cutaneous calciphylaxis can be induced in rats but not in mice, indicating that there are species differences in the predisposition for ectopic calcification (60). Our own data show that the IC50 values for inhibition of BCP precipitation obtained with human serum are at least twofold higher than those obtained with mouse serum (Figure 5, b and e). Therefore it is likely that mice are better protected against ectopic calcification than are rats or humans.
Although it is impossible to fully address this issue experimentally, one important general conclusion can be drawn from this study. The concentration of ions, especially of calcium and phosphate, is not the sole determining factor for ectopic calcification. Here we demonstrate that the presence or absence of serum inhibitors of BCP precipitation plays a more critical role than previously anticipated. In fact, in an initial screen of hemodialysis patients, we have already determined that Ahsg serum concentrations are significantly lower than those in healthy controls, and correlate inversely with all-cause mortality (57). Future studies are required to underscore the clinical significance of these findings and to analyze the potential of using Ahsg as a therapeutic agent to reduce ectopic calcification.
Acknowledgments
We thank B. Lecher for help with animal experimentation, H.G. Hollweg for electron microscopy, and P. Catala-Lehnen for bone histomorphometry analyses. Financial support was obtained from the Deutsche Forschungsgemeinschaft (to W. Jahnen-Dechent).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: α2–Heremans-Schmid glycoprotein (Ahsg); basic calcium phosphate (BCP).
References
- 1.Schinke T, McKee MD, Karsenty G. Extracellular matrix calcification: where is the action? Nat. Genet. 1999;21:150–151. doi: 10.1038/5928. [DOI] [PubMed] [Google Scholar]
- 2.Luo G, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 3.Speer MY, et al. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 5.Okawa A, et al. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 6.Bostrom K, Demer LL. Regulatory mechanisms in vascular calcification. Crit. Rev. Eukaryot. Gene Expr. 2000;10:151–158. [PubMed] [Google Scholar]
- 7.Shanahan CM, et al. Expression of mineralisation-regulating proteins in association with human vascular calcification. Z. Kardiol. 2000;89(Suppl. 2):63–68. doi: 10.1007/s003920070101. [DOI] [PubMed] [Google Scholar]
- 8.Le Saux O, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet. 2000;25:223–227. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 9.Munroe PB, et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura I, et al. Association of the human NPPS gene with ossification of the posterior longitudinal ligament of the spine (OPLL) Hum. Genet. 1999;104:492–497. doi: 10.1007/s004390050993. [DOI] [PubMed] [Google Scholar]
- 11.Halverson PB. Calcium crystal-associated diseases. Curr. Opin. Rheumatol. 1996;8:259–261. doi: 10.1097/00002281-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Goodman WG, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N. Engl. J. Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 13.Rosenhek R, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N. Engl. J. Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 14.Faverly DR, Hendriks JH, Holland R. Breast carcinomas of limited extent: frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer. 2001;91:647–659. doi: 10.1002/1097-0142(20010215)91:4<647::aid-cncr1053>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Hackeng TM, Rosing J, Spronk HM, Vermeer C. Total chemical synthesis of human matrix Gla protein. Protein Sci. 2001;10:864–870. doi: 10.1110/ps.44701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen KO. Fetuin, a new globin isolated from serum. Nature. 1944;154:575. [Google Scholar]
- 17.Schultze HE, Heide K, Haupt H. Charakterisierung eines niedermolekularen α2-Mukoids aus Humanserum. Naturwiss. 1962;49:15–17. [Google Scholar]
- 18.Elzanowski A, Barker WC, Hunt LT, Seibel-Ross E. Cystatin domains in α2-HS glycoprotein and fetuin. FEBS Lett. 1988;227:167–170. doi: 10.1016/0014-5793(88)80890-1. [DOI] [PubMed] [Google Scholar]
- 19.Olivier E, et al. Fetuin-B, a second member of the fetuin family in mammals. Biochem. J. 2000;350:589–597. [PMC free article] [PubMed] [Google Scholar]
- 20.Dziegielewska, K.M., and Brown, W.M. 1995. Fetuin. Springer-Verlag. Heidelberg, Germany. 178 pp.
- 21.Terkelsen OB, et al. Rat fetuin: distribution of protein and mRNA in embryonic and neonatal rat tissues. Anat. Embryol. (Berlin) 1998;197:125–133. doi: 10.1007/s004290050124. [DOI] [PubMed] [Google Scholar]
- 22.Triffitt JT, Owen ME, Ashton BA, Wilson JM. Plasma disappearance of rabbit α2-HS glycoprotein and its uptake by bone tissue. Calcif. Tissue Res. 1978;26:155–161. doi: 10.1007/BF02013251. [DOI] [PubMed] [Google Scholar]
- 23.Schinke T, et al. The serum protein α2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J. Biol. Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 24.Heiss A, et al. Structural basis of calcification inhibition by α2-HS glycoprotein/fetuin-A: formation of colloidal calciprotein particles. J. Biol. Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 25.Jahnen-Dechent W, et al. Cloning and targeted deletion of the mouse fetuin gene. J. Biol. Chem. 1997;272:31496–31503. doi: 10.1074/jbc.272.50.31496. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 27.van den Broek FA, Beynen AC. The influence of dietary phosphorus and magnesium concentrations on the calcium content of heart and kidneys of DBA/2 and NMRI mice. Lab. Anim. 1998;32:483–491. doi: 10.1258/002367798780599758. [DOI] [PubMed] [Google Scholar]
- 28.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 29.Brunnert SR, Shi S, Chang B. Chromosomal localization of the loci responsible for dystrophic cardiac calcinosis in DBA/2 mice. Genomics. 1999;59:105–107. doi: 10.1006/geno.1999.5862. [DOI] [PubMed] [Google Scholar]
- 30.Colinayo VV, et al. Genetic characterization of the Dyscalc locus. Mamm. Genome. 2002;13:283–288. doi: 10.1007/s00335-001-2148-1. [DOI] [PubMed] [Google Scholar]
- 31.Drueke TB. Genetic aspects of secondary hyperparathyroidism in uremia. Am. J. Kidney Dis. 2001;38:S143–S146. doi: 10.1053/ajkd.2001.27424. [DOI] [PubMed] [Google Scholar]
- 32.London GM, Pannier B, Marchais SJ, Guerin AP. Calcification of the aortic valve in the dialyzed patient. J. Am. Soc. Nephrol. 2000;11:778–783. doi: 10.1681/ASN.V114778. [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal NC. Mechanisms of inhibition of calcification. Clin. Orthop. 1989;247:279–289. [PubMed] [Google Scholar]
- 34.Angelis M, Wong LL, Myers SA, Wong LM. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083–1089; discussion 1089–1090. doi: 10.1016/s0039-6060(97)90212-9. [DOI] [PubMed] [Google Scholar]
- 35.Khafif RA, DeLima C, Silverberg A, Frankel R. Calciphylaxis and systemic calcinosis. Collective review. Arch. Intern. Med. 1990;150:956–959. [PubMed] [Google Scholar]
- 36.Gilson RT, Milum E. Calciphylaxis: case report and treatment review. Cutis. 1999;63:149–153. [PubMed] [Google Scholar]
- 37.Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210–2217. doi: 10.1046/j.1523-1755.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 38.Vasku V, Vasku J. The development of the pathophysiological concept of calciphylaxis in experiment and clinic. Pathophysiology. 2001;7:231–244. doi: 10.1016/s0928-4680(00)00064-x. [DOI] [PubMed] [Google Scholar]
- 39.Neuman, W.F. 1980. Bone material and calcification mechanisms. In Fundamental and clinical bone physiology. M.R. Urist, editor. J.B. Lippincott Co. Philadelphia, Pennsylvania, USA. 83–107.
- 40.Schinke T, Koide T, Jahnen-Dechent W. Human histidine-rich glycoprotein expressed in SF9 insect cells inhibits apatite formation. FEBS Lett. 1997;412:559–562. doi: 10.1016/s0014-5793(97)00827-2. [DOI] [PubMed] [Google Scholar]
- 41.Price PA, et al. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J. Biol. Chem. 2002;277:3926–3934. doi: 10.1074/jbc.M106366200. [DOI] [PubMed] [Google Scholar]
- 42.Price PA, Caputo JM, Williamson MK. Bone origin of the serum complex of calcium, phosphate, fetuin, and matrix Gla protein: biochemical evidence for the cancellous bone-remodeling compartment. J. Bone Miner. Res. 2002;17:1171–1179. doi: 10.1359/jbmr.2002.17.7.1171. [DOI] [PubMed] [Google Scholar]
- 43.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J. Biol. Chem. 2003;278:22144–22152. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 44.Price PA, Nguyen TM, Williamson MK. Biochemical characterization of the serum fetuin-mineral complex. J. Biol. Chem. 2003;278:22153–22160. doi: 10.1074/jbc.M300739200. [DOI] [PubMed] [Google Scholar]
- 45.Mathews ST, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 46.Szweras M, et al. alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 47.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J. Biol. Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 48.Binkert C, et al. Regulation of osteogenesis by fetuin. J. Biol. Chem. 1999;274:28514–28520. doi: 10.1074/jbc.274.40.28514. [DOI] [PubMed] [Google Scholar]
- 49.Demer LL. A skeleton in the atherosclerosis closet. Circulation. 1995;92:2029–2032. doi: 10.1161/01.cir.92.8.2029. [DOI] [PubMed] [Google Scholar]
- 50.Shafritz AB, et al. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N. Engl. J. Med. 1996;335:555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 51.van Oss CJ, Gillman CF, Bronson PM, Border JR. Opsonic properties of human serum α2-HS glycoprotein. Immunol. Commun. 1974;3:329–335. doi: 10.3109/08820137409061113. [DOI] [PubMed] [Google Scholar]
- 52.Terkeltaub RA, Santoro DA, Mandel G, Mandel N. Serum and plasma inhibit neutrophil stimulation by hydroxyapatite crystals. Evidence that serum α2-HS glycoprotein is a potent and specific crystal-bound inhibitor. Arthritis Rheum. 1988;31:1081–1089. doi: 10.1002/art.1780310901. [DOI] [PubMed] [Google Scholar]
- 53.Hart SP, et al. Specific binding of an antigen-antibody complex to apoptotic human neutrophils. Am. J. Pathol. 2003;162:1011–1018. doi: 10.1016/S0002-9440(10)63895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baskies AM, et al. Serum glycoproteins in cancer patients: first report of correlations with in vitro and in vivo parameters of cellular immunity. Cancer. 1980;45:3050–3060. doi: 10.1002/1097-0142(19800615)45:12<3050::aid-cncr2820451229>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 55.Kalabay L, et al. Diagnostic value of the determination of serum alpha2-HS-glycoprotein. Orv. Hetil. 1992;133:1553–1554. [PubMed] [Google Scholar]
- 56.Kalabay L, et al. Human fetuin/alpha2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur. J. Gastroenterol. Hepatol. 2002;14:389–394. doi: 10.1097/00042737-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Ketteler M, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 58.Gorgani NN, Smith BA, Kono DH, Theofilopoulos AN. Histidine-rich glycoprotein binds to DNA and Fc gamma RI and potentiates the ingestion of apoptotic cells by macrophages. J. Immunol. 2002;169:4745–4751. doi: 10.4049/jimmunol.169.9.4745. [DOI] [PubMed] [Google Scholar]
- 59.Lebreton JP, et al. Serum concentration of human α2-HS glycoprotein during inflammatory process. Evidence that α2-HS glycoprotein is a negative acute-phase reactant. J. Clin. Invest. 1979;64:1118–1129. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller S, Vernon-Roberts E, McClure J. Cutaneous calciphylactic reactions in the mouse and the rat and the effects of diphosphonates on the reaction in the rat. J. Pathol. 1984;142:7–13. doi: 10.1002/path.1711420105. [DOI] [PubMed] [Google Scholar]